Method Article
A Reproducible Cartilage Impact Model to Generate Post-Traumatic Osteoarthritis in the Rabbit
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The open medial femoral condyle impact model in rabbits is reliable for studying post-traumatic osteoarthritis (PTOA) and novel therapeutic strategies to mitigate PTOA progression. This protocol generates an isolated cartilage defect of the posterior medial femoral condyle in rabbits using a carriage-based drop tower with an impactor head.
Streszczenie
Post-traumatic osteoarthritis (PTOA) is responsible for 12% of all osteoarthritis cases in the United States. PTOA can be initiated by a single traumatic event, such as a high-impact load acting on articular cartilage, or by joint instability, as occurs with anterior cruciate ligament rupture. There are no effective therapeutics to prevent PTOA currently. Developing a reliable animal model of PTOA is necessary to better understand the mechanisms by which cartilage damage proceeds and to investigate novel treatment strategies to alleviate or prevent the progression of PTOA. This protocol describes an open, drop tower-based rabbit femoral condyle impact model to induce cartilage damage. This model delivered peak loads of 579.1 ± 71.1 N, and peak stresses of 81.9 ± 10.1 MPa with a time-to-peak load of 2.4 ± 0.5 ms. Articular cartilage from impacted medial femoral condyles (MFCs) had higher rates of apoptotic cells (p = 0.0058) and possessed higher Osteoarthritis Research Society International (OARSI) scores of 3.38 ± 1.43 compared to the non-impacted contralateral MFCs (0.56 ± 0.42), and other cartilage surfaces of the impacted knee (p < 0.0001). No differences in OARSI scores were detected among the non-impacted articular surfaces (p > 0.05).
Wprowadzenie
Post-traumatic osteoarthritis (PTOA) is a leading cause of disability worldwide, and accounts for 12%-16% of symptomatic osteoarthritis (OA)1. The current gold standard for end-stage OA management is total knee and hip arthroplasty2 or arthrodesis, as in the case of end-stage tibiotalar or subtalar arthritis. Although largely successful, arthroplasty can have costly and morbid complications3. In addition, arthroplasty is less desirable in patients under 50 years, given the low revision-free implant survivorship of 77%-83%4,5. Currently, there are no FDA-approved treatments to prevent or mitigate the progression of PTOA.
PTOA affects the whole joint, including the synovial tissue, subchondral bone, and articular cartilage. It is characterized by articular cartilage degeneration, synovial inflammation, subchondral bone remodeling, and osteophyte formation6,7. The phenotype of PTOA develops via a complex process of interplay between cartilage, synovium, and subchondral bone. The current understanding is that cartilage injury leads to the liberation of extra-cellular matrix (ECM) components such as type 2 collagen (COL2) and aggrecan (ACAN). These ECM component fragments are pro-inflammatory and cause increased production of IL-6, IL-1β, and reactive oxygen species. These mediators act on chondrocytes, causing upregulation of matrix metalloproteinases (MMPs), such as MMP-13, which degrade articular cartilage while also decreasing matrix synthesis, leading to an overall catabolic environment for the articular cartilage8. In addition, there is evidence of increased chondrocyte apoptosis in primary osteoarthritis and PTOA9,10. Mitochondrial dysfunction occurs after supraphysiological loading of cartilage11,12,13,14, which can lead to increased chondrocyte apoptosis12,15. Enhanced chondrocyte apoptosis has been associated with increased proteoglycan depletion and cartilage catabolism and has been shown to precede changes in cartilage and subchondral bone remodeling16,17,18.
As with most human diseases, reliable and translational models of PTOA are needed to further understand the pathophysiology of the disease and test novel therapeutics. Large animals such as swine and canines have been used in intra-articular fracture and impact models of PTOA17,19, but they are costly. Smaller animal models, such as mice, rats, and rabbits are less expensive and are used to study PTOA generated through joint destabilization, which typically involves surgical transection of the anterior cruciate ligament (ACL) and/or disruption of the medial meniscus20,21,22,23,24,25. Although joint trauma can lead to various consequences, including ligamentous injury26, mechanical overload of the cartilage occurs in nearly all cases.
There is emerging evidence that the pathology behind the development of PTOA after ligamentous instability (as in ACL transection) and acute chondral injury is due to distinct mechanisms27. Therefore, developing models of direct injury to cartilage is important. There are currently a limited number of impact models generating osteochondral or chondral injury in rats and mice28,29. However, murine cartilage is not well-suited for generating isolated chondral defects. This is because murine articular cartilage is only 3-5 cell layers thick and lacks organized superficial, radial, and transitional cartilage zones, as well as the thick calcified cartilage layer found in humans and larger animals. Murine models also display spontaneous resolution of partial cartilage defects30,31. Hence, we chose the rabbit for this impact model as its cartilage thickness and organization are similar to those of humans, and it is the smallest animal model that will allow for the delivery of a consistent chondral impact that results in PTOA. Prior open surgical models of femoral condyle impact in the rabbit have employed a pendulum32, a hand-held spring-loaded cartilage impaction device33, and a drop tower that allowed rabbit-specific impactor creation34. However, these studies lacked in vivo data. Others have reported in vivo data with pendulum-based35, pneumatic36, and spring-loaded37 impact devices10, and these studies show a high rate of variability in peak stress and loading rates between the methods. Still, the field lacks a consistent approach to reliably model acute cartilage trauma in vivo.
The current protocol employs a drop-tower-based system to deliver a consistent impact to the posterior medial condyle of the rabbit knee. A posterior approach to the knee is employed to expose the posterior medial femoral condyle. A Steinman pin is then placed across the femoral condyles from medial to lateral in line with the joint surface and secured to the platform. Once secured, a load is delivered to the posterior medial femoral condyle. This method allows for consistent cartilage damage to be delivered to the weight-bearing surface of the rabbit distal femur.
Protokół
The following procedure was performed with approval from the Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC). All survival surgeries were performed under sterile conditions, as outlined by the NIH guidelines. Pain and infection risks were managed with proper analgesics and antibiotics to optimize successful outcomes. Skeletally mature male New Zealand White rabbits, weighing 3.0-4.0 kg, were used for the present study.
1. Drop tower fabrication
- Generate CAD drawings for components of the drop tower, base platform, and mechanism to secure the Steinman pin (Supplementary Figures 1-14).
- Purchase commercially available components (See Table of Materials).
- Procure the machine parts of the device or give CAD drawings to a machinist for fabrication.
NOTE: A high-precision machinist with toolmaking capability is required to fabricate the 3 mm diameter impactor tip (Supplementary Figure 1, part 20 and Supplementary Figures 13,14). The impacting face of the impactor head had curvatures of 7.14 mm and 5.56 mm in the sagittal and coronal planes, respectively, to conform to the curvature of the medial rabbit condyle35(Supplementary Figures 13,14). - Assemble parts such that the drop tower consists of a carriage traveling on two vertical rods via fixed-alignment linear ball bearings, and the base platform supports the rabbit and secures the Steinman pin (Figure 1 and Figure 2).
NOTE: The carriage crossbeam of this design has a bending stiffness equal to that of a previous drop tower38 with an acceptable vibration level.
2. Animal preparation
- Weigh the rabbit and anesthetize it using 2.5 mg/kg alfaxalone and 0.15 mg/kg midazolam IM (see Table of Materials). Apply eye ointment to both eyes after induction. Maintain anesthesia using ~2%-3% isoflurane. Give buprenorphine SR (0.1 mg/kg) SQ for analgesia and perioperative enrofloxacin (10 mg/kg) SQ. In place of Buprenorphine, NSAIDs such as Carprofen, 4mg/kg or Meloxicam, 0.2 – 0.3mg/kg or Ketoprofen, 3mg/kg can be given as SQ injections.
- Shave the rabbit's hind limb from the ankle to the hindquarters. Extra caution is urged in rabbit hair removal to prevent contamination of the incision. Using a set of dedicated, sharp rabbit hair clippers is important.
- Place the stainless steel front leg block (Supplementary Figure 1, part No. 2, and Supplementary Figure 4) under the end of the impact platform and cover the platform with a heating pad. Place the rabbit sternal (i.e., prone) on the heating pad. Place a padded bump under the contralateral hip.
- Ensure the operative extremity has the knee centered and resting on the polyethylene block (Figure 2A1). Use silk tape to gently retract the tail superior and contralateral to the operative extremity.
- Wipe down the surgical site with chlorohexidine and 70% alcohol-soaked sterile gauze. Scrub the surgical site, starting at the posterior knee, with a circular sweep outward. Repeat at least 3 times with fresh scrubs, ending with 70% alcohol.
- Place a sterile glove over the operative foot up to the ankle and wrap it with a sterile cohesive wrap.
- Sterilely drape out the surgical site with three drapes: one directly under the operative extremity and the other two to cover the rest of the body. Secure the drapes with towel clamps.
3. Surgical exposure
NOTE: Prior to the surgery and impact, the weight and the drop height that deliver visible cartilage damage without subchondral bone fracture should be empirically determined for the specific strain, age, and sex of the rabbit.
- Palpate the position of the patella anteriorly to estimate the position of the knee joint, which is located distal to the patella. Using a 15-blade, make a 3-4 cm incision along the posterior aspect of the extended knee from the level of the superior pole of the patella distally.
- Perform blunt and sharp dissections through the underlying superficial fascia. Develop the interval between the skin medially and the medial gastrocnemius laterally. Place a self-retaining Weitlaner retractor in this interval (see Table of Materials).
- A secondary fascial layer will become visible just overlying the saphenous artery and vein. Dissect lateral to the saphenous and retract the vasculature medially and the posterior gastrocsoleus complex laterally.
NOTE: Take care not to cut this vasculature. If this artery is damaged, ensure proper ligation, as post-operative hemorrhagic shock can occur.
- A secondary fascial layer will become visible just overlying the saphenous artery and vein. Dissect lateral to the saphenous and retract the vasculature medially and the posterior gastrocsoleus complex laterally.
- Dissect distally until a small mobile fabella is identified over the posterior medial femoral condyle. Perform an arthrotomy to mobilize the fabella superolateral, exposing the underlying medial femoral condyle. Gently remove soft tissue by blunt and sharp dissection to expose the posterior aspect of the medial femoral condyle. Use a Freer and Cricket self-retainer (see Table of Materials) to retract soft tissues at this level.
- While keeping the condyle exposed, advance a 0.062 inch Steinman pin (see Table of Materials) across the distal femur, beginning at the superior aspect of the medial femoral condyle and centered in the anterior-posterior direction of the medial femoral condyle, roughly 5 mm from the posterior aspect of the condyle.
- Drive the wire laterally through the bone and lateral skin parallel to the joint surface using a battery-powered Steinman pin driver. Palpation of the lateral epicondyle will ensure the appropriate trajectory of the Steinman pin.
- Remove the retractors and close the skin with a 3-0 polysorb suture (see Table of Materials) in a running fashion. Cover the incision with sterile gauze.
4. Impact of the femoral condyle
- Remove the drape under the operative limb and secure the Steinman pin to a customizable and adjustable height impact platform. First, place the height-adjustable, lower aspect of the Steinman pin securing apparatus under the pin (Figure 2A2). Ensure the wire is parallel with the ground on this platform by adjusting the screw heights as needed.
- After ensuring the Steinman pin is parallel to the ground, place the top screw-based aspect of the secure platform (Figure 2A3) onto the lower screw-based aspect of the height-adjustable piece. Ensure the Steinman pin is tightly secured by screwing the top bar into the lower height adjustable portion of the pin-holding platform (Figure 2A2).
- Once the Steinman pin is secured to the platform, remove the suture, and reopen the incision. Expose the medial femoral condyle with self-retaining Weitlaner and cricket retractors. An additional Freer may be needed to retract additional soft tissue out of the path of the impactor tip (Figure 2B).
- Wipe down the drop tower with an approved disinfectant. Attach the sterile 3 mm impactor head (Figure 2A4) to the drop tower carriage. Bring the drop tower over the operative extremity and place its base (Figure 2A6) underneath the impacting platform (Figure 3A).
- Gently lower the impactor (Supplementary Figure 2, part 20, and Supplementary Figure 13) onto the center of the posterior medial femoral condyle. Ensure no soft tissue is in the path of the impactor.
- Move the rabbit or tower as needed to ensure the impactor head is centered over the posterior medial femoral condyle (Figure 3B). Any time the rabbit is moved or re-positioned that surgical site should be assessed for any possible breaks to sterility and the area re-sterilized if needed.
- Once appropriate trajectory is ensured, clamp the tower onto the platform with the toggle clamps (Figure 2A5, see Table of Materials).
- Administer one dose of intravenous alfaxalone (0.5-0.7 mg/kg) 5-10 min prior to impact for deeper anesthesia without increasing inhalant anesthesia.
NOTE: A lack of palpebral reflex, pedal withdrawal, and pinna reflex evidences deeper anesthetization. This deeper anesthesia helps prevent possible limb reactions during placement in the apparatus and during impact.
CAUTION: If given too quickly, alfaxalone can cause transient apnea and hypoxia in rabbits and should be given slowly over 1-2 min. If hypoxia does occur, ensure adequate oxygenation and restoration of vitals before proceeding. - Set the impactor on the drop tower at the desired height above the medial femoral condyle. For the current carriage assembly, including the bearings, with a mass of 1.41 kg, this is a height of 7 cm.
NOTE: Drop tower height was determined from pilot studies on cadaver tissue. This height generated visible cartilage damage but not subchondral bone fracture for the rabbits in this study. - Click on the Start button on the LabVIEW data acquisition software (Supplementary Coding File 1) just before freeing the spindle stop (Supplementary Figure 2, item No. 14) to release the carriage and allow it to drop under the force of gravity.
NOTE: The data acquisition software will collect the data from a load cell (Figure 1, 6) positioned between the impactor and carriage and an accelerometer (Figure 1, 7) during the impact at 100 kHz using a laptop connected to a data acquisition module. - Place the txt file generated by the data acquisition software in the same folder with the Matlab data analysis code (Supplementary Coding File 2) and run the data analysis code to filter raw data and calculate impact parameters.
- Ensure that the maximum load is identified. The associated time point is considered the time of maximum deformation and zero velocity.
NOTE: Data analysis code will analyze all txt files in the folder and report the results for each file. The code will determine the beginning and end of impact based on changes in the load-time data. Data from the accelerometer will be integrated numerically to calculate velocity and integrated again to calculate displacement. Data analysis code will numerically calculate the impulse, work, and kinetic energy from the following formulas:
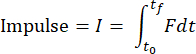


where F is the force measured by the load sensor, x0 and t0 are the displacement and time at the beginning of the impact, and x, and tf are the displacement and time at the end of the impact. The loading rate will be numerically calculated as the average of dσ/dt in the loading phase of impact. Peak stress will be calculated by dividing the peak load by the contact area of the impactor head. - Perform visualization of the cartilage surface to determine whether appropriate cartilage damage has occurred (Figure 4A).
5. Surgical site closure
- Remove the drop tower from over the operative extremity. Place all used surgical tools aside and change into new sterile gloves.
NOTE: Given that the drop tower is not sterile, all tools used up to the impact should now be considered contaminated. - Reapply a sterile drape to the lower extremity and obtain unused sterile self-retractors.
- Re-expose the medial femoral condyle and thoroughly irrigate the surgical site with 50-60 mL of sterile saline.
- Close the posterior capsule with a 5-0 polysorb suture, followed by skin closure with a 4-0 monosorb suture (see Table of Materials).
- Inject 2 mL of lidocaine/bupivacaine for local analgesia around the incision intradermally.
- Remove the Steinman pin with a power driver set (see Table of Materials) by oscillating to minimize soft tissue injury.
- Dress the wound with a non-adhesive dressing, followed by adhesive tape. Perform an x-ray of the operative extremity to ensure no fracture occurred and appropriate pin placement (Figure 4B).
6. Post-operative management
- Return the rabbit to its cage and monitor it on heated blankets until it recovers from anesthesia (~25 min).
- Continue closely monitoring the rabbits for several days after the surgery to ensure they heal properly and regain mobility. Administer enrofloxacin (10 mg/kg) for 2 days post-operatively for infection prophylaxis. Administer buprenorphine SR analgesia (0.1 mg/kg) subcutaneously every 2-3 days after the surgery and as needed. In place of Buprenorphine, NSAIDs such as Carprofen, 4mg/kg SQ daily, Meloxicam, 0.2 – 0.3mg/kg SQ daily up to 3 days or Ketoprofen, 3mg/kg SQ daily can be administered 3-5 days after surgery and as needed.
NOTE: We have succeeded in preventing postoperative wound dehiscence, due to rabbit licking or chewing, with the placement of human neonatal pants over the hindlimbs39. If the rabbit chews through the pants, an Elizabethan collar (see Table of Materials) may be placed to prevent chewing of the incision.
7. Histological evaluation
- At 16 weeks post-injury, harvest knees from euthanized rabbits, fix them in 10% neutral buffered formalin for 48 h, followed by paraffin embedding and sectioning into 5 µm thick slices.
- After de-paraffinization and rehydration, stain the sections with safranin O fast green per standard protocols40,41.
- Perform the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay on the sections using TUNEL Chromogenic Apoptosis Detection kit following the manufacturer's instructions, counterstained with Hematoxylin42 (see Table of Materials).
Wyniki
The success of this procedure was monitored immediately after impact by visualization of the condyle by the surgeon (Figure 4A) and by radiography to ensure no fracture occurred (Figure 4B). There is a risk of impact failure leading to an intra-operative fracture of the condyle. This was typically due to improper Steinman pin placement (Figure 5). Using this model, there was a fracture failure rate secondary to intra-operative fracture of 9.0% (6 of 67 surgeries). The average peak impact stress was 81.9 ± 10.1 MPa (CV = 12.3%), and the average loading rate was 36.6 ± 11.0 MPa/ms (CV = 30.1%). Other parameters were also consistent, with the CVs ranging from 5%-23.5% (Table 1).
Safranin O-fast green stained histological sections of the knee joints from n = 8 rabbits were evaluated for cartilage degradation and osteoarthritis pathology using the Osteoarthritis Research Society International (OARSI) scoring system43. Cartilage damage was not observed in the contralateral uninjured femoral condyle (Figure 6A) and was mainly localized to the site of the impact (Figure 6B). Impacted 16-week medial femoral condyles (MFCs) had higher OARSI scores of 3.38 ± 1.43 compared to the contralateral control MFCs with an OARSI score of 0.56 ± 0.42 (p < 0.0001) (Figure 6C). Further, impacted knee MFCs also displayed higher OARSI scores than the medial tibial plateau (MTP; 0.71 ± 0.59), lateral tibial plateau (LTP; 0.88 ± 0.64), and lateral femoral condyle (LFC; 0.81 ± 1.00) of the same knee (p < 0.0001) (Figure 6D). In contrast, no differences in OARSI scores were observed among the MFC (0.56 ± 0.42), LTP (0.50 ± 0.46), MTP (0.28 ± 0.45), and LFC (0.25 ± 0.46) compartments of the contralateral non-impacted knee (p > 0.05) (Figure 6E). There were also no significant differences between the impacted and non-impacted LFC, MTP, and LTP joint surfaces (p >0.05) (Figure 6F).
Articular cartilage from impacted MFC harvested at 16 weeks had higher levels of TUNEL positivity (69.1 ± 14.4%), indicating increased chondrocyte apoptosis, compared to non-impacted MFCs (53.4% ± 12.4%) (p = 0.0058) (Figure 7).
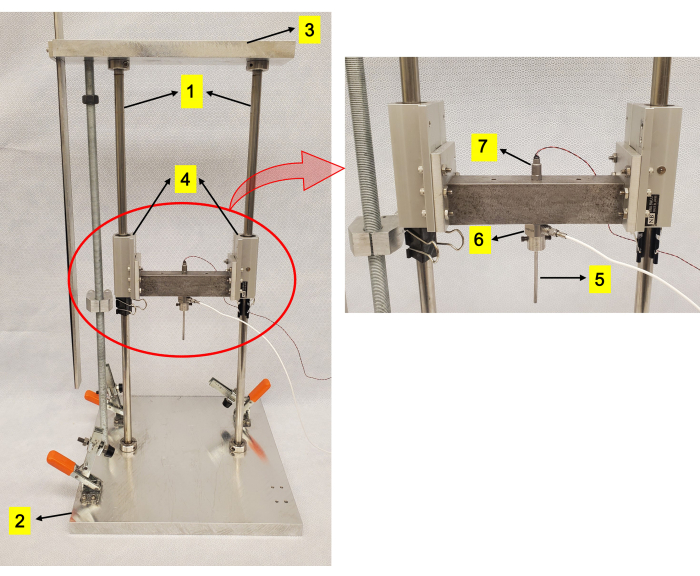
Figure 1: Drop-tower apparatus. (1) Vertical rods. (2) An aluminum platform into which rods are press-fit. (3) A plate to further restrain the rods. (4) Fixed alignment linear ball bearings. (5) Impactor head mounted on the carriage. (6) Load cell. (7) Accelerometer. Please click here to view a larger version of this figure.

Figure 2: Components used during surgical procedures and placement of the rabbit on the impact apparatus. (A) Apparatus used to generate cartilage injury and identification of the components: (1) polyethylene impact platform, (2) height-adjustable portion of the Steinman pin holding apparatus, (3) upper aspect of the height-adjustable Steinman pin holding apparatus, (4) 3 mm diameter sterile impactor head, (5) toggle clamps to hold the impact platform to the drop tower apparatus, and (6) base of the impact platform. (B) Positioning of the rabbit hind limb with the Steinman pin (indicated with red arrows) fixed to the platform prior to impact of the posterior medial femoral condyle. Drapes were omitted from figures for demonstration purposes. A cadaver was used to generate the pictures. Please click here to view a larger version of this figure.

Figure 3: Proper impactor placement on the medial femoral condyle. (A) Impact apparatus over the rabbit hindlimb that is secured to the platform. (B) Proper placement of the impactor tip on the medial femoral condyle prior to impact. Drapes were omitted from figures for demonstration purposes. Please click here to view a larger version of this figure.
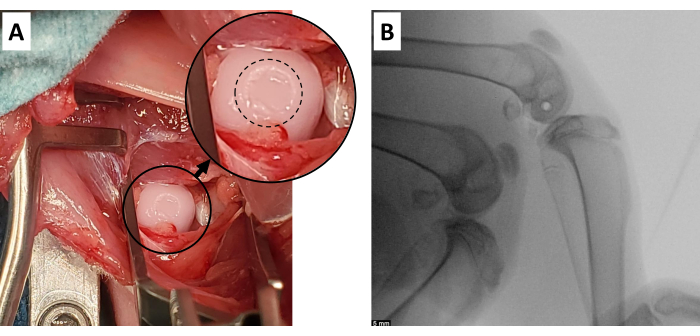
Figure 4: Successful cartilage defect. (A) Expected gross appearance of cartilage injury generated with this model. Inset is an enlarged area of the impacted cartilage surface, with the defect outlined with a dashed circle. (B) Appropriate Steinman pin position in the distal femur, with at least 5 mm of distance from the posterior cartilage surface and closely approximated to the angle of the joint surface (radiolucent circle in femoral condyles). Scale bar = 5 mm. Please click here to view a larger version of this figure.
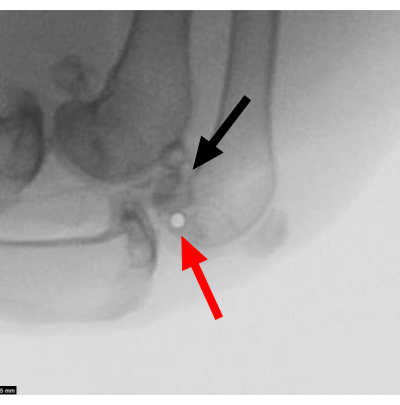
Figure 5: Unsuccessful cartilage defect. Radiograph showing a misplaced pin in the medial femoral condyle, resulting in an osteochondral fracture on impact. The red arrow points to the misplacement of the Steinman pin. The black arrow points to the fractured medial femoral condyle. Scale bar = 5 mm. Please click here to view a larger version of this figure.

Figure 6: Increased osteoarthritis severity in the impacted medial femoral condyle. Representative (A) contralateral and (B) impacted medial femoral condyles (MFC) sections stained with safranin-O (red stain of proteoglycan content) and Fast Green (blue-green stain of connective tissue with lower proteoglycan content). Magnification: 400x; scale bar = 62.3 µm. (C) OARSI scoring of the impacted and control MFC. (D) OARSI scores of all joint compartments from the impacted knee joint. (E) OARSI scores of the joint compartments from the non-impacted contralateral knee joint. (F) OARSI scores of the joint compartments from impacted and non-impacted knees. Medial femoral condyle (MFC), medial tibial plateau (MTP), lateral tibial plateau (LTP), and lateral femoral condyle (LFC). Group comparisons were performed using Student's t-test or ANOVA, followed by Tukey's HSD post-hoc test. Please click here to view a larger version of this figure.
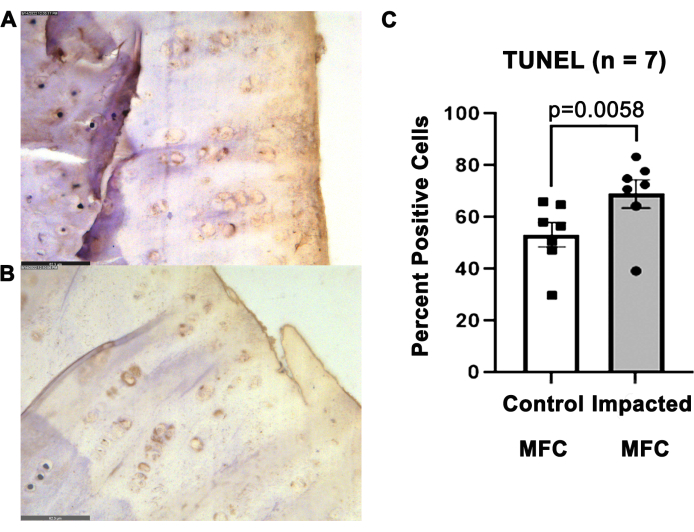
Figure 7: Increased apoptotic chondrocytes in the impacted MFC. Representative images depicting TUNEL-stained sections of (A) contralateral uninjured MFC and (B) injured MFC at 16 weeks post-impact at 400x magnification. Scale bar = 62.3 µm. TUNEL positivity is indicated by brown-colored nuclei. (C) Quantification of TUNEL-positive cells in the impacted and control MFCs. Groups were compared by paired Student's t-test. Please click here to view a larger version of this figure.
Table 1: Impact parameters of the study. This includes Peak Stress (Megapascals; MPa), Peak Load (Newtons; N), Loading Rate (Megapascals per millisecond; MPa/ms), Impact Duration (milliseconds; ms), Work (Joules; J), Impulse (Newton seconds; N·s), Kinetic Energy (Joules; J), Acceleration (meters per second squared; m/s2), and Time to Peak Load (milliseconds; ms). Please click here to download this Table.
Table 2: Impact surgery times. Please click here to download this Table.
Table 3: Advantages and disadvantages of the currently described model. Please click here to download this Table.
Supplementary Figure 1: Detailed parts description and parts list of Base Platform. Please click here to download this File.
Supplementary Figure 2: Detailed parts description and parts list of Drop Tower. Please click here to download this File.
Supplementary Figure 3: Drawing of Part 01-Rabbit holder table. Please click here to download this File.
Supplementary Figure 4: Drawing of Part 02-Front leg. Please click here to download this File.
Supplementary Figure 5: Drawing of Part 03-Main leg. Please click here to download this File.
Supplementary Figure 6: Drawing of Part 04-K-wire holder base. Please click here to download this File.
Supplementary Figure 7: Drawing of Part 05-Screw head K-wire holder. Please click here to download this File.
Supplementary Figure 8: Drawing of Part 06-Polyethylene plate. Please click here to download this File.
Supplementary Figure 9: Drawing of Part 07-Plate. Please click here to download this File.
Supplementary Figure 10: Drawing of Part 11-Top holder. Please click here to download this File.
Supplementary Figure 11: Drawing of Part 16-Impactor plate. Please click here to download this File.
Supplementary Figure 12: Drawing of Part 17-Impactor beam. Please click here to download this File.
Supplementary Figure 13: Drawing of Part 20-Impactor Tip. Please click here to download this File.
Supplementary Figure 14: Drawing of curvature of the impactor tip head. Please click here to download this File.
Supplementary Coding File 1: DropTestVIManual(1).vi. Please click here to download this File.
Supplementary Coding File 2: ImpactAnalysis(1).m. Please click here to download this File.
Dyskusje
This surgical procedure aims to generate consistent cartilage damage to the weight-bearing surface of the rabbit medial femoral condyle in a model of PTOA. An advantage of this procedure is that the posterior approach to the knee allows for direct visualization of the complete posterior medial femoral condyle, and it can be performed in approximately 37 min (Table 2). It should also be noted that this is an open injury model and may lead to acute inflammatory changes beyond just the impact due to potential damage to the synovium and joint capsule17,44. The advantages and disadvantages of the model are summarized in Table 3. Care was taken to avoid damaging ligamentous and meniscal structures to ensure joint stability. As a result, no differences were detected between contralateral control limbs and impacted limbs in joint compartments outside the zone of impact (medial and lateral tibial plateaus and lateral femoral condyles).
The most critical aspect of this protocol is the generation of an isolated cartilage lesion in the femoral condyle. Steinman pin trajectory heavily influences the success of this method. If the wire is not parallel to the joint surface or if it is placed too posteriorly relative to the center of the medial femoral condyle, it can lead to an osteochondral fracture of the femoral condyle (Figure 5). The lateral epicondyle is a consistently palpable landmark that can be used for an appropriate pin trajectory.
Animals with fractures of the subchondral bone should be removed from the study. For the current study method, we have had a failure rate secondary to intra-operative fracture of 9.0% (6 of 67 surgeries). This fracture rate is lower than a recent open pendulum-based impact model of the MFC, which had a fracture rate of 28%45. We recommend trialing this method with cadaveric specimens until the surgeon and study team feel comfortable with the approach and outcome. This method was trialed in cadaveric specimens of hind limbs and whole New Zealand White Rabbits prior to in vivo experimentation.
This method is comparable to prior published lapine acute cartilage damage generation methods. The loading rate of this impact model of 51.0 ± 16.0 MPa/ms was higher than previous works using a pendulum (around 0.5 to 6 MPa/ms)35,46,47, or a pneumatic cylinder (~0.4 MPa/ms)36 and lower than that from a spring-loaded impact device (~530 MPa/ms)37. The current impact technique models a moderate load compared to previous models, resulting in a peak stress of 81.9 ± 10.1 MPa with a CV of 12.3% that is consistent with prior models of the pendulum, spring-loaded, and pneumatic cylinder-delivered loads, with four prior models delivering stresses of 10.1-169 MPa, with CVs ranging from 0.85-40.5%36,37,45,46.
One limitation of this model is that it did not generate osteochondral fractures and hence did not fully mimic the typical intra-articular fractures seen in the clinical setting17. It was also noted that the mean acceleration of the drop tower carriage before impact was 6.4 ± 0.4 m/s2, lower than the gravitational free-fall acceleration of 9.8 m/s2, likely due to friction of the ball bearings. Still, the method allows one to isolate the impacted cartilage-mediated effects of PTOA pathogenesis, which are not fully understood.
Although several described lapine models deliver a chondral injury, utilizing the posterior approach to the knee with the drop tower model stands out as a simple, efficient, and clinically relevant method of generating PTOA, enabling the study of its pathogenesis and testing novel therapeutics. Overall, the lapine open posteromedial femoral condyle impact injury model is a promising platform for studying the cellular and molecular events associated with PTOA and identifying novel therapeutic targets48,49 to prevent or mitigate cartilage injury.
Ujawnienia
Roman Natoli delivers lectures for AO Trauma North America, is a section editor for Current Osteoporosis Reports, and received textbook royalties from Morgan and Claypool. Todd McKinley receives royalties from Innomed. The remaining authors have nothing to disclose.
Podziękowania
This study was supported by DoD Peer Reviewed Medical Research Program - Investigator-Initiated Research Award W81XWH-20-1-0304 from the U.S. ARMY MEDICAL RESEARCH ACQUISITION ACTIVITY, by NIH NIAMS R01AR076477 and a Comprehensive Musculoskeletal T32 Training Program from the NIH (AR065971) and by NIH NIAMS Grant R01 AR069657. The authors would like to thank Kevin Carr for providing his expertise in machining and fabrication to this project, and Drew Brown and the Indiana Center for Musculoskeletal Health Bone Histology Core for aiding with histology.
Materiały
| Name | Company | Catalog Number | Comments |
| Flat head screw | McMaster-Carr | 92210A194 | Stainless steel hex drive flat head screw, 8-32, 1/2" |
| #15 scalpel blades | McKesson | 1029066 | Scalpel McKesson No. 15 Stainless Steel / Plastic Classic Grip Handle Sterile Disposable |
| 1/2”-20 threaded rod | McMaster-Carr | 99065A120 | 1/2”-20 threaded rod |
| 10 mL syringe | McKesson | 1031801 | For irrigation; General Purpose Syringe McKesson 10 mL Blister Pack Luer Lock Tip Without Safety |
| 3 mL syringe | McKesson | 1031804 | For lidocaine/bupiviacaine injection; General Purpose Syringe McKesson 3 mL Blister Pack Luer Lock Tip Without Safety. |
| 3-0 polysorb | Ethicon | J332H | 3-0 Vircryl, CT-2, 1/2 circle, 26 mm, tapered |
| 4-0 monosorb | Ethicon | Z397H | 4-0 PDS 2, FS-2, 3/8 circle, 19mm, cutting edge |
| 5-0 polysorb | Med Vet International | NC9335902 | Med Vet International 5-0 ETHICON COATED VICRYL C-3 |
| Accelerometer | Kistler | 8743A5 | Accelerometer |
| Adson-Browns Forceps | World precision tools | 500177 | Adson-Brown Forceps, 12 cm, Straight, TC Jaws, 7 x 7 Teeth |
| Alfaxalone | Jurox | 49480-002-01 | Alfaxan Multidose by Jurox : 10 mg/mL |
| Buprenorphine | Par Pharmaceuticals | 42023-0179-05 | Buprenorphine HCL injection: 0.3 mg/mL |
| Butorphanol | Zoetis | 54771-2033 | Butorphanol tartrate 10mg/ml by Zoetis |
| Chlorhexidine Hand Scrub | BD | 371073 | BD E-Z Scrub 107 Surgical Scrub Brush/Sponge, 4% CHG, Red |
| Collet | STRYKER | 14023 | Stryker 4100-62 wire Collet 0.28-0.71'' |
| Cordless Driver handpiece | STRYKER | OR-S4300 | Stryker 4300 CD3 Cordless Driver 3 handpiece |
| Cricket Retractors | Novosurgical | G3510 21 | 2x Heiss (Holzheimer) Cross Action Retractor |
| Dissector Scissors | Jorvet labs | J0662 | Aesculap AG, Metzenbaum, Scissors, Straight 5 3/4″ |
| Elizabethian Collar | ElizaSoft | 62054 | ElizaSoft Elizabethan Recovery Collar |
| Enrofloxacin | Custom Meds | Enrofloxacin compounded by Custom Meds | |
| Eye Ointment | Pivetal | 46066-753-55 | Pivetal Articifical Tears- recently recalled |
| Face-mount shaft collar | McMaster-Carr | 5631T11 | Face-mount shaft collar |
| Fast green | Millipore Sigma | F7258 | Fast green |
| Freer | Jorvet labs | J0226Q | Freer elevator |
| Head screw -1 | McMaster-Carr | 91251A197 | Black-oxide alloy steel socket head screw, 8-32, 3/4" |
| Head screw -2 | McMaster-Carr | 92196A194 | Stainless steel socket head screw, 8-32, 1/2" |
| Head screw -3 | McMaster-Carr | 92196A146 | Stainless steel socket head screw, 8-32, 1/2" |
| Head screw -4 | McMaster-Carr | 92196A151 | Stainless steel socket head screw, 6-32, 3/4" |
| Hematoxylin Solution, Gill No. 1 | Millipore Sigma | GHS132-1L | Hematoxylin Solution, Gill No. 1 |
| Hex nut | McMaster-Carr | 91841A007 | Stainless steel hex nut, 6-32 |
| Hold-down toggle clamp | McMaster-Carr | 5126A71 | Hold-down toggle clamp |
| Impact device | n/a | n/a | custom made |
| Impact platform | n/a | n/a | custom made |
| K-wires | Jorvet Labs | J0250A | JorVet Intramedullary Steinman Pins, Trocar-Trocar 1/16" x 7" |
| Lab View | National Instruments | n/a | n/a |
| Load cell | Kistler | 9712B5000 | Load cell |
| MATLAB | The MathWorks Inc. | n/a | n/a |
| Microscope | Leica | DMi-8 | Leica DMi8 microscope with LAS-X software |
| Midazolam | Almaject | 72611-749-10 | Midazolam Hydrochloride injection: 5mg/ml by Almaject |
| milling machine depth stops | McMaster-Carr | 2949A71 | Clamp-on milling machine depth stops |
| Mobile C-arm | Philips | 718095 | BV Pulsera, Mobile C-arm |
| Mounted linear ball bearing | McMaster-Carr | 9338T7 | Mounted linear ball bearing |
| Needle Driver | A2Z Scilab | A2ZTCIN39 | TC Webster Needle Holder Smooth Jaws 5", Premium |
| Pentobarbital | Vortech | 0298-9373-68 | Pentobarbital 390 mg/mL by Vortech |
| Safranin O | Millipore Sigma | HT90432 | Safranin O |
| Small Battery pack | STRYKER | NS014036 | 6212 Small Battery pack- 9.6 V |
| Steel rod, 2’ | McMaster-Carr | 89535K25 | Steel rod, 2’ |
| Sterile Saline | ICU Medical | 6139-22 | AquaLite Solution Pour Bottles, 250 mL |
| Stryker 6110-120 System 6 Battery Charger | STRYKER | OR-S6110-120 | |
| Surgical gloves | McKesson | 1044729 | Surgical Glove McKesson Perry Size 6.5 Sterile Pair Latex Extended Cuff Length Smooth Brown Not Chemo Approved |
| Surgical gown | McKesson | 1104452 | Non-Reinforced Surgical Gown with Towel McKesson Large Blue Sterile AAMI Level 3 Disposable |
| Suture scissors | Jorvet Labs | J0910SA | Super Cut Scissors, Mayo, Straight, 5 1/2″ |
| TUNEL staining kit | ABP Bioscience | A049 | TUNEL Chromogenic Apoptosis Detection Kit |
| Weitlaner Retractors | Fine Science Tools | 17012-11 | 2x Weitlaner-Locktite Retractors |
Odniesienia
- Thomas, A. C., Hubbard-Turner, T., Wikstrom, E. A., Palmieri-Smith, R. M. Epidemiology of posttraumatic osteoarthritis. Journal of Athletic Training. 52 (6), 491-496 (2017).
- Pasquale, M. K., et al. Healthcare Utilization and costs of knee or hip replacements versus pain-relief injections. American Health Drug Benefits. 8 (7), 384-394 (2015).
- Yao, J. J., et al. Direct Inpatient medical costs of operative treatment of periprosthetic hip and knee infections are twofold higher than those of aseptic revisions. Journal of Bone and Joint Surgery America. 103 (4), 312-318 (2021).
- Anatone, A. J., et al. Decreased implant survival is associated with younger patients undergoing total knee arthroplasty. HSS Journal. 18 (2), 290-296 (2022).
- Stone, B., Nugent, M., Young, S. W., Frampton, C., Hooper, G. J. The lifetime risk of revision following total knee arthroplasty : a New Zealand Joint Registry study. The Bone and Joint Journal. 104-B (2), 235-241 (2022).
- Chen, D., et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Research. 5, 16044 (2017).
- Robinson, W. H., et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature Review Rheumatology. 12 (10), 580-592 (2016).
- Perez-Garcia, S., et al. Profile of matrix-remodeling proteinases in osteoarthritis: impact of fibronectin. Cells. 9 (1), 40 (2019).
- Hashimoto, S., Ochs, R. L., Komiya, S., Lotz, M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheumatology. 41 (9), 1632-1638 (1998).
- Natoli, R. M., Athanasiou, K. A. Traumatic loading of articular cartilage: Mechanical and biological responses and post-injury treatment. Biorheology. 46 (6), 451-485 (2009).
- Coleman, M. C., Brouillette, M. J., Andresen, N. S., Oberley-Deegan, R. E., Martin, J. M. Differential effects of superoxide dismutase mimetics after mechanical overload of articular cartilage. Antioxidants (Basel). 6 (4), 98 (2017).
- Goodwin, W., et al. Rotenone prevents impact-induced chondrocyte death. Journal of Orthopaedic Research. 28 (8), 1057-1063 (2010).
- Wolff, K. J., et al. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. Journal of Orthopaedic Research. 31 (2), 191-196 (2013).
- Delco, M. L., Bonnevie, E. D., Bonassar, L. J., Fortier, L. A. Mitochondrial dysfunction is an acute response of articular chondrocytes to mechanical injury. Journal of Orthopaedic Research. 36 (2), 739-750 (2018).
- Coleman, M. C., Ramakrishnan, P. S., Brouillette, M. J., Martin, J. A. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheumatology. 68 (3), 662-671 (2016).
- Bobinac, D., Spanjol, J., Zoricic, S., Maric, I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone. 32 (3), 284-290 (2003).
- Coleman, M. C., et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Science Translational Medicine. 10 (427), eaan5372 (2018).
- Heraud, F., Heraud, A., Harmand, M. F. Apoptosis in normal and osteoarthritic human articular cartilage. Annals of Rheumatological Diseases. 59 (12), 959-965 (2000).
- Narez, G. E., Fischenich, K. M., Donahue, T. L. H. Experimental animal models of post-traumatic osteoarthritis of the knee. Orthopedic Reviews (Pavia). 12 (2), 8448 (2020).
- Fischenich, K. M., et al. Chronic changes in the articular cartilage and meniscus following traumatic impact to the lapine knee. Journal of Biomechanics. 48 (2), 246-253 (2015).
- Isaac, D. I., Meyer, E. G., Kopke, K. S., Haut, R. C. Chronic changes in the rabbit tibial plateau following blunt trauma to the tibiofemoral joint. Journal of Biomechanics. 43 (9), 1682-1688 (2010).
- Wei, F., et al. Post-traumatic osteoarthritis in rabbits following traumatic injury and surgical reconstruction of the knee. Annals of Biomedical Engineering. 50 (2), 169-182 (2022).
- Terracciano, R., et al. Quantitative high-resolution 7T MRI to assess longitudinal changes in articular cartilage after anterior cruciate ligament injury in a rabbit model of post-traumatic osteoarthritis. Osteoarthritis and Cartilage Open. 4 (2), 100259 (2022).
- Huang, K., Cai, H. L., Zhang, P. L., Wu, L. D. Comparison between two rabbit models of posttraumatic osteoarthritis: A longitudinal tear in the medial meniscus and anterior cruciate ligament transection. Journal of Orthopaedic Research. 38 (12), 2721-2730 (2020).
- Sun, Z. B., Peng, H. Experimental Study on the prevention of posttraumatic osteoarthritis in the rabbit knee using a hinged external fixator in combination with exercises. Journal of Investigative Surgery. 32 (6), 552-559 (2019).
- Gardner, M. J., et al. The incidence of soft tissue injury in operative tibial plateau fractures: a magnetic resonance imaging analysis of 103 patients. Journal of Orthopedic Trauma. 19 (2), 79-84 (2005).
- Dilley, J. E. B. . M. A., Roman, N., McKinley, T. O., Sankar, U. Post-traumatic osteoarthritis: A review of pathogenic mechanisms and novel targets for mitigation. Bone Reports. 18, 101658 (2023).
- Seol, D., et al. Effects of knockout of the receptor for advanced glycation end-products on bone mineral density and synovitis in mice with intra-articular fractures. Journal of Orthopedic Research. 36 (9), 2439-2449 (2018).
- Furman, B. D., et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. Journal of Orthopedic Research. 25 (5), 578-592 (2007).
- Glasson, S. S., Chambers, M. G., Van Den Berg, W. B., Little, C. B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 18 Suppl 3, S17-S23 (2010).
- McCoy, A. M. Animal models of osteoarthritis: comparisons and key considerations. Veterinary Pathology. 52 (5), 803-818 (2015).
- Fening, S. D., Jones, M. H., Moutzouros, V., Downs, B., Miniaci, A. Method for Delivering a controlled impact to articular cartilage in the rabbit knee. Cartilage. 1 (3), 211-216 (2010).
- Leucht, F., et al. Development of a new biomechanically defined single impact rabbit cartilage trauma model for in vivo-studies. Journal of Investigative Surgery. 25 (4), 235-241 (2012).
- Vrahas, M. S., Smith, G. A., Rosler, D. M., Baratta, R. V. Method to impact in vivo rabbit femoral cartilage with blows of quantifiable stress. Journal of Orthopedic Research. 15 (2), 314-317 (1997).
- Borrelli, J., Burns, M. E., Ricci, W. M., Silva, M. J. A method for delivering variable impact stresses to the articular cartilage of rabbit knees. Journal of Orthopedic Trauma. 16 (3), 182-188 (2002).
- Milentijevic, D., Rubel, I. F., Liew, A. S., Helfet, D. L., Torzilli, P. A. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. Journal of Orthopedic Trauma. 19 (7), 466-473 (2005).
- Alexander, P. G., et al. An In vivo lapine model for impact-induced injury and osteoarthritic degeneration of articular cartilage. Cartilage. 3 (4), 323-333 (2012).
- Bonitsky, C. M., et al. Genipin crosslinking decreases the mechanical wear and biochemical degradation of impacted cartilage in vitro. Journal of Orthopedic Research. 35 (3), 558-565 (2017).
- Bartley, K. A., Johnson, C. H. Human Infant pants for postoperative protection during social housing of new zealand white rabbits (Oryctolagus cuniculus). Journal of the American Association for Laboratory Animal Science. 58 (4), 510-516 (2019).
- Lillie, R. D., Fullmer, H. M. . Histopathologic technic and practical histochemistry. , (1976).
- Prophet, E., Mills, B., Arrington, J. B., Sobin, L. H. . Armed Forces Institute of Pathology: Laboratory Methods in Histotechnology. Washington DC: American Registry of Pathology. , (1992).
- Dilley, J. E., et al. CAMKK2 is upregulated in primary human osteoarthritis and its inhibition protects against chondrocyte apoptosis. Osteoarthritis and Cartilage. 31 (7), 908-918 (2023).
- Pritzker, K. P., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 14 (1), 13-29 (2006).
- Christiansen, B. A., et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 23 (10), 1627-1638 (2015).
- Borrelli, J., Zaegel, M. A., Martinez, M. D., Silva, M. J. Diminished cartilage creep properties and increased trabecular bone density following a single, sub-fracture impact of the rabbit femoral condyle. Journal of Orthopaedic Research. 28 (10), 1307-1314 (2010).
- Borrelli, J., Silva, M. J., Zaegel, M. A., Franz, C., Sandell, L. J. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. Journal of Orthopedic Research. 27 (3), 347-352 (2009).
- Borrelli, J., Zhu, Y., Burns, M., Sandell, L., Silva, M. J. Cartilage tolerates single impact loads of as much as half the joint fracture threshold. Clinical Orthopedics and Related Research. 426, 266-273 (2004).
- Karnik, S., et al. Decreased SIRT1 activity is involved in the acute injury response of chondrocytes to ex vivo injurious mechanical overload. International Journal of Molecular Sciences. 24 (7), 6521 (2023).
- Mevel, E., et al. Systemic inhibition or global deletion of CaMKK2 protects against post-traumatic osteoarthritis. Osteoarthritis Cartilage. 30 (1), 124-136 (2022).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone