Method Article
Planarian as an Animal Model for Experimental Acute Seizure
W tym Artykule
Podsumowanie
Seizures negatively impact various functions and life quality. Planaria worms were exposed to varying concentrations of chemoconvulsants to evaluate their seizure phenotypes and disruptive motility. This study proposes using planaria worms as a model for acute seizures in humans and holds significance in drug development for epilepsy.
Streszczenie
Epilepsy is among the most prevalent neurological disorders characterized by recurring spontaneous seizures. Seizures represent a clinical manifestation of uncontrolled, excessively synchronized neural cell activity. The extent of brain damage from seizures depends on their duration and intensity. Regrettably, there is no effective remedy for epilepsy. The aim of this investigation is to assess whether the planaria worm Dugesia dorotocephala could serve as a model to aid in identifying and developing treatments for epilepsy that can target acute seizures. Currently, various models, such as marine models, are used to evaluate antiseizure medications (ASM). However, they are very expensive, and there are ethical concerns. Alternatively, invertebrate models offer a cost-effective research opportunity in the drug discovery process for ASM. Planaria belong to the flatworm family and inhabit marine freshwater and terrestrial environments. Dugesia dorotocephala is the dominant species of aquatic planaria across North America. D. dorotocephala presents as a viable invertebrate model for epilepsy studies due to its cost-effectiveness, vertebrate-like neurons, and quantifiable behaviors, unlike other invertebrates or larger animals. They have been used in various pharmacology and environmental toxicology studies related to age, memory, and regeneration. In this study, planaria were exposed to different concentrations of pilocarpine, a common chemoconvulsant to study their behavior upon exposure. Following the observation, planaria were euthanized and preserved in either formaldehyde or Golgi solution for neurohistological assessment. Six distinct behavioral phenotypes were observed in planaria: dorsal oscillations, head oscillations, tail dorsal expansion, C-shape, head flick, and tail flick. Dorsal oscillation frequencies were significantly increased among experimental groups compared to the control and exhibited dose dependence. Additionally, pilocarpine disrupted the motility of the planaria. Pilocarpine-induced seizures in planaria can serve as a model to evaluate acute seizures and antiseizure medication, which is essential in developing therapeutic interventions for human patients suffering from epilepsy.
Wprowadzenie
Epilepsy, characterized by two or more seizures within 24 h without an apparent cause, impacts ~50 million people globally1. Among them, 10-15 million individuals are reported to have drug-resistant epilepsy2. Therefore, epilepsy drug investigation is crucial. The condition entails brief episodes of either partial or generalized involuntary movement, ranging from blank staring to body stiffening and shaking, and is linked with a surge of electrical activity in the brain3.
Historically, epilepsy research has relied on rodents and other mammals due to their evolutionary similarities to humans. However, these methods can be time-consuming and expensive, necessitating an alternative approach4,5. Non-mammalian creatures like fruit flies, leeches, tadpoles, zebrafish, and roundworms have been utilized in studies and have shown promising outcomes6. Furthermore, it was shown that planaria could provide a comparative genomic study model between invertebrate and human genomes alongside the capability to test pro-convulsant, anti-seizure medications (ASM), and behavioral patterns6. Planaria (Phylum Platyhelminthes), known as flatworms and members of the Turbellaria class, are primarily renowned for their regenerative abilities; however, this investigation concentrates on their response to seizure-inducing substances.
Planaria share fundamental neurological mechanisms with humans, such as responsiveness to serotonin and dopamine, showing a 95% similarity to the nervous system-related genes in the mammalian brain and possessing a recognizable brain structure7. Additionally, they exhibit observable motions in laboratory conditions and are cost-effective, time-efficient, and ethical compared to rodents or other mammals. These observable behaviors, such as screw-like, C-like, and walnut-shaped motions, have been extensively documented for decades and are associated with substances like cocaine, nicotine, dopamine, and pilocarpine7,8,9,10,11,12,13,14. Hence, planaria emerge as a viable model for epilepsy drug research in humans.
This method aims to characterize neurons of planaria that have been exposed to pilocarpine using a Golgi stain. The Golgi stain is used to visualize neurons under light microscopy and has been used to investigate whether a change in morphology is related to seizures15,16,17. Current literature has no evidence of Golgi staining being performed on planarian brains. Although previous studies have documented pharmacological effects by observing behavioral phenotypes, this manuscript is the first to characterize the neurons of planaria exposed to pilocarpine using Golgi staining11,18. This technique proves valuable in visualizing and understanding the morphological changes associated with seizures. This study noted a significant increase in the frequency of oscillating dorsal oscillation behavior in planarian worms as the concentration of pilocarpine was increased.
Protokół
NOTE: The overall experimental design is described in Figure 1.
1. Behavior phenotype assay
- Prepare concentrations of 1 mM and 2 mM pilocarpine dissolved in spring water as well as control with Springwater only.
- Pipette 3 mL of each solution into a 4 x 3 well plate, with each row representing a different concentration of pilocarpine.
- Place one lab-reared Dugesia dorotocephala that is about 2 weeks old in each of the 12 wells using a transfer pipette with the tip cut off.
NOTE: Cut the tip so the end is large enough to fit the planaria without damaging it. - Record the behavior of the planaria for 1 h using any camera that records the behaviors well and is observable to the human eye, positioned over the 4 x 3 well plate in normal indoor lighting conditions.
- Repeat steps 1.1-1.4 with concentrations of 3 mM, 4 mM, and 6 mM pilocarpine dissolved in spring water.
2. Motility analysis
NOTE: Planaria behaviors were recorded in 2.5 cm diameter wells. The 1 h long video recordings were split into 30 min parts and cropped using commercial software to analyze planaria individually.
- Begin a new experiment using automated behavior analysis software. Open the enhanced automated behavior tracking system. Click New under New experiment. Name the experiment.
- Set up the experimental settings as described below.
- Click Experiment Settings in the menu prompt. Ensure that settings are default: video source (from video file), Number of Arenas (1), Tracked Features (Center-point detection), Body Point Detection Technique (Contour-based), Analysis Options (none), Units (cm, s, deg).
- Set up the arena settings as described below.
- Click Arena Settings under the Arena Settings tab in the top left Setup panel. When prompted to upload a video, select recorded footage of planaria from the stored file location. Once the video is selected, click Grab.
- Follow the prompts on the top right panel of the screen. Click on 1. Draw Scale to Calibrate. On the video image of the well, drag a scale line from edge to edge of the specimen well. Enter the real-world distance of the well (2.5 cm).
- Click on 2. Select Shape and Draw Arena. Ensure that Arena 1 is selected in the right panel. Move the mouse cursor to the top middle of the screen and click the Circle Icon.
- Create a circle in the uploaded video image by dragging from one perimeter of the arena to another. Ensure the arena is in the orange zone. Adjust the shape so that it fits the arena. Ensure that the Arena 1 label is within the bounds of the arena.
- At the bottom of the right panel, change the Shape, Size, and Position (Width:2.75, Height:2.75, X:0, Y:0) to the desired measurements.
- Click 3. Select Shape and Draw Zones (Optional). Zone Group 1 within the right panel will now be selected. Move the mouse cursor to the top middle of the screen and select the Circle Icon. Create a smaller circle in the arena image by dragging it within.
- In the bottom right panel, change Shape, Size, and Position to desired measurements (Width:1.75, Height:1.75, X:0, Y:0).
- Click the Add Zone Label button in the same menu bar as the circle. Click Within the Center of the Smaller Circle. Right-click the Zone 1 tag in the right panel to rename the Zone Center.
- Click the Add Zone Label button again. Click on the Perimeter of the Arena, Outside of the Center Zone. Right click Zone 2 in the right panel to rename the zone Perimeter.
- Click 4. Validate Setup to ensure Valid Arena Settings.
- Set up the Trial control settings as described below.
- Click Trial Control Settings under the Trial Control Settings tab in the top left Setup panel. Six component boxes will appear. Click Settings within the fourth box labeled Condition, which includes Time (1) and Infinite time (condition never met).
- Click the Bubble beside After, then enter the duration (30 min) in the right text box. There will also be a drop-down menu to the right of the time text box to select between h, min, and s.
NOTE: Detection settings for recorded footage will depend on footage quality. The following methods can be manipulated to observe the best results, specific to individual footage.
- Set up the detection settings as described below.
- Click Detection Settings under the Detection Settings tab in the top left Setup panel. Click the Select Video button in the top right panel. Upload the desired video to base detection settings on.
- Click the Advanced tab in the same right panel. Now, under the Method tab, ensure it is set to Gray Scaling. Change the range (0 - 100).
- Under the Smoothing tab of the right panel, ensure Video pixel smoothing is None, Dropped frames correction is Off, and Track noise reduction is Off.
- Under the Subject Contour tab of the right panel, adjust the sequence setting and change Erosion, Dilation, and Erosion to 1, 1, and 0, respectively.
- Under the Subject Size tab, change Minimum (0) and Maximum (125000). Click Save in the bottom right.
- Set up a sequence of video trials for observation as described below.
- Click Trial List in the top left Setup panel. Upload video footage of specimens in the desired sequence by clicking the Ellipses under the System Video file column. To add more trials, click Add Trials near the top left of the Trial List Panel.
- Set up Acquisition settings.
- Click Acquisition under the top left Acquisition tab. Under Acquisition settings in the right panel, Click Track all planned trials.
- To begin acquisition, click on the Red Button in the lower middle of the screen next to Ready for Start. Video acquisition will now begin and take a certain amount of time, depending on the quantity and length of video trials uploaded.
- Set up the analysis settings as described below.
- Click Analysis Profile under the left panel Analysis tab. Click on the default selected dependent variables, Velocity and Distance Moved, and delete them.
- Within the dependent variables panel, under the Location tab, click In Zone. Click the Center and Perimeter Zone boxes so that they are check marked.
- Click the Trial Statistics tab within the In Zone box. Click to uncheck Latency to First. Click Add.
- Under the Body tab, click the Rotation button. Click Clockwise and leave the Threshold (50.00 degrees) and minimum distance moved (2.00 cm) at default. Click Add.
- Click the Rotation button again and click Counterclockwise. Click Add.
- Analyze tracks as described below.
- Under the Results tab, click Track Visualization. On the right panel, under Filter, uncheck Last. The playback control bar in the bottom middle panel of the screen will depict track visualization of specimens during specific times of observation.
- Analyze and export results. Results can be found in the remainder of the bottom left panel tabs. To export data, click Raw Data or Statistics under Export within the left panel.
3. Euthanasia
- Prepare a 22 mM solution of eugenol in spring water. Place planaria into Petri dishes with 9 mL of 22 mM eugenol solution using the cut transfer pipette, keeping each concentration separate. Euthanize for 3 min or until all movement has ceased.
- Place planaria into a fresh Petri dish filled with 9 mL of spring water to rinse 1x-2x, and place into either the Golgi solution described below (Golgi stain) or 4% paraformaldehyde (immunofluorescence stain).
4. Histological analysis
- Golgi staining
- After planaria are euthanized, place them into a beaker or Petri dish filled with a 1:1 mixture of Solutions A and B from the Golgi Stain Kit. Replace solutions A and B the following day by placing planaria into a new beaker filled with a mixture of solutions A and B.
- Allow planaria to sit for 1 week in solutions A and B.
- Remove planaria from solutions A and B and place it into solution C in another beaker. Replace solution C the following day. Allow planaria to sit for a minimum of 72 h and up to 1 week in solution C.
- Remove planaria from solution C with a transfer pipette and embed by placing a small amount of OCT compound on a chuck, adding the planaria, and then surrounding the planaria with OCT compound. Transversally cut planaria into 5 µM sections with the cryostat machine at -24° C.
- Mount planaria onto gelatin-coated slides using a transfer pipette and solution C and allow it to dry in the dark at RT for up to 3 days.
- Stain slides using the staining solution (1 part solution D, 1 part solution E, 2 parts Springwater) by submerging the slides in beakers with the solutions as follows: Staining solution for 10 min, then in Spring water 2x for 4 min each, then 50% ethanol for 4 min, then 75% ethanol for 4 min, then 95% ethanol for 4 min, then in 100% ethanol 4x for 4 min, and finally in xylene 3x for 4 min.
- Coverslip slides with histological mounting media and observe tissue under Bright field Microscopy.
- Immunofluorescence staining
- After planaria are euthanized, place into 4% paraformaldehyde for at least 24 h. It can also be stored in a fridge after this step.
- Transfer planaria to 20% sucrose from 4% PFA and leave in it for 1 day. Remove planaria from sucrose and place it into PBS buffer for 5 min.
- Rinse with 3-4 washes of PBS buffer and transfer to 20% sucrose for storage until ready to stain.
- Place into 70%, 95%, and 100% alcohol solutions for 1 min each. Place in xylene for 1 min.
- Place in 95% and 70% alcohol for 1 min each. Place into PBS buffer 3x for 5 min each. Place into a 60:40 methanol and hydrogen peroxide solution for 10-15 min.
- Rinse in PBS 3x for 5 min each. Add the volume of 4% paraformaldehyde used previously in 4.2.1 into this PBS buffer.
- Rinse in PBS 3x for 5 min each. Block for 1 h with powdered milk, by covering the planaria with it.
- Dilute primary antibody (1H6) to 5 µg/mL with PBS and incubate the slides overnight at 4 °C in the antibody solution. The following day, rinse planaria in PBS for 10 min.
- Dilute secondary antibody (goat anti-mouse IgG) to 1 µg/mL in PBS and incubate the slides overnight at RT in the antibody solution. The following day, rinse planaria in PBS for 15 min.
NOTE: Different antibodies have different optimal concentrations; determine this before starting the experiment. - Mount whole planaria on the slide, cover with a coverslip, seal with aqueous mounting media, and observe the tissue under a fluorescent microscope.
5. Image analysis
- Open the open-source image analysis software application. Install the colour_deconvolution2.jar plugin to the open-source image analysis software application by dragging the plugin to the application.
- Select the image to analyze and drag it to the open-source image analysis software application. Go to the Image tab. Click color. Click Colour Deconvolution2 v2.1.
- In the vectors option, click H DAB. In the output option click 8bit_Transmittance. Make sure the Simulated LUTs, Cross product for Colour 3, Show matrices, and Hide legend are all selected. Click OK.
- Select the Image in Black and White to ensure the open-source image analysis software application can effectively analyze the image.
- Go to the Image tab. Click Adjust. Click Threshold. Adjust the threshold so that the DAB staining or the foreground is red in color with a white background. Make sure the threshold is the same for each image if multiple images are being quantified.
- Click Analyze. Click Set Measurements. Select Area, Min & max gray value, Limit to threshold, Display label, Median, and Mean gray value. Click OK. Click Analyze. Click Measure. Obtain the result.
- Repeat steps 1.2 to 1. U6ntil all the images are quantified. Click the Results Image. Click File. Click Save As. Save file as a spreadsheet and view the data on the spreadsheet software.
6. Statistical Analysis
- Calculate the mean and standard errors of the mean (SEM) for the planaria's behavior using Analysis of Variance (ANOVA) and Student's t-test for statistical significance (p < 0.05).
Wyniki
The following behavior was observed by the planaria exposed to varying concentrations of pilocarpine:
Dorsal oscillations: a bubble-like formation that travels from the cranial end of the planarian's body to the caudal end.
Head oscillations: bubble-like formation by the head of the planarian that forms a tadpole-like appearance.
C-Shape: head moving clockwise and tail moving counterclockwise to form a C.
Head flick: the head of the planarian abruptly jerks to the left or the right.
Tail dorsal oscillation: bubble-like formation by the tail of the planarian that forms a tadpole-like appearance.
Tail flick: the tail of the planarian abruptly jerks to the left or the right.
We observed that the frequency of behaviors increased with increasing pilocarpine concentration. The results are the number of particular behaviors noticed in the whole recording. Dorsal oscillations at 6 mM pilocarpine (mean = 16 ± 4.10, p < 0.0001) and 4 mM (mean = 11.25 ± 2.17, p < 0.0001) were statistically significant compared to control (mean = 0; Figure 2A). This movement exhibited dose-dependent behavior (R2 = 0.87; Figure 2G). Additionally, it took less time for planaria to display this behavior when exposed to 6 mM of pilocarpine compared to other concentrations.
For head oscillations, significant differences were observed between 6 mM and control (mean = 10.25 ± 3.57, p = 0.0070), 3 mM and control (mean = 1.50 ± 0.96, p = 0.0187), and 1 mM and control (mean = 2.75 ± 1.80, p = 0.0405). At 4 mM pilocarpine, there were no statistical differences observed (mean = 6.75 ± 3.4004; Figure 2B).
Significant differences in the C-shape phenotype were observed between control and 6 mM (mean = 8.50 ± 2.47, p = 0.0034), 1 mM (mean = 5.75 ± 0.48, p = 0.0368), and between 6 mM and 2 mM (mean = 3.75 ± 1.28, p = 0.0454). There were no statistical differences observed between 3 mM Pilocarpine (mean = 3.50 ± 1.8484) and 4 mM Pilocarpine (mean = 5.00 ± 2.6771; Figure 2D).
A significant difference in the head flick phenotype was observed between 6 mM and control (mean = 6.50 ± 1.19, p = 0.0072) and 6 mM and 4 mM (mean = 1.75 ± 0.85, p = 0.0416). There were no statistical differences among 1 mM Pilocarpine (mean = 4.250 ± 1.2500), 2 mM Pilocarpine (mean = 3.8750 ± 1.6630), and 3 mM Pilocarpine (mean = 2.75000 ± 1.0308; Figure 2E).
Significant differences in the tail dorsal oscillations phenotype were observed between 4 mM and control (mean = 5.50 ± 2.63, p = 0.0087), 2 mM (mean = 0.50 ± 0.19, p = 0.0063), and between 6 mM and control (mean = 5.00 ± 2.45, p = 0.0157), 2 mM (mean = 0.50 ± 0.19, p = 0.0125), and 1 mM (mean = 0.75 ± 0.48, p = 0.0366). There were no statistical differences at 3 mM Pilocarpine (mean = 1.7500 ± 0.250; Figure 2C).
For the tail-flick phenotype, significant differences were observed between 6 mM and control (mean = 15.25 ± 7.20, p = 0.0037), 4 mM (mean = 2.50 ± 0.87, p = 0.0125), 1 mM (mean = 4.50 ± 1.55, p = 0.0318), and 2 mM pilocarpine (mean = 4.63 ± 1.67, p = 0.0158). There were no statistical differences at 3 mM Pilocarpine (mean = 7.2500 ± 3.5444; Figure 2F)
These results support that pilocarpine induces different types of behavior in planaria. It is recognized that dorsal oscillations represent reliable behavior after exposure to pilocarpine.
Using automatic tracking for motility (Figure 3A), we observed that planaria in 3 mM solutions spent more time in the center of the wells compared to the control group (Control, mean = 101.938 ± 32.219; 3 mM Pilocarpine, mean = 187.966 ± 24.908, 6 mM Pilocarpine, mean = 176.467 ± 22.980; Figure 3B).
Planaria in 6 mM solutions spent more time in the perimeter of the wells than both the 3 mM group and the control group, with a significant difference between 6 mM and control (Control, mean = 708.958 ± 59.506; 3 mM Pilocarpine, mean = 881.562 ± 80.604; mean = 968.712 ± 84.267, p = 0.0241; Figure 3C).
Planaria treated with 3 mM and 6 mM Pilocarpine also entered the center versus perimeter zones more frequently than the control group (Control, mean = 104.250 ± 11.436; 3 mM Pilocarpine, mean = 159.625 ± 13.368; 6 mM Pilocarpine, mean = 132.500 ± 28.126; Figure 3D).
Although there is a small sample size, the normal quantile plot generated using statistical analysis software demonstrates that most data values fall near the solid red line. The data values also fall within the dotted red confidence bounds, indicating a normal distribution. Statistical analysis software was selected for this analysis because it offers a powerful combination of data visualization and statistical tools. The software is particularly effective for creating quantile plots, which are essential for visually assessing normality in small datasets. Its intuitive interface and built-in statistical tests, such as ANOVA, make it ideal for quickly running analyses and ensuring accuracy in graphical representations, which aids in robust data interpretation9,13,14,19.
Significant differences were observed in the mean perimeter durations between the control group and the 6 mM group. This study provides valuable insights into the motility changes in planaria when exposed to different solution concentrations. Statistical analysis revealed significant differences in the mean perimeter durations between the control group and the 6 mM group, providing insights into the motility changes in planaria when exposed to different solution concentrations.
To support the behavioral analysis, histological analysis was performed using two methods. The Golgi stain was utilized to visualize the neurons in the planarian nervous system, to determine whether the seizure-like behavior had an effect on the presence or morphology of neurons. Second, to confirm that it was indeed neurons that were being observed, an immunofluorescent assay was performed using an antibody that recognizes an antigen found on axonal projections of sensory neurons20. Histological studies using Golgi staining and immunofluorescence using anti-1H6 showed the potential to identify and quantify nerves using both transverse sections and whole-mount preparations (Figure 4)21. Pilocarpine (6 mM) treatment reduced the number of neural structures compared to Control (Control (n = 3): mean = 764.14 ± 260.46; pilocarpine (n = 3): mean = 162.12 ± 86.22; p < 0.05)

Figure 1: Experimental procedure overview for Dugesia dorotocephala. Overall procedure of this study. Planaria are placed into various concentrations of pilocarpine, and their behavior is recorded and analyzed. This is followed by histological analysis using the Golgi stain and an immunofluorescent assay. Please click here to view a larger version of this figure.

Figure 2: Pilocarpine-induced changes in planaria movement and oscillations. (A-F) Changes of planaria movements with increasing pilocarpine concentrations, noting statistically significant values with *. Individual values (black dots), mean (green line), standard error mean (SEM, blue bar), standard deviation (blue line), and quartile (red box) are noted. (G) Frequency of dorsal oscillations with increasing pilocarpine concentration with individual values (green dots) and the line of best fit (black). R = correlation coefficient. Analysis of Variance was used to calculate significance. Please click here to view a larger version of this figure.
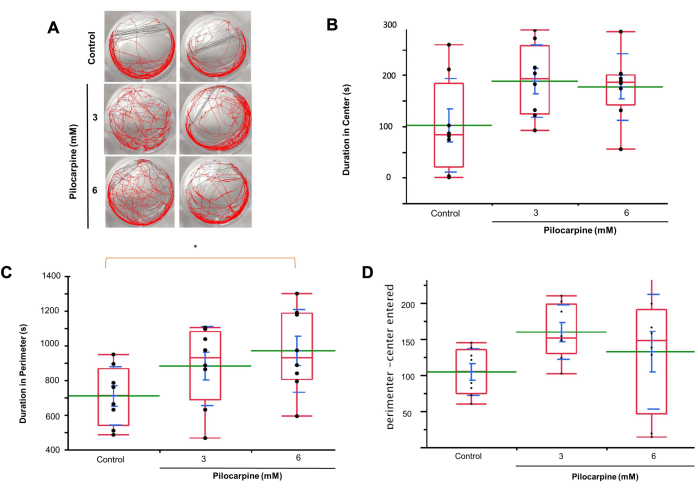
Figure 3: Planaria behavior in control and pilocarpine solutions using motion tracking analysis. (A) Observations of planaria behavior in control, 3 mM, and 6 mM solutions. (B) Duration of time planaria spent in the center of the wells in 3 mM solutions compared to the control group. (C) Duration of time planaria spent in the perimeter of the wells in 6 mM solutions compared to both the 3 mM group and the control group. (D) Frequency of planaria entering the center and perimeter zones in 3 mM solutions compared to the control group. Data shows individual values (black dots), mean (green line), standard error mean (SEM, blue bar), Standard deviation (blue line), and quartile (red box). Analysis of Variance was used to calculate significance. Please click here to view a larger version of this figure.

Figure 4: Neural structural analysis of planaria. (A) The graphic depicts the planaria gross structure highlighting key neurological features, following the transverse section of planaria through the gut highlighting the digestive and muscular structures, and the transverse section of the planarian brain highlighting key neurological structures. (B) Control versus pilocarpine neuronal structure using immunofluorescence. Estimation of the nerve density decreased (0.16 nerves/pixels in the control group to 0.11 nerves/pixels) in the pilocarpine-treated group. (C) Representative sections of planaria using Golgi staining; 1: head arrow showing brain; 2: arrow showing ventral nerve cord; 3 neuron-like showing protrusion (arrow). (D) Note that lateral branches (asterisk) and (E) neural structures are reduced in pilocarpine. (F) The number of neural cell profiles is reduced in pilocarpine. Data shows individual values (black dots), mean (green line), standard error mean (SEM, blue bar), Standard deviation (blue line), and quartile (red box). Student's t-Test was used to calculate significance. Please click here to view a larger version of this figure.
Dyskusje
This study demonstrated pilocarpine-induced behavior in planaria at different concentrations. The most pertinent behavior was oscillating dorsal expansion, as this behavior has not been documented in other studies regarding seizure-like behavior in planaria9,10,11,12. The mechanism by which pilocarpine induces seizure-like behaviors in planaria is still unknown. However, this study demonstrates that different behaviors may correspond to different degrees of neural excitability. This effect is thought to be caused by muscarinic receptor agonism22.
The key steps in the protocol involved the preparation of pilocarpine solutions for dose-dependent analysis. Following the procedure here ensured the planarias used in the behavioral recording would have consistent conditions. Placing the planaria in separate well plates containing either pilocarpine solutions or Springwater allowed key behaviors such as dorsal oscillations, C-shape movements, and head flicks to be observed. The focus on one planarian per well plate also reduced potential errors by the automated tracking software for the one-hour recordings. In addition, it was essential to calibrate and analyze the video footage with specific settings to quantify movement patterns, including time spent in the center versus perimeter zones for the motility analysis. Euthanasia performed by rinsing planaria in a potent solution before preserving them in Golgi stain was crucial for histological analysis as it allowed for the humane termination of the planaria and prevented potential physiological changes that could occur in the organisms that could affect the integrity of the neural tissues. The Golgi staining was performed stepwise using solutions A, B, and C, followed by embedding and sectioning to and sectioning to examine neuronal structures. This technique was significant because it provided detailed visualization of neuronal morphology and enabled observable changes in neural structures. Immunofluorescence staining uses specific antibodies to identify the neuronal markers, with thorough washing and blocking steps to enhance clarity. Image analysis performed used software to quantify neuronal changes, ensuring consistent thresholds for reliable comparisons. Statistical analysis, including ANOVA and t-tests, evaluates significant differences in behaviors and neural structures. All these steps provide a robust methodology for assessing seizure-like activity and its neural basis in planaria.
There were several factors that needed troubleshooting. First, a sufficient concentration of pilocarpine was needed to induce the seizure-like activity but was not to be exceedingly damaging so that all activity was ceased. For that reason, several concentrations were first tested to determine the optimal concentration to obtain these results. These concentrations were also used to determine if the behavior exhibited dose dependency. Second, the Golgi assay was optimized for loss of tissue. After mounting to the slide using solution C, the tissue would fall off during the staining process. Gelatin-coated slides were incorporated, and the slides were carefully handled during the staining process; these two modifications minimized tissue loss. Next, to get cross sections, the planaria were placed flat into a temporary mold, frozen in the cryostat, and then mounted onto the chuck perpendicular to their original position so transverse sections could be cut. Regarding the immunofluorescence assay, the optimal concentration of the antibody needed to be determined. A serial dilution is recommended to determine the optimal dilution concentration. Luckily, the manufacturer's recommendations worked well for this assay, so a serial dilution was not necessary. This protocol used milk as a blocking buffer as this is a cheaper and more affordable option and works just as well as a buffer23,24,25. Finally, troubleshooting was required for video artifacts; the planaria exposed to 2 mM pilocarpine were excluded due to tracking disruptions from container movement, lighting issues, and angle changes.
As with any procedure, there are limitations. Although planaria exhibit similar neuronal pathways as humans, they are invertebrates and lack the complexity of mammalian brains26. Therefore, results observed in planaria may not correlate directly with humans. Second, this assay does allow quantification of neurons but does not allow analysis of the morphology of neurons and their dendrites, which is often diagnostic in patients with epilepsy15,19. The results of this research, therefore, serve only as preliminary analysis for human epilepsy.
The quantification of the 0 mM and 6 mM pilocarpine planarias in Figure 4 holds significant implications in determining whether the planaria experienced seizures. By carefully analyzing and comparing the values obtained from the experimental and control groups, there was a statistically significant difference between the two groups. This suggests that the pilocarpine applied to the experimental group may have directly contributed to the observed behavior in the planaria. Integrating further improvements into the video analysis software could also enhance the accuracy and reliability of the data collected from planaria observations. Such software could automate the tracking and quantification of motion patterns, providing objective measurements of movements such as dorsal oscillation and reducing the reliance on video quality alone for these observations.
Current studies have demonstrated seizure-like behavior in planaria; however, to our knowledge, the oscillating dorsal expansion movement has not been noted9,10,11. Other behaviors observed, such as the C-shape, are consistent with current research9,10,11,12. Immunofluorescence assays have been performed on planaria, and the results are consistent with this assay20,27; however, to our knowledge, a Golgi stain has not been performed on planaria before, and optimization of this stain may provide the opportunity to analyze neuronal morphology. This may, in turn, provide insights into the pathophysiology of epilepsy. It has already been demonstrated that pilocarpine induces seizure-like behavior in planaria. With further modifications and repetition, this assay has the potential to be used in drug screening for anti-seizure medication.
Ujawnienia
The authors have nothing to declare.
Podziękowania
We want to thank EVMS Research Incentive Fund (PI: A.E. Musto) and Dr. Jorge Jacot for his helpful suggestions with optimizing the immunofluorescence protocol.
Materiały
| Name | Company | Catalog Number | Comments |
| 1.5 mL centrifuge tubes | |||
| 4% paraformaldehyde solution | Himedia | TCL119 | |
| Aqueous mounting media | Clini Sciences | NB-47-02240-30ML | |
| Beakers (one for each concentration tested) | |||
| Carolina Springwater | carolina | 132450 | |
| Cryostat | |||
| Diluted primary and secondary antibodies | |||
| Ethanol (100%) | sigma Aldrich | ||
| EthosVision XT 16 | noldus | ||
| Fiji Version 2.9.0 | |||
| Gelatin-coated slides | sigma Aldrich | 643203 | |
| Golgi Antibody 1H6 | DSHB | AB_2619608 | |
| Golgi stain kit_ | Neuroscience Associate | PK 401/401A | |
| Hydrogen Peroxide | |||
| Methanol | |||
| Mounting media | thermo fischer scientific | ||
| OCT compound | |||
| PBS buffer | sigma Aldrich | P4417 | |
| Powdered Milk | |||
| Tin foil | |||
| Transfer pipettes | |||
| Xylene |
Odniesienia
- World Health Organization. . Epilepsy. , (2024).
- Dalic, L., Cook, M. J. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat. 12, 2605-2616 (2016).
- Mayo Clinic. . Seizures - Symptoms and causes. , (2023).
- Shomrat, T., Levin, M. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. J Exp Biol. 216 (20), 3799-3810 (2013).
- Moreira-Lobo, D. C., et al. Eugenol modifies the excitability of rat sciatic nerve and superior cervical ganglion neurons. Neurosci Lett. 472 (3), 220-224 (2010).
- Johan Arief, M. F., Choo, B. K. M., Yap, J. L., Kumari, Y., Shaikh, M. F. A systematic review on non-mammalian models in epilepsy research. Front Pharmacol. 9, 655 (2018).
- Mineta, K., et al. Origin and evolutionary process of the CNS elucidated by comparative genomics analysis of planarian ESTs. Proc Natl Acad Sci U S A. 100, 7666-7671 (2003).
- Wu, J. P., Lee, H. L., Li, M. H. Cadmium neurotoxicity to a freshwater planarian. Arch Environ Contam Toxicol. 67 (4), 639-650 (2014).
- Kim, A., Rawls, S. M. Nicotine-induced C-shape movements in planarians are reduced by antinociceptive drugs: Implications for pain in planarian paroxysm etiology. Brain Res. 1778, 147770 (2022).
- Reho, G., Lelièvre, V., Cadiou, H. Planarian nociception: Lessons from a scrunching flatworm. Front Mol Neurosci. 15, 935918 (2022).
- Pagán, O. R., et al. Planarians require an intact brain to behaviorally react to cocaine, but not to react to nicotine. Neuroscience. 246, 265-270 (2013).
- Raffa, R. B., Holland, L. J., Schulingkamp, R. J. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. J Pharmacol Toxicol Methods. 45 (3), 223-226 (2001).
- Bhatt, P., Reitz, A. B., Tallarida, C. Mephedrone ("bath salt") pharmacology: insights from invertebrates. Neuroscience. 208, 79-84 (2012).
- Neufeld, T., Carniello, T., Dotta, B. A single hypoxic event ameliorates pilocarpine-induced hyperkinetic movements in Planaria. Nat Sci. 14, 149-156 (2022).
- Rossini, L., et al. Dendritic pathology, spine loss, and synaptic reorganization in human cortex from epilepsy patients. Brain. 144 (1), 251-265 (2021).
- Owuor, K., et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 42 (4), 448-457 (2009).
- Dubey, V., et al. Dendritic reorganization in the hippocampus, anterior temporal lobe, and frontal neocortex of lithium-pilocarpine induced Status Epilepticus (SE). J Chem Neuroanat. 133, 102329 (2023).
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 20 (5), 359-368 (2011).
- Musto, A. E., Walker, C. P., Petasis, N. A., Bazan, N. G. Hippocampal neuro-networks and dendritic spine perturbations in epileptogenesis are attenuated by neuroprotectin D1. PLoS One. 10 (1), e0116543 (2015).
- Omuro, K. G., et al. Novel monoclonal antibodies to study tissue regeneration in planarians. BMC Dev Biol. 15 (2), (2015).
- Keenan, C. L., et al. Cytoarchitecture of primitive brains: Golgi studies in flatworms. J Comp Neurol. 195, 4 (1981).
- Pronin, A. N., Wang, Q., Slepak, V. Z. Teaching an old drug new trick: Agonism, antagonism, and biased signaling of pilocarpine through M3 muscarinic acetylcholine receptor. Mol Pharmacol. 92 (5), 601-612 (2017).
- Im, K., Mareninov, S., Diaz, M. F. P., Yong, W. H. An introduction to performing immunofluorescence staining. Methods Mol Biol. 1897, 299-311 (2019).
- Vogt, R. V., Phillips, D. L., Henderson, L. O., Whitfield, W., Spierto, F. W. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J Immunol Meth. 101 (1), 43-50 (1987).
- Duhamel, R. C., Johnson, D. A. Use of nonfat dry milk to block nonspecific nuclear and membrane staining by avidin conjugates. J Histochem Cytochem. 33 (7), 711-714 (1985).
- Chen, T. S., Huang, T. H., Lai, M. C., Huang, C. W. The role of glutamate receptors in epilepsy. Biomedicines. 11 (3), 783 (2023).
- Kreshchenko, N. Immunocytochemical identification of serotoninergic neurons in Planaria Girardia tigrina. Biochem. Moscow Suppl Ser A. 11, 68-76 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone