Method Article
Using Near-Infrared Spectroscopy Wearable Devices to Identify Central Versus Peripheral Limitations During Exercise
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol integrates near-infrared spectroscopy (NIRS) technology to assess localized hematological and oxygenation changes at the prefrontal cortex, respiratory (m.Intercostales), and locomotor (m.Vastus Lateralis) muscles during cardiopulmonary exercise testing, enabling the identification of central and peripheral limiting factors affecting exercise performance.
Streszczenie
The gold standard to assess the aerobic capacity in physically active subjects and athletes is the maximal oxygen consumption test (VO2–max), which involves analysis of exhaled–gases and cardiorespiratory variables obtained via the breath-by-breath method in an ergospirometer during an incremental exercise. However, this method cannot elucidate metabolic changes at the muscular level. Near-infrared spectroscopy (NIRS) has emerged as a valuable technology to evaluate local oxygen levels (Tissular Saturation Index, TSI) by quantifying the concentrations of oxygenated (O2-Hb) and deoxygenated (H-Hb) hemoglobin in the microvasculature of tissues. NIRS applications extend to respiratory and locomotor muscles, assessing metabolic changes associated with the cost of breathing (COB) and peripheral workload, respectively. Additionally, cerebral regions, such as the prefrontal cortex, have been explored with NIRS technology to assess physiological changes related to cognitive demand associated with planning or ideation of motor tasks linked to sports performance. Thus, by analyzing exercise-induced changes (D) in O2-Hb, H-Hb, and TSI, it is possible to identify central and peripheral exercise limitations, particularly when endurance training is the main component of physical fitness (e.g., running, cycling, triathlon, etc.). Addressing these factors is paramount for coaches and exercise physiologists to optimize athletic performance, incorporating training strategies focused on the primary exercise-limiting factors. This study outlines a protocol for utilizing wearables devices equipped with NIRS technology to analyze exercise changes in TSI, O2-Hb, and H-Hb, alongside cardiorespiratory variables typically registered in athletes during VO2–max tests. This approach offers a comprehensive method for identifying the primary systems involved in stopping exercise progression and sports performance improvement.
Wprowadzenie
Endurance athletes rely on an efficient balance of oxygen delivery and uptake to sustain high–intensity exercise and enhance their athletic performance1,2. The maximal oxygen uptake test (VO2-max) is a vital physiological assessment that determines sports performance by analyzing exhaled–gases and cardiorespiratory variables during incremental exercise intensity1. This assessment, known as ergospirometry or Cardiopulmonary Exercise Testing (CPET), reflects the exercise response of the cardiovascular, respiratory, and muscular systems3. Along these lines, the increased energy cost associated with breathing, referred to as the cost of breathing (COB), heightens the demand for nutrients and oxygen in the surrounding tissues. This phenomenon has been documented to potentially reduce blood flow to the muscles involved in active movements, resulting in a decreased tolerance to physical exertion and an early cessation of exercise progression due to the metabolic reflex4.
During a VO2–max test, it is also possible to identify the ventilatory thresholds (VTs), which correspond to specific exercise intensities marking the transition from aerobic to anaerobic metabolism (aerobic threshold or ventilatory threshold 1 [VT1], and anaerobic threshold or respiratory compensation point [RCP] or ventilatory threshold 2 [VT2])5. The VTs reflect the ventilatory responses that compensate for metabolic changes during incremental exercise6. By identifying these thresholds, CPET offers a comprehensive evaluation by integrating the responses of multiple biological systems critically engaged during high–intensity exercise.
However, while ergospirometry is widely considered the gold standard for evaluating CPET, it does not capture metabolic changes occurring at the muscle level. These changes are crucial for understanding the physiological limiting factors associated with the lack of progression during high–intensity exercise in endurance athletes. In this context, NIRS technology has emerged as a valuable tool in exercise science, aiding in analyzing hemodynamic variables at the microvascular muscle level7.
In recent years, sports professionals and researchers have used a wide range of commercial wearables equipped with NIRS technology to explore non–invasive muscular changes during exercise, providing the ability to determine VT1 and VT2 with this technology8. Thus, the integrative analysis of data from NIRS and CPET offers a comprehensive understanding of physiological responses to exercise.
The NIRS technology utilizes the modified Beer-Lambert law to quantify changes (D) in the concentrations of oxyhemoglobin (O2-Hb) and deoxyhemoglobin (H-Hb) during exercise7. At the local tissue level, a decrease in O2-Hb reflects an increase in local metabolic demand, while an increase in H-Hb reflects an increase in oxygen extraction. Total hemoglobin (tHb), the sum of O2-Hb and H-Hb, is used as an index of local tissue blood flow. Conversely, the difference between O2-Hb and H-Hb (Hbdiff) provides an index of tissue oxygen extraction9. The tissular saturation index (TSI), calculated as the ratio of O2-Hb to tHb, reflects the tissue oxygen saturation level and indicates the balance between local oxygen delivery and uptake10,11. Thus, NIRS data give critical insights into the physiological status at the microvascular level, providing a detailed understanding of tissue oxygenation and hemodynamics that complements the information obtained from CPET.
This detailed understanding provided by NIRS technology extends to many practical applications. Recent research highlights the versatility of NIRS and demonstrates its practical application in monitoring respiratory12,13 and locomotor muscles7, as well as brain regions involved in motor act ideation, such as the prefrontal cortex (PFC)14,15. This broad applicability underscores the ability of NIRS to provide comprehensive insight into physiological responses to various types of muscle contractions (concentric or, eccentric, or isometric contractions) and exercise.
By analyzing exercise-induced DTSI at both the muscular and cerebral levels, NIRS provides a valuable potential for identifying associations between peripheral and central limiting factors that affect the progression of exercise16,17. For example, among central limiting factors, decreased blood flow resulting from cerebral vasoconstriction caused by compensatory hyperventilation due to elevated hydrogen levels from anaerobic metabolism and increased blood lactate during high-intensity exercise is a significant contributor to the reduction in TSI in the prefrontal cortex17,18. In contrast, peripheral limiting factors are characterized by an imbalance between oxygen supply and demand in the exercising musculature19. Reduced local oxygen delivery and increased oxygen consumption can lead to tissue deoxygenation, as evidenced by decreased TSI20. This distinction highlights the multifaceted nature of performance limitations during high–intensity exercise, where both central and peripheral mechanisms are critical. This understanding suggests that delaying the onset of these limiting factors during exercise may contribute to improved athletic performance.
To fully leverage the potential of NIRS technology in identifying these limitations, standardized procedures are essential to ensure high-quality data collection and analysis. This document outlines methods for conducting maximal endurance exercise testing using NIRS technology to collect physiological data and elucidate the relationship between central and peripheral limiting factors during high-intensity exercise in endurance athletes. The proposed protocol provides a standardized approach to ensure consistency and accuracy in assessing the physiological phenomena underlying these limiting factors.
Protokół
The protocol was approved by the Institutional Review Board of the Pontificia Universidad Católica de Chile (projects nº 210525001 and 220608010), and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the testing described.
1. Placement and setup of NIRS wearables
NOTE: Various NIRS wearables and data acquisition software can be utilized. Researchers should thoroughly consult the manufacturer's instructions and guidelines to ensure proper setup and usage. In this study, the devices that use a continuous wave register of the NIRS signal are used. These commercial devices are easy to use, but they can only detect changes in light attenuation relative to the reference or baseline phase and cannot detect absolute concentrations like other devices that employ a time-domain register of NIRS.
- NIRS wearables preparation and general placement guidelines
- Before placing the devices and starting the measurements, ensure that all wearables are fully charged.
NOTE: For the devices used in this study, the manufacturer reports that a battery with a full charge can register 6–8 h continuously. - Apply double-sided adhesive tape to all wearables to secure them to the participant's skin, ensuring that the tape does not obstruct the light emitters and detectors.
- Cover all wearables with a layer of cling film, followed by a layer of a waterproof adhesive dressing to protect them from sweat.
- Before placing the devices, clean the target area with an alcohol pad to remove any residue that may interfere with the register (e.g., creams, cosmetics, etc.). If necessary, shave the area around the target site, as hair can interfere with NIRS signals.
NOTE: It is recommended that a thorough hand wash be performed before placing any device on the participant's skin to prevent potential contamination. Wearing gloves is encouraged, as it can further reduce the risk of contamination. - Once all wearables are correctly placed on the participant's skin (see section 1.2), secure them with a layer of elastic therapeutic tape. If additional fixation is needed, use an elastic bandage wrap of dark color, ensuring that excessive compression does not alter the measurements (less than the 25 mm Hg capillary occlusion pressure measured by a conventional sphygmomanometer).
- Place a black cloth over all wearables to prevent ambient light from penetrating. If covering the area with a cloth is not possible (around 6 cm2), use black elastic therapeutic tape to block ambient light.
- Before placing the devices and starting the measurements, ensure that all wearables are fully charged.
- NIRS device placement
NOTE: Ensure that NIRS wearables devices are placed so that ON/OFF and setting buttons are easily accessible.- Prefrontal Cortex: Place the NIRS probe on the left or right dorsolateral prefrontal cortex, approximately 10 mm above the participant's superciliary arch, similar to Fp1 electrode placement according to the modified international EEG 10-20 system21.
- m.Intercostales: Place the NIRS probe over the 7th intercostal space at the right anterior axillary line22,23,24. If, for some reason, it is not positioned over the right hemithorax, position it over the left hemithorax, but the signal from the heart rate may be more pronounced on the left side.
- To confirm NIRS penetration depth, use a B–mode ultrasound to verify the distance from subcutaneous tissue to the outer border of the m.Intercostales. For measurements at m.Intercostales, ensure that the distance between skin and muscle is less than 15 mm.
- m.Vastus Lateralis: Place the NIRS probe 5 cm lateral to the imaginary line's midpoint, connecting the patella's upper edge and the greater trochanter of the femur24,25,26.
- To ensure that the adipose tissue thickness (ATT) does not alter the register of the NIRS signal, measure skinfold thickness to confirm the NIRS penetration depth27. For measurements at m.Vastus Lateralis, ensure that the ATT is less than 20 mm.
- NIRS software setup
- Once all NIRS wearables are correctly placed (see section 1.2), power them ON before starting the measurement.
- Launch the data acquisition software provided by the manufacturer, create a new file, and link the NIRS wearables.
- After all NIRS wearables are successfully linked, set the sampling rate to 10 Hz for data acquisition and analog-to-digital conversion for the assessed tissues. For prefrontal cortex measurements, adjust the differential pathlength factor (DPF) according to the age-dependent DPF for each participant28. For muscle measurement, set the DPF to 4, as used in previous protocols with athletes as subjects of study29,30.
2. Calibration and setup of ergospirometer
- Volume calibration
- Open the ergospirometer's software provided by the manufacturer to begin the calibration process.
- Attach the flow meter to a 28 mm turbine with a syringe adapter. Connect one corrugated tube to the syringe adapter and the other to a 3 L calibration syringe.
- Perform six withdrawal/injection maneuvers, maintaining a constant flow rate. Upon completion, the software will automatically confirm if the calibration test has passed.
- Gas calibration
NOTE: Ensure the flow calibration is done before starting the gas calibration.- Air calibration
- Ensure the sample line from the gas analyzer is disconnected from the calibration port and hanging freely. Then, initialize the calibration process.
- During calibration, a stable flat line is observed as the concentrations of oxygen (O2) and carbon dioxide (CO2) do not vary significantly (less than 5%). Once air calibration has been successfully completed, proceed to the metabolic gas calibration.
- Metabolic gas calibration
- Open the gas valves and verify that adequate pressure is delivered to the system by checking the manometer (consult the manufacturer for specific instructions).
- Connect the sample line to the calibration port and initialize the calibration process. Perform a 3 min pre-heating before starting the calibration, as advised by the manufacturer.
- If done correctly, after the 3 min pre-heating period, two flat lines should be observed: one fluctuating between room air (approximately 21.00% O2 and 0.04% CO2) and the other between the calibration gas (16.00% O2 and 5.00% CO2).
- Finally, disconnect the sample line from the calibration port and attach it to the mouthpiece that will be used for the upcoming test.
- Air calibration
3. ECG electrode placement (12 leads)
- Prepare the skin by exfoliating with a cream and/or shaving any hair from the electrode placement sites if necessary. Clean the areas with an alcohol pad to remove any superficial tissue residues.
- Place the ECG electrodes as follows31:
- Place the bipolar leads (Limb lead electrodes) as follows: Left arm (LA): left side of subclavicular fossa; Right arm (RA): right side of subclavicular fossa; Left leg (LL): anterior projection of left femoral head; Right leg (RL): anterior projection of right femoral head.
- Place the precordial lead electrodes as follows: V1: 4th intercostal space to the right of the sternum; V2: 4th intercostal space to the left of the sternum (in line with V1); V3: Midway between V2 and V4; V4: 5th intercostal space at the midclavicular line; V5: anterior axillary line at the same level as V4; V6: midaxillary line at the same level as V4 and V5.
4. Incremental maximal exercise test (cardiopulmonary exercise testing, CPET)
- Ask the participant to sit on the bike, ensuring that the seat and handlebars are adjusted to their height for optimal comfort and positioning.
NOTE: It is advised to set the seat height so that the knee is slightly bent at full extension32. The handlebars should be positioned to allow for a slight flexion of the elbows. - Attach a pulse oximeter to the participant's ear lobe, ensuring the site is clean by wiping it with an alcohol pad.
- Explain the protocol and instruct the participant to breathe through the mask before, during, and after the test.
NOTE: The participant must avoid talking or whistling while wearing the mask, as this can affect the ergospirometer's readings33. - Once the participant is positioned and prepared, have the participant extend the right leg and wait 2 min for the start instruction (initial rest stage). Have the participant pedal at a cadence between 80–100 rpm for 6 min at 0.6 W·kg-1 and 0.8 W·kg-1 for women/men, respectively (warm-up phase). Then, increment the workload at a rate of 20 W·min-¹ for women and 25 W·min-¹ for men until the participant reaches exhaustion (exercise phase).
- After completing the exercise phase, instruct the participant to remain still and continue breathing into the mask for 3 min (cool-down or recovery phase).
- Once the exercise protocol is finished, carefully remove the pulse oximeter from the earlobe, the mask, all three NIRS wearables, and the ECG electrodes.
NOTE: To keep the laboratory ambient condition (e.g., air temperature ~20 ± 2 °C, relative humidity ~40% ± 5%), it is a crucial criterion. Some participants can show a high sweat rate, which interferes with the fixation of devices on the skin and affects NIRS data recording. The use of ventilators can help to reduce hot thermoregulation by sweating.
Wyniki
During the completion of a CPET, the symptoms of dyspnea, leg fatigue, and rate of perceived exertion (RPE) were reported in all subjects. The complementary use of the NIRS devices did not add any discomfort to the subjects' sensation assessment. Also, we did not stop the CPET assessments by any risk event associated with excessive physiological stress.
We studied two competitive male cyclists recruited from a national cycling club. The inclusion criteria for this study were physically active participants (≥150 min of moderate or ≥75 min of vigorous physical activity per week) with a normal body mass index (20–25 kg·m-2). Exclusion criteria for this study were a history of respiratory, cardiovascular, metabolic, musculoskeletal, or neoplastic disease or an infectious or inflammatory process at least 2 weeks before the study assessments.
The completion of a CPET, accompanied by non–invasive recording of hemodynamic and tissue oxygenation changes resulting from metabolic changes induced by increased exercise intensity using devices equipped with NIRS technology, allows the identification of central limiting factors associated with changes in brain areas versus peripheral limiting factors related to respiratory or musculoskeletal responses to increased exercise intensity. The testing protocol encompasses a series of steps, including the preparation of the participant, the execution of the exercise test, and the collection of relevant physiological data.
Successful execution of the CPET–NIRS protocol is demonstrated by clear and consistent data across several parameters. During the initial rest stage, measurements such as heart rate (HR), pulse oxygen saturation (SpO2), and NIRS readings are recorded to establish a baseline. The warm-up phase, characterized by low workload pedaling, prepares the participant for the incremental exercise phase, where the workload progressively increases (see Figure 1).

Figure 1: Experimental design of exercise protocol scheme. Schematic representation of the stages of the exercise protocol used in the study, highlighting key events such as rest (R), warm-up (W), exercise (E), finalize (F), and stop (S), which correspond to the flow of the cardiopulmonary exercise testing protocol. Please click here to view a larger version of this figure.
Representative results of the CPET (Figure 2) and NIRS (Figure 3 and Figure 4) data from two male athletes assessed under controlled laboratory ambient conditions (air temperature ~20 ± 2 °C; relative humidity ~40% ± 5 %) are shown: (i) Participant 1 (Figure 2A and Figure 3) (age: 33 years, weight: 80 kg, height: 178 cm, Workload max: 300 W, VO2-max: 46 mL·kg-1·min-1, VE: 177 L·min-1, HR-max (%predicted, 220-years): 100%, PetCO2: 27 mmHg); and (ii) Participant 2 (Figure 2B and Figure 4) (age: 26 years, weight: 67 kg, height: 178 cm, Workload max: 300 W, VO2-max: 51 mL·kg-1·min-1, VE: 131 L·min-1, HR-max (% predicted, 220-years): 93%, PetCO2: 33 mmHg).
In both participants, the VO2 (oxygen consumption), VCO2 (carbon dioxide production), RQ (respiratory quotient, VCO2·VO2-1), HR, VE (lung ventilation), and RR (respiratory rate) exhibit a continuous rise as the exercise intensity increases until the maximal value of VO2 is reached (see Figure 2).
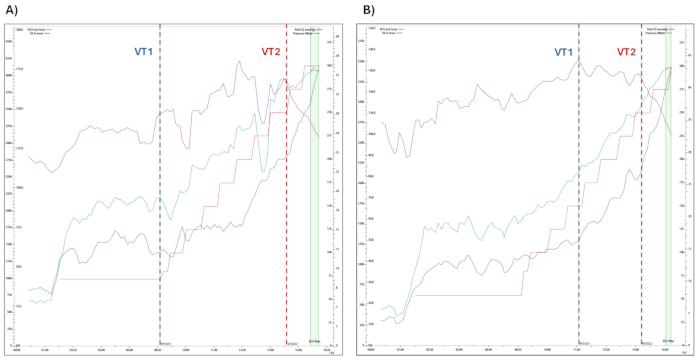
Figure 2: Changes in physiological variables assessed during CPET. The progression of physiological variables measured during cardiopulmonary exercise testing, including oxygen consumption (VO2), minute ventilation (VE), end-tidal CO2 pressure (PetCO2), and power output (Watts), are shown. The transitions between ventilatory thresholds 1 and 2 (VT1 and VT2) are indicated within the exercise stages. (A) Participant 1 and (B) Participant 2. Please click here to view a larger version of this figure.
The NIRS data provided insights into the local metabolic demand during CPET. The exercise-induced changes observed in the target tissue (muscular versus brain) vary depending on the specific tissue and the intensity of the exercise analyzed. Therefore, a useful physiological framework to interpret the exercise-induced NIRS data is the triphasic model of exercise intensity proposed by Skinner and McLellan34. In this model, the authors delineate three zones defined by VTs: Phase I or aerobic, (ii) Phase II or aerobic-anerobic transition, and (ii) Phase III or "metabolic instability".
At exercise intensities below VT2 (Phases I and II), a decrease in O2-Hb and an increase in H-Hb will occur at the muscle level – if there is no significant variation in T-Hb as a parameter of local blood flow. Our CPET-NIRS protocol consists of cyclic exercises with repetitive muscle contractions/relaxations, so minimal variation in T-Hb is expected. However, exercise-induced changes vary depending on the target muscle tissue being evaluated. In locomotor muscles, as the m.Vastus Lateralis, the progressive increase in exercise intensity, induces NIRS data changes concomitant with the workload (see Figure 3A). In contrast, in accessory respiratory muscles, such as the m.Intercostales, changes are concurrent with ventilatory changes rather than workload (see Figure 3B). In PFC, an increase of O2-Hb, H-Hb, and T-Hb is observed because blood flow exceeds the local demand induced by exercise; also, a slight decrease in TSI could be seen (see Figure 3C). In all tissues evaluated, the TSI parameter decreases as exercise intensity increases, making the changes in m.Vastus Lateralis more notorious than m.Intercostales and PFC (see Figure 3D).

Figure 3: Example of central limitation (Participant 1). NIRS data during CPET protocol (Events: W = Warm-up, E = Exercise, VT1 = Ventilatory threshold 1 or aerobic ventilatory threshold, VT2 = Ventilatory threshold 2 or anaerobic ventilatory threshold, F = Finalized exercise or VO2-max). (A) m.Vastus Lateralis, (B) m.Intercostales, and (C) Prefrontal cortex (PFC). Please click here to view a larger version of this figure.
As exercise-induced metabolic demand increases, particularly at intensities above VT2 (Phase III or "metabolic instability"), interesting physiological responses to be studied occur both at the muscular (locomotor and respiratory muscles) and PFC levels. These consist of a marked decrease in O2-Hb and tHb, alongside a remarkable increase in H-Hb, aspects that support the pronounced decline in TSI.
In subjects with high ventilatory demand during high-intensity exercise, the exponential rise in VE and RR causes elevated hyperventilation because of the increase in CO2 of "metabolic origin". This hyperventilation can induce pronounced brain vasoconstriction, thereby limiting performance by central limitation, as seen in this representative subject. Theoretically, the changes observed in NIRS data result from brain vasoconstriction induced by the hypocapnia inferred from the abrupt decrease of pressure end-tidal of CO2 (PetCO2) registered in the CPET (see Figure 2). These physiological changes have been shown to have a high relationship with increased dyspnea induced by exercise, registered using the modified Borg's scale35,36.
On the other hand, subjects with high locomotor demand but not high respiratory demand do not exhibit brain vasoconstriction by hypocapnia. Consequently, NIRS data may continue to reflect changes like those observed at moderate exercise intensities. In these subjects, exercise performance is limited by peripheral rather than central limiting factors (see Figure 4). These physiological changes have been shown to have a high relationship with increased leg fatigue induced by exercise.
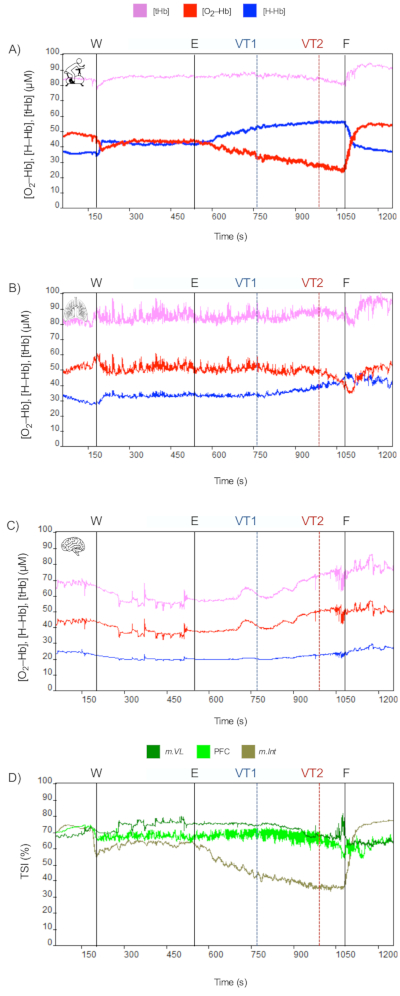
Figure 4: Example of peripheral limitation (Participant 2). NIRS data during CPET protocol (Events: W = Warm up, E = Exercise, VT1 = Ventilatory threshold 1 or aerobic ventilatory threshold, VT2 = Ventilatory threshold 2 or anaerobic ventilatory threshold, F = Finalized exercise or VO2–max). (A) m.Vastus Lateralis, (B) m.Intercostales, and (C) Prefrontal cortex (PFC). Please click here to view a larger version of this figure.
Dyskusje
There is significant potential in using NIRS wearables as a complementary tool to CPET for evaluating athletic performance and identifying central and peripheral exercise–limiting factors in aerobic or endurance athletes, given that NIRS technology has proven its validity and reliability in assessing microvascular hemodynamics in both cerebral and muscular regions37,38. However, to maximize the benefits of this technology, several considerations must be addressed to ensure accurate measurements.
General guidelines for NIRS wearable device placement involve adapting the positioning to the target tissue and ensuring secure attachment to the participant's skin, as movement during exercise may impact NIRS signals (artifacts)7. To achieve this, the device should be in complete contact with the skin and affixed using double-sided adhesive tape, ensuring that light emitters and detectors are unobstructed. For added stability, elastic therapeutic tape can be applied over the device. When placing the device on a limb that will be actively engaged during exercise, such as the m.Vastus Lateralis, an elastic bandage wrap, can be used for added stability during cycling. However, avoiding excessive compression around the NIRS optodes is crucial as it may alter local blood flow and potentially affect the accuracy of the NIRS measurements (the pressure do not be higher than capillary perfusion pressure, ~25 mm Hg)7. It is recommended that any tape or bandage used over the NIRS device be black to prevent interference from ambient light39,40. Additionally, dimmed lighting in the testing environment can minimize potential disruptions to NIRS signal accuracy.
While proper placement and securing of the devices are essential, it is equally important to consider individual anatomical characteristics that may influence NIRS measurements. One major limitation is that the accuracy of NIRS measurements can be affected by factors such as adipose tissue thickness (ATT)41,42. The maximum penetration depth for NIRS is approximately half the distance between the light source and detector43. As ATT increases, the proportion of the NIRS signal originating from the underlying skeletal muscle decreases11. This reduction in signal contribution results in lower levels of O2-Hb and H-Hb, among other chromophores42. Therefore, it is recommended that ATT be measured to ensure proper light–penetration into the muscle. A caliper or ultrasound can be used for this purpose, as both methods accurately assess body composition in athletes; however, the latter provides superior accuracy and may be preferred44.
In addition to ATT, the accuracy of NIRS measurements is also influenced by the differential path length factor (DPF), which is used to calculate concentrations of O2–Hb and H–Hb through the modified Beer-Lambert law45. Most commercial NIRS devices utilize continuous–wave systems that emit light at a constant intensity and assume a constant DPF11. However, DPF is not a fixed value, as it varies due to individual anatomical differences, including skull and ATT41,46. Furthermore, variability in DPF among individuals and differences in anatomical characteristics between sexes such as variations in bone, muscle mass, and adipose tissue distribution– can also influence the accuracy of measurements28. Due to the assumption of a constant DPF, these devices can only measure relative changes in O2-Hb and H-Hb from a baseline rather than providing absolute values11. Therefore, while NIRS technology is valuable for monitoring trends in tissue oxygenation, caution must be exercised when interpreting these measurements. Further research should focus on developing methods to estimate DPF in cerebral and muscular tissues accurately. In the interim, documenting DPF values used in studies is recommended to improve the reproducibility of results.
Another anatomical characteristic that can affect NIRS measurements is skin melanin concentration. Melanin, along with hemoglobin, is a primary chromophore in the skin47. Individuals with darker skin pigmentation have larger and more concentrated melanosomes, which can lead to greater signal attenuation due to increased light absorption7. The strength of the detected signal depends on the light absorbed by chromophores, tissue light scattering properties, and the distance between the light source and detector47. Consequently, higher melanin concentrations can interfere with NIRS signal quality, leading to attenuated oxygen tissue saturation readings, mainly at muscular level48,49. To account for these variations and enhance the interpretability of NIRS data across diverse populations, it is recommended that skin pigmentation be reported using the Fitzpatrick skin type classification scale7.
Regarding the applicability of this protocol in exercise prescription, NIRS technology has primarily been used to assess muscle metabolism during endurance exercise, particularly in protocols where substrate oxidation serves as the main energy source for ATP resynthesis. Limited evidence is available regarding its application in resistance training, but a literature review suggests that the acute effects of strength training on TSI depend on muscle fiber composition. Specifically, muscles with a higher proportion of type I fibers, such as the m.Vastus Lateralis shows a greater ΔTSI compared to other muscle groups trained at the same intensity. Nonetheless, considerable heterogeneity in study methodologies continues to limit the generalization of reported findings. The preliminary results of this study, along with future publications of standardized protocols, will support broader applications of this technology for prescribing exercise intensity across diverse contexts50.
In conclusion, NIRS wearable devices represent a significant advancement in non-invasive monitoring of hemodynamic responses at the microvascular level during exercise, complementing the cardiopulmonary variables assessed by CPET. Unlike invasive methods, NIRS provides real-time data on the balance between oxygen delivery and consumption without disrupting the athlete's natural movement. This technology effectively identifies central and peripheral exercise-limiting factors by detecting changes in O2-Hb, H-Hb, and TSI across various tissues and exercise intensities. The detailed insights into variations in metabolic demand and physiological responses highlight NIRS's potential to optimize training programs and enhance athletic performance. Additionally, NIRS's ability to assess cerebral and muscular microvascular hemodynamics offers new opportunities to explore physiological responses to different exercise modalities and intensities. Overall, NIRS technology holds considerable promise for advancing our understanding of human physiology and contributing to research in exercise science, providing a valuable tool for improving athletic performance and refining training strategies.
Ujawnienia
The authors declare no conflict of interest.
Podziękowania
We thank all participants in this study and technical laboratory staff for their support in the measurements taken at the Laboratory of Exercise Physiology. The authors FC-B and ME-R were partially supported by the III, IV, and V Research & Innovation Competitions of the School Health Sciences (Faculty of Medicine, Pontificia Universidad Católica de Chile). The author RC-C was funded by Project supported by the Competition for Research Regular Projects, year 2023, code LPR23-17, Universidad Tecnológica Metropolitana.
Materiały
| Name | Company | Catalog Number | Comments |
| Column Scale | SECA | 711 | There are numerous alternatives to this item |
| Portable Stadiometer | SECA | 217 | There are numerous alternatives to this item |
| 12-lead ECG | COSMED | Quark T12x | A 12-lead ECG provides a better understanding of HR during exercise and facilitates the detection of arrhythmias. |
| Pulse Oxymeter | COSMED | Integrated pulse oxymeter | |
| Ergoespirometer | COSMED | Quark-CPET | Calibration gases and calibration syringe are included |
| Cycle-ergometer | Ergoline GmH | ViaSprint 150P | There are numerous alternatives to this item. Must ensure compatibility with provided software |
| NIRS weareable | Artinis Medical Systems | Portalite | Articulated NIRS weareable fits the surface where it's placed upon. |
| NIRS weareable | Artinis Medical Systems | Portamon | Portamon device provides better results on high adipose-tissue surfaces. |
| Metabolic Data Management Software (OMNIA) | COSMED | Software will vary upon system choice | |
| NIRS Data Management Software (Oxysoft) | Artinis Medical Systems | Software will vary upon device choice | |
| Wireless Probe Type Ultrasound Scanner | SONUS | Duo LC | There are numerous alternatives to this item |
Odniesienia
- Bassett, D. R. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 70 (1), 12-25 (2000).
- Seiler, S. What is best practice for training intensity and duration distribution in endurance athletes. Int J Sports Physiol Perform. 5 (3), 276-291 (2010).
- Palange, P., et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 29 (1), 185-209 (2006).
- Contreras-Briceño, F., et al. Intercostal muscles oxygenation and breathing pattern during exercise in competitive marathon runners. Int J Environ Res Public Health. 18 (16), 8287 (2021).
- Mier, C. M., Alexander, R. P., Mageean, A. L. Achievement of VO2max criteria during a continuous graded exercise test and a verification stage performed by college athletes. J Strength Cond Res. 26 (10), 2648-2654 (2012).
- Racinais, S., Buchheit, M., Girard, O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol. 5, 142 (2014).
- Perrey, S., Quaresima, V., Ferrari, M. Muscle oximetry in sports science: An updated systematic review. Sports Med. 54 (4), 975-996 (2024).
- Contreras-Briceño, F., et al. Determination of the respiratory compensation point by detecting changes in intercostal muscles oxygenation by using near-infrared spectroscopy. Life (Basel). 12 (3), 444 (2022).
- Kozlova, S. G. The use of near-infrared spectroscopy in the sport-scientific context. J Neurol Neurol Diord. 4 (2), 203 (2018).
- Perrey, S. Non-invasive NIR spectroscopy of human brain function during exercise. Methods. 45 (4), 289-299 (2008).
- Barstow, T. J. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol. 126 (5), 1360-1376 (2019).
- Kowalski, T., et al. Respiratory muscle training induces additional stress and training load in well-trained triathletes—randomized controlled trial. Front Physiol. 14, 1264265 (2023).
- Espinosa-Ramírez, M., et al. Sex-differences in the oxygenation levels of intercostal and vastus lateralis muscles during incremental exercise. Front Physiol. 12, 738063 (2021).
- Perrey, S. Evaluating brain functioning with NIRS in sports: Cerebral oxygenation and cortical activation are two sides of the same coin. Front Neuroergonomics. 3, 1022924 (2022).
- Thomas, R., Perrey, S. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur J Appl Physiol. 102 (2), 153-163 (2007).
- Kirby, B. S., Clark, D. A., Bradley, E. M., Wilkins, B. W. The balance of muscle oxygen supply and demand reveals critical metabolic rate and predicts time to exhaustion. J Appl Physiol. 130 (6), 1915-1927 (2021).
- Perrey, S. Training monitoring in sports: It is time to embrace cognitive demand. Sports (Basel). 10 (4), 56 (2022).
- Angius, L., et al. Transcranial direct current stimulation over the left dorsolateral prefrontal cortex improves inhibitory control and endurance performance in healthy individuals. Neuroscience. 419, 34-45 (2019).
- Dempsey, J. A., McKenzie, D. C., Haverkamp, H. C., Eldridge, M. W. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest. 134 (3), 613-622 (2008).
- Peltonen, J. E., et al. Cerebral and muscle deoxygenation, hypoxic ventilatory chemosensitivity and cerebrovascular responsiveness during incremental exercise. Respir Physiol Neurobiol. 169 (1), 24-35 (2009).
- Klem, G. H., Lüders, H. O., Jasper, H. H., Elger, C. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol Suppl. 52, 3-6 (1999).
- Vogiatzis, I., et al. Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol. 587 (14), 3665-3677 (2009).
- Vogiatzis, I., et al. Intercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 182 (9), 1105-1113 (2010).
- Contreras-Briceño, F., et al. Reliability of NIRS portable device for measuring intercostal muscles oxygenation during exercise. J Sports Sci. 37 (23), 2653-2659 (2019).
- Crum, E. M., O'Connor, W. J., Van Loo, L., Valckx, M., Stannard, S. R. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur J Sport Sci. 17 (8), 1037-1043 (2017).
- Vogiatzis, I., et al. Quadriceps muscle blood flow and oxygen availability during repetitive bouts of isometric exercise in simulated sailing. J Sports Sci. 29 (10), 1041-1049 (2011).
- Grassi, B., Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J Biomed Opt. 21 (9), 091313 (2016).
- Duncan, A., et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. 39 (5), 889-894 (1996).
- Aebi, M. R., Willis, S. J., Girard, O., Borrani, F., Millet, G. P. Active preconditioning with blood flow restriction or/and systemic hypoxic exposure does not improve repeated sprint cycling performance. Front Physiol. 10, 1393 (2019).
- Cocking, S., et al. Repeated sprint cycling performance is not enhanced by ischaemic preconditioning or muscle heating strategies. Eur J Sport Sci. 21 (2), 166-175 (2021).
- Kligfield, P., et al. Recommendations for the standardization and interpretation of the electrocardiogram. J Am Coll Cardiol. 49 (10), 1109-1127 (2007).
- Dillon, H. T., et al. The effect of posture on maximal oxygen uptake in active healthy individuals. Eur J Appl Physiol. 121 (5), 1487-1498 (2021).
- DeCato, T. W., Haverkamp, H., Hegewald, M. J. Cardiopulmonary exercise testing (CPET). Am J Respir Crit Care Med. 201 (1), P1-P2 (2020).
- Skinner, J. S., Mclellan, T. H. The transition from aerobic to anaerobic metabolism. Res Q Exerc Sport. 51 (1), 234-248 (1980).
- Elmberg, V., et al. Reference equations for breathlessness during incremental cycle exercise testing. ERJ Open Res. 9 (2), 00566-02022 (2023).
- Borg, G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 14 (5), 377-381 (1982).
- Perrey, S. Could near infrared spectroscopy be the new weapon in our understanding of the cerebral and muscle microvascular oxygen demand during exercise. J Sport Health Sci. 13 (4), 457-458 (2024).
- Orcioli-Silva, D., et al. Cerebral and muscle tissue oxygenation during exercise in healthy adults: A systematic review. J Sport Health Sci. 13 (4), 459-471 (2024).
- Kovalenko, B., Roskosky, M., Freedman, B. A. Effect of ambient light on near infrared spectroscopy. J Trauma Treat. 04 (03), (2014).
- Wik, L. Near-infrared spectroscopy during cardiopulmonary resuscitation and after restoration of spontaneous circulation: A valid technology. Curr Opin Crit Care. 22 (3), 191-198 (2016).
- Pirovano, I., et al. Effect of adipose tissue thickness and tissue optical properties on the differential pathlength factor estimation for NIRS studies on human skeletal muscle. Biomed Opt Express. 12 (1), 571 (2021).
- Van Beekvelt, M. C. P., Borghuis, M. S., Van Engelen, B. G. M., Wevers, R. A., Colier, W. N. J. M. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond). 101 (1), 21-28 (2001).
- Homma, S. Influence of adipose tissue thickness on near infrared spectroscopic signal in the measurement of human muscle. J Biomed Opt. 1 (4), 418 (1996).
- Gomes, A. C., et al. Body composition assessment in athletes: Comparison of a novel ultrasound technique to traditional skinfold measures and criterion DXA measure. J Sci Med Sport. 23 (11), 1006-1010 (2020).
- Delpy, D. T., Cope, M., Zee, P. V. D., Arridge, S., Wray, S., Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 33 (12), 1433-1442 (1988).
- Talukdar, T., Moore, J. H., Diamond, S. G. Continuous correction of differential path length factor in near-infrared spectroscopy. J Biomed Opt. 18 (5), 056001 (2013).
- Zonios, G., Bykowski, J., Kollias, N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Dermatol. 117 (6), 1452-1457 (2001).
- Patel, N. A., Bhattal, H. S., Griesdale, D. E., Hoiland, R. L., Sekhon, M. S. Impact of skin pigmentation on cerebral regional saturation of oxygen using near-infrared spectroscopy: A systematic review. Crit Care Explor. 6 (2), e1049 (2024).
- Wassenaar, E. B., Van Den Brand, J. G. H. Reliability of near-infrared spectroscopy in people with dark skin pigmentation. J Clin Monit Comput. 19 (3), 195-199 (2005).
- Miranda-Fuentes, C., et al. Changes in muscle oxygen saturation measured using wireless near-infrared spectroscopy in resistance training: A systematic review. Int J Environ Res Public Health. 18 (8), 4293 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone