Method Article
Isolation and Characterization of Intact Phycobilisome in Cyanobacteria
W tym Artykule
Podsumowanie
The current protocol details the isolation of phycobilisomes from cyanobacteria by centrifugation through a discontinuous sucrose density gradient. The fractions of intact phycobilisomes are confirmed by 77K fluorescent emission spectrum and SDS-PAGE analysis. The resulting phycobilisome fractions are suitable for negative staining of TEM and mass spectrometry analysis.
Streszczenie
In cyanobacteria, phycobilisome is a vital antenna protein complex that harvests light and transfers energy to photosystem I and II for photochemistry. Studying the structure and composition of phycobilisome is of great interest to scientists because it reveals the evolution and divergence of photosynthesis in cyanobacteria. This protocol provides a detailed and optimized method to break cyanobacterial cells at low cost by a bead-beater efficiently. The intact phycobilisome can then be isolated from the cell extract by sucrose gradient ultracentrifugation. This method has demonstrated being suitable for both model and non-model cyanobacteria with different cell types. A step-by-step procedure is also provided to confirm the integrity and property of phycobiliproteins by 77K fluorescence spectroscopy and SDS-PAGE stained by zinc sulfate and Coomassie Blue. The isolated phycobilisome can also be subjected to further structural and compositional analyses. Overall, this protocol provides a helpful starting guide that allows researchers unfamiliar with cyanobacteria to quickly isolate and characterize intact phycobilisome.
Wprowadzenie
Phycobilisome (PBS) is a huge water-soluble pigment-protein complex that attaches to the cytoplasmic side of the photosystems in the thylakoid membranes of cyanobacteria1. PBS is primarily composed of colored phycobiliproteins and colorless linker proteins1,2. The phycobiliproteins can be divided into four major groups: phycoerythrin, phycoerythrocyanin, phycocyanin, and allophycocyanin3. The four major groups absorb different wavelengths of light energy in the range of 490-650 nm, which chlorophylls absorbed inefficiently3. The PBS can serve as a light-harvesting antenna for collecting light energy and delivering it to Photosystem II and I4.
The structure and composition of PBS vary from species to species. Collectively, three shapes of phycobilisome (hemidiscodial, bundle-shaped, and rod-shaped) have been identified in different cyanobacterial species5. Even in the same species, the composition of PBS changes in response to the environment, such as light quality and nutrient depletion6,7,8,9,10,11. Therefore, the experimental procedure to isolate PBS from cyanobacteria has been instrumental in studying PBS12. Over several decades, many different protocols have isolated PBS and analyzed its structure, composition, and function6,7,8,12,13,14,15,16,17. The wide variety of methods for PBS isolation indeed provides flexibility in isolating the complex in different species with different reagents and instruments. However, it also makes choosing a suitable protocol more difficult for scientists unfamiliar with cyanobacteria and PBS. Therefore, a generalized and straightforward protocol is developed in this work for those interested in starting PBS isolation from cyanobacteria.
The methods for isolating PBS from previous publications are summarized here. Since PBS is a water-soluble protein complex and is readily dissociated, a high ionic strength phosphate buffer is required to stabilize PBS during extraction18. Several research articles that describe methods for the isolation of PBS from cyanobacterium have been published in the past. Most of the methods require a high concentration of phosphate buffer8,14,15,18,19. However, the procedures for mechanical disrupting of the cells vary, such as glass beads-assisted extraction, sonication20, and French press6,8,14. Different phycobiliproteins can be obtained by precipitation with ammonium sulfate20 and purified by HPLC21 or a chromatographic column22. On the other hand, intact PBS can be easily isolated by sucrose density gradient ultracentrifugation6,8,15.
In this protocol, one model cyanobacterium and one non-model cyanobacterium were used as the materials for PBS isolation. They are model unicellular glucose-tolerant Synechocystis sp. PCC 6803 (hereafter Syn6803) and non-model filamentous Leptolyngbya sp. JSC-1 (hereafter JSC-1), respectively7,23,24. The protocol begins by disruption of the unicellular and filamentous cyanobacteria in a high-ionic-strength phosphate buffer. After lysis, the supernatants are collected by centrifugation and then treated with a nonionic detergent (Triton X-100) to solubilize the water-soluble proteins from the thylakoid membranes. The total water-soluble proteins are applied to a discontinuous sucrose density gradient to fractionate the PBS. The discontinuous sucrose gradient in this protocol consists of four sucrose solutions and partitions the intact PBS in the lowest fractions of sucrose layer25. The integrity of PBS can be analyzed by SDS-PAGE, zinc-staining, and 77K fluorescence spectroscopy6,7,8,26. This method is suitable for scientists who aim to isolate intact PBS from cyanobacteria and study its spectral, structural, and compositional properties.
There are several advantages of this protocol. (1) This method is standardized and can be used for isolating intact PBS from both unicellular and filamentous cyanobacteria. Most of the articles describe the method that applied in either one type of cyanobacteria4,7,8,12,13,14,16,18. (2) This method is performed at room temperature, as PBS dissociates at low temperature19,27. (3) This method describes using a bead-beater to disrupt the cells; therefore, it is cheaper and safer than high-pressured French press and possible hearing damage from sonicator in other methods8,13,14,20. (4) This method isolates intact PBS by sucrose gradient ultra-centrifugation. In this way, intact PBS with different sizes and partially dissociated PBS can be separated based on sucrose concentration.
Protokół
The Synechocystis sp. PCC 6803, the model glucose-tolerant strain, was obtained from Dr. Chu, Hsiu-An at Academia Sinica, Taiwan. Leptolyngbya sp. JSC-1, the non-model filamentous,was obtained from Dr. Donald A. Bryant at Pennsylvania State University, USA.
1. Cell culture and harvesting
- Inoculate Syn6803 or JSC-1 cells using a metallic inoculation loop into a 100 mL conical flask containing 50 mL of B-HEPES medium28. Culture the cells at 30 °C under 50 µmol photons m-2 s-1 (white-light LED) in 1% (v/v) CO2 with constant stirring by a magnetic stirrer (120 rpm) until the culture reaches mid-exponential growth phase (OD750 = 0.6-0.8).
NOTE: Harvest the cell culture when the optical density of the culture at 750 nm (OD750) reaches 0.6-0.8 by transferring to a new flask. Optical density was routinely used to estimate microbial cell density in liquid cultures29. The wavelengths from 720-750 nm were used in various studies for measuring cyanobacterial growth30,31,32. In this protocol, OD750 is used because JSC-1 can produce pigments that absorb far-red light7. Alternatively, chlorophyll concentration was also used to measure the growth of cyanobacteria33. - Transfer the cell culture (OD750 ~0.8) into a 1L conical flask and dilute it to OD750 = 0.2 in 500 mL of B-HEPES medium. Grow the culture until the late exponential growth phase (OD750 = 0.8-1.0) in the same condition mentioned above (step 1.1) and then harvest the culture by centrifugation.
- Centrifuge the culture at 10,000 x g for 20 min at room temperature and completely discard the supernatant.
NOTE: The cells pellets can be stored at -80 °C for up to 6 months.
2. Cells lysis
- Suspend the cell pellets and wash twice with 0.75 M K-phosphate buffer, pH 7.
NOTE: To make 0.75 M K-phosphate buffer at pH 7, prepare 1.5 M of K2HPO4 and 1.5 M of KH2PO4 separately. Mix 615 mL of 1.5 M K2HPO4 and 385 ml of 1.5M KH2PO4 to get 1.5 M K-phosphate buffer at pH 7. Simply dilute it with the same amount of ddH2O to make 0.75 M K-phosphate buffer at pH 7. - Pellet the cells in 50 mL centrifuge tubes by centrifugation at 10,000 x g for 20 min at room temperature. Discard the supernatant and resuspend the cells with 0.75 M K-phosphate buffer. Measure the wet weight of the pellet by an electronic balance and resuspend 1 g wet weight of the pellet in 5 mL of the buffer.
NOTE: Wet weight of a pellet was measured by subtracting the weight of an empty centrifuge tube from the weight of the centrifuge tube containing the cell pellet. - Add 1 mL of cell suspension and 0.4-0.6 g of 0.1 mm glass beads (see Table of Materials) into a 2 mL screw cap vial.
- Break the cells for 30 s by a bead-beater (see Table of Materials). Allow the tubes to cool in a room temperature water bath for 2 min and repeat the breaking cycle 5 times.
- After lysis, centrifuge the vials at 500 x g for 10 s at room temperature. Collect the supernatant with a pipette into a 15 mL centrifuge tube. Wash the beads with 0.5 mL of 0.75 M K-phosphate buffer one time, then transfer to the same centrifuge tube.
- Add Triton X-100 (2% w/v, final concentration) to the lysed cell suspension and incubate on a rocking shaker (40 rpm) at room temperature until the solution becomes homogenous (~20 min).
- Centrifuge the tubes at 17,210 g for 20 min to remove the intact cells and large cell debris. Store the supernatant without the upper Triton micelle layer at room temperature for up to 1 h.
NOTE: The supernatant contains two separated layers. The upper hydrophobic Triton X layer contains chlorophyll-binding proteins and hydrophobic rod-shaped phycobilisomes6. The lower aqueous layer contains water-soluble PBS.
3. Preparation of the sucrose gradient buffers and PBS isolation
- Make four concentrations of sucrose (2.0 M, 1.0 M, 0.75 M and 0.5 M) in 0.75 M K-phosphate buffer at pH 7.
NOTE: It is suggested to use 1.5 M K-phosphate buffer at pH 7 to prepare the sucrose gradient because adding sucrose dilutes the concentration of K-phosphate buffer. After sucrose is fully dissolved, add ddH2O to adjust the concentration to 0.75 M, pH 7. - Place 2.8 mL of 2.0 M sucrose buffer at the bottom of the 40 mL centrifuge tube and overlay with three layers of sucrose solutions (8 mL of 1.0 M; 12 mL of 0.75 M and 11 mL of 0.5 M for 40 mL centrifuge tube), and finally PBS-containing the supernatant fraction (3.0 mL) (Figure 1A).
NOTE: Carefully add the sucrose solution and allow sucrose to drop very slowly inside of the tube. Hold the pipette tip just above the surface of the solution in the tube while loading the solution. Sucrose layers can be observed when they are layered slowly. - Centrifuge the resulting gradients at 125,800 x g for ~16h-20 h at 25 °C.NOTE: An ultracentrifuge (see Table of Materials) is required for this step.
- Upon ultra-centrifugation, condense the fractionated PBS and phycobiliproteins between the layers. Blue bands in Syn6803 (purple bands showed in JSC-1) were observed in the interfaces (Figure 1D).
- Slowly collect the fractions with a pipette from the top of the sucrose gradients. Remove the sucrose by repeatedly concentrating (3,500 x g for 20 min) and diluting the fractions with 0.75 M K-phosphate buffer in membrane centrifugal filter units (10 K molecular weight cut-off, see Table of Materials).
4. Measurement of PBS fluorescence at 77K
NOTE: A fluorimeter equipped with a liquid nitrogen container (see Table of Materials) is used to measure fluorescence spectra at 77K.
- Dilute the concentrated PBS sample with 0.75 M K-phosphate buffer to gain at least 500 µL.
NOTE: The concentrations of phycocyanin of samples were ~4.2 µg mL-1 based on the formula: (OD615 0.474 x OD652)/5.34 [mg/mL]34. - Add 500 µL of the PBS sample to a transparent glass tube and freeze the tube in liquid nitrogen until it is completely frozen. Move the frozen tube to a transparent Dewar container (see Table of Materials) pre-filled with liquid nitrogen.
NOTE: The inner diameter of the glass tube is 3 mm in this protocol. Thin glass tubes were used to minimize re-absorption of short-wavelength emission in the sample. - Choose the excitation wavelengths for phycoerythrin and phycocyanin to be 550 nm and 580 nm, respectively.
NOTE: Fluorescence emissions were recorded from 560-800 nm (for 550 nm excitation) or 600-800 nm (for 580 nm excitation).
5. SDS-PAGE analysis of PBS
- Buffer exchange the concentrated PBS samples in 0.75 M of K-phosphate with 50 mM of Tris buffer (pH 8.0) in membrane centrifugal filter units (3K molecular weight cut-off, see Table of Materials).
- Mix 50 µL of PBS solution with 10 µL of 6x SDS loading buffer [300 mM Tris pH 6.8, 12% (w/v) Sodium dodecyl sulfate, 0.06% (w/v) Bromphenol blue, 50% (v/v) Glycerol, 6% (v/v) β-Mercaptoethanol] (see Table of Materials) in a microcentrifuge tube and incubate at 95 °C for 10 min in a heat block.
- Separate the protein samples by SDS-PAGE (8%-20% (w/v) polyacrylamide gel) and continue with zinc and Coomassie staining. The detailed procedure has been described in Reference8.
- For zinc staining, incubate the gel in 50 mM ZnSO4 solution for 10 min at room temperature, wash the gel with distilled water, and visualize zinc-induced fluorescence under UV irradiation (312 nm).
- For Coomassie blue staining, incubate the gel in Coomassie Blue staining buffer [0.25% (w/v) of Coomassie R-250, 10% (v/v) of Acetic acid, 50% (v/v) of Methanol, see Table of Materials] for 1 h at room temperature. Wash the gel with distilled water twice to remove the residual staining buffer and incubate the gel with destaining buffer [10% (v/v) Acetic acid, 30% (v/v) Methanol] on a rocking shaker (40 rpm) at room temperature overnight. Visualize the fully-destained gel with a digital camera or a scanner.
Wyniki
The Syn6803 and JSC-1 cells were cultivated in conical flasks with constant stirring in B-HEPES medium at 30 °C, under a LED white light (50 µmol photons m-2s-1) in a growth chamber filled with 1% (v/v) CO2. At the exponential growth phase (OD750 = ~0.5), the cells were subcultured into fresh medium with a final optical density OD750 = ~0.2. After reaching the late exponential growth phase (OD750 = 0.6-0.8), the cultures were collected and centrifuged (10,000 x g at room temperature for 20 min). The supernatant was discarded, and the cell pellets were stored at -80 °C until the next step was available.
To break the cells, the cells were first thawed at room temperature. The cells were disrupted by bead-beating with 0.1 mm glass beads within the 0.75 M K-phosphate buffer. Complete cell lysis shows the supernatant in dark blue-green color before centrifugation (Figure 1B). After solubilization by Triton X-100 and centrifugation to remove the insoluble membranes and cell debris, the supernatant was loaded on the top of sucrose gradient solution in a 40 mL centrifuge tube (Figure 1C). After 18 h of centrifugation, the intact PBS moved to the gradient's center as a clear blue band for Syn6803 and a purple band for JSC-1. The difference in their color results from the different compositions of PBS in Syn6803 and JSC-1. The PBS from Syn6803 carries phycocyanin35, whereas the PBS of JSC-1 carries phycocyanin and phycoerythrin7. The result of sucrose density-gradient separation of isolated PBS shows several fractions of dissociated phycobiliproteins, and the lowest fraction represents the intact PBS (Figure 1D).
The absorption spectra of the different fractions of PBS isolated from JSC-1 and Syn6803 are compared in Figure 2A. The detailed procedure of absorption spectra has been described in the articles7,8. The absorption spectra from the dissociated PBS and intact PBS of JSC-1 and Syn6803 have the same peak at 622 nm, corresponding to the phycocyanin. And the phycoerythrin absorption peak at 571 nm is found in both dissociated PBS and intact PBS of JSC-1. Allophycocyanin shows a minor peak with a shoulder at 670 nm in the intact PBS fractions of JSC-1 and Syn6803. The spectral differences indicate that the rod (phycocyanin) to core (allophycocyanin) ratio is higher in the dissociated PBS than in the intact PBS fraction, as the spectral properties of phycobiliproteins have been determined previously5. Since the dissociated PBS and the intact PBS fractions show similar absorption spectra and fluorescent emission features at room temperature, 77K fluorescence spectroscopy was then used to analyze the different fractions isolated from the sucrose gradient. 77K fluorescent emission spectra of the dissociated PBS and the intact PBS fractions were excited by 580 nm. The emission spectra of the dissociated PBS have one strong peak at ~650 nm in Syn6803 and JSC-1, representing the emission from phycocyanin (Figure 2B). The fluorescent emission spectra of the intact PBS show two fluorescent emission maximal peaks: the weak one at 651 nm and the strong one at 684 nm, revealing the energy harvested by phycocyanin was transferred efficiently to allophycocyanin and terminal emitters (ApcD and ApcE) in the core9 (Figure 2C). These features demonstrate that the lowest fractions in the sucrose gradient contain intact PBS.
The dissociated PBS and the intact PBS were separated by SDS-PAGE on an 8%-20% (w/v) SDS polyacrylamide linear gradient gel. The α and β subunits of phycocyanin and allophycocyanin were visible on the gel without staining (Figure 3A). The gel was subsequently stained with 10 mM ZnSO4 for 10 min. The chromophore-containing phycobiliproteins were observed by the zinc staining during UV irradiation8. Phycobiliprotein subunits (14-21 kDa) are strongly fluorescent, and the ApcE (arrow) showed weak fluorescence (Figure 3B). Further, Coomassie Blue staining shows the different protein composition between the dissociated PBS fractions and the intact PBS fractions (Figure 3C). The upper fractions contain other non-PBS water-soluble proteins as more proteins are stained than in intact PBS fractions. The intact PBS in Syn6803 shows a prominent ApcE band (arrow) lacking in the dissociated PBS fractions (Figure 3C).
The volume ratio between the PBS extract and the sucrose gradient is critical for obtaining sharp bands in the sucrose gradient after ultracentrifugation. The suggested ratio of solubilized PBS extracts to sucrose gradient solution is 1:10. Based on previous experience, making the sucrose gradient in 40 mL centrifuge tubes shows a higher resolution than the sucrose gradient made in the 13 mL tubes. The discontinuous sucrose gradient diffuses over time because of vibration on the bench. Therefore, the 13 mL centrifuge tube can be a good choice for handling lots of samples simultaneously. To demonstrate that, the sucrose gradients in 13 mL centrifuge tubes at the ratios as 1:3 and 1:10 have been prepared between the crude extracts of Syn6803 or JSC-1 to the sucrose gradient solution (Figure 4A). After ultracentrifugation, the sucrose gradient prepared at ratio 1:3 displays wider bands than the gradient made at ratio 1:10 (Figure 4B). The lowest fractions of each gradient contain intact PBS, confirmed by 77K fluorescent emission spectroscopy.
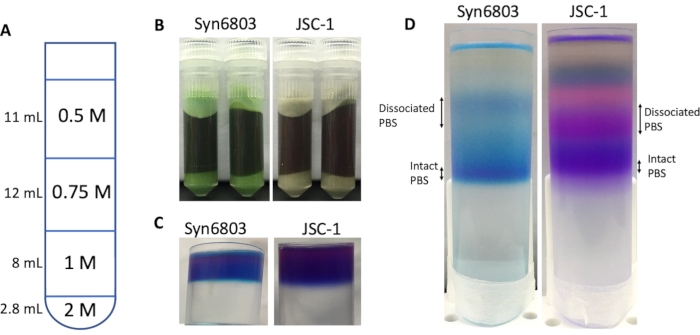
Figure 1: Disruption of Syn6803 and JSC-1 cells and sucrose gradient ultracentrifugation. (A) Schematic illustration of preparing the discontinued sucrose gradient in a 40 mL centrifuge tube. (B) The screw-cap vials were filled with 0.4 g of glass beads and 1 mL of cell suspension of Syn6803 or JSC-1, and then the cells were lysed by a bead-beater. (C) The supernatant of Triton X-100 solubilized extract was layered on the top of a sucrose density gradient. (D) After centrifugation for 18 h, the PBS fractions were visualized as clear blue bands in the gradient in Syn6803 and as purple bands in JSC-1. Please click here to view a larger version of this figure.
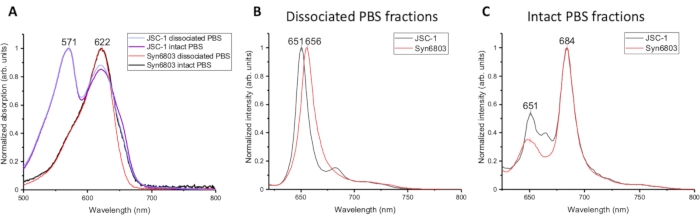
Figure 2: Spectroscopic characterization of different fractions of sucrose gradients. (A) Absorption spectra of dissociated PBS and intact PBS fractions from JSC-1 and Syn6803. Fluorescence emission spectra were measured at 77 K using the excitation wavelength at 580 nm for (B) the dissociated PBS fractions and (C) the intact PBS fractions of Syn6803 and JSC-1. Please click here to view a larger version of this figure.
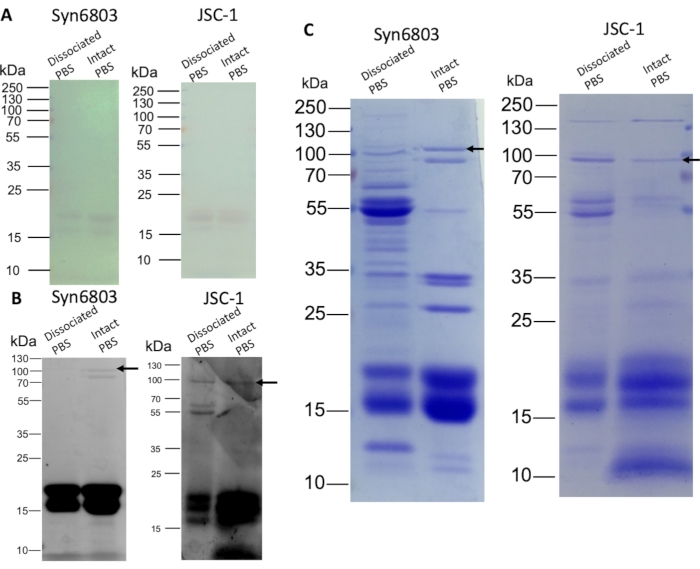
Figure 3: SDS-PAGE analysis of PBS isolated from sucrose gradients. The dissociated and the intact PBS fractions were quantified (20 µg protein per lane) and loaded onto an 8%-20% (w/v) gradient polyacrylamide gel. After gel electrophoresis, the gel was visualized by zinc staining and Coomassie Blue staining, subsequently. (A) The color of phycobiliproteins is visible on the gel without staining. (B) Zinc-stained gel shows the fluorescence of phycobiliproteins. (C) The gel stained with Coomassie Blue show phycobiliproteins and other proteins. The arrows indicate the positions of the ApcE. Please click here to view a larger version of this figure.
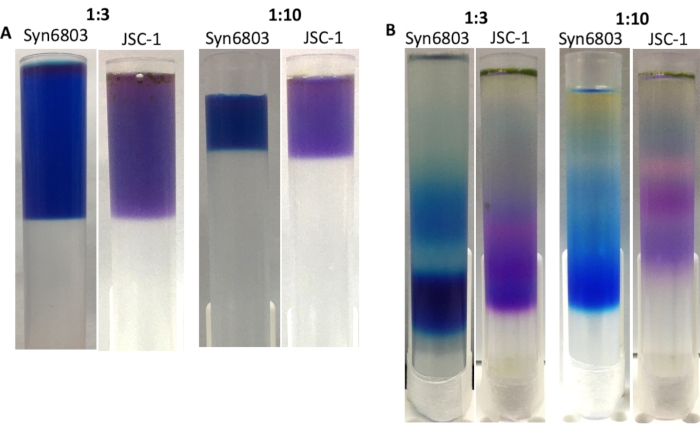
Figure 4: The sucrose gradient made in 13 mL centrifuge tubes. The ratios between the crude extract and the sucrose gradient solution are marked on the top of the images. (A) The supernatant of Triton X-100 solubilized extracts of Syn6803 and JSC-1 was layered over sucrose density gradients with different ratios of the solubilized extracts to sucrose gradient solution. (B) After centrifugation for 18 h, the PBS fractions in Syn6803 and JSC-1 were observed in the sucrose gradients. Please click here to view a larger version of this figure.
Dyskusje
This protocol describes a simple and standard method for isolating intact PBS in two types of cyanobacteria, unicellular model Syn6803, and filamentous non-model JSC-1. The critical steps of the protocol are cell homogenization and ultracentrifugation on a discontinuous density gradient of sucrose. Generally, the disruption of filamentous cells is more complicated than unicellular ones. Increasing the amount of the starting material (the wet weight of the cell pellet) and the repetition of bead-beating were helpful to increase the yield of PBS from filamentous cyanobacterial cells. For the filamentous cyanobacterium, JSC-1, three times more cells were used than unicellular one, Syn6803. In addition, to completely break the filamentous cyanobacterium, it requires bead-beating more times than the unicellular cyanobacteria to observe the dark bluish-purple color in the extraction buffer.
The duration of bead-beating is not suggested to exceed 30 s since the samples are heated up during each bead-beating cycle. The tubes are recommended to cool at room temperature on a bench (or in a water bath) between each cycle, as the intact PBS is readily dissociated at low temperature25. Therefore, every procedure in this protocol is suggested to be performed at room temperature. Many research articles have described different cell disruption methods, including a high-pressure homogenizer (French-Press) or a sonicator6,8,20. A bead mill homogenizer is suggested because it is cheaper, safer, easier to handle, faster, more accessible, and more efficient in processing multiple samples simultaneously.
Phycobilisomes show three different shapes, hemidiscoidal, bundle-shaped, and rod-shaped5. The intact phycobilisomes isolated in Figure 2 belong to the hemidiscoidal shape as discussed in earlier publications7,13,36. The upper fraction that represents dissociated PBS, however, can also contain rod-shaped PBS. Syn6803 and JSC-1 encode a specific rod-core linker CpcL, which suggests the presence of rod-shaped PBS in addition to the hemidiscoidal PBS6,37. Rod-shaped PBS has been directly identified in Syn6803 but not in JSC-17,13,37. On the other hand, JSC-1 remodels its PBS by performing type III chromatic acclimation in green/red light and far-red light photoacclimation in red/far-red light6,7. All these diversities indicate that there may be other types of PBS in the sucrose gradients, in addition to the lowest intact hemidiscoidal PBS fraction. For example, two types of PBS are present at the same time when Synechococcus sp. PCC 7335 and JSC-1 acclimates to far-red light8,13. It is suggested that the person isolating PBS from an uncharacterized cyanobacterial strain should be more cautious in analyzing each fraction in the sucrose gradient to identify any possible types of PBS.
Overall, this protocol provides a cheap, simple, and fast method for cell disruption. It has also been demonstrated that this method is reliable to isolate PBS from different cyanobacteria with different cell types and PBS compositions.
Ujawnienia
The authors declare no competing interest.
Podziękowania
The authors thank Technology Commons, College of Life Science, National Taiwan University for the convenient use of the ultracentrifuge. The cyanobacterial strains Synechocystis sp. PCC 6803 and Leptolyngbya sp. JSC-1 was gifted from Dr. Chu, Hsiu-An at Academia Sinica, Taiwan, and Dr. Donald A. Bryant at Pennsylvania State University, USA, respectively. This work was funded by the Ministry of Science and Technology (Taiwan) (109-2636-B-002-013- and 110-2628-B-002-065-) and the Ministry of Education (Taiwan) Yushan Young Scholar Program (109V1102 and 110V1102).
Materiały
| Name | Company | Catalog Number | Comments |
| 0.1 mm glass beads | BioSpec | 11079101 | for PBS extraction |
| 13 mL centrifugation tube | Hitachi | 13PA | ultracentrifugation |
| 40 mL centrifugation tube | Hitachi | 40PA | ultracentrifugation |
| Acetic acid | Merck | 8.1875.2500 | for Coomassie Blue staining |
| B-HEPES medium | A modified cyanobacterial medium from BG-11 medium | ||
| Brilliant Blue R-250 | Sigma | B-0149 | for Coomassie Blue staining |
| Bromophenol blue | Wako pure chemical industries | 2-291 | protein loading buffer |
| Electronic balance | Radwag | WLC 2/A2/C/2 | for the wet weight measurement of cell pellets |
| Fluorescence spectrophotometer | Hitachi | F-7000 | Spectrophotometer |
| Glycerol | BioShop | Gly001.500 | protein loading buffer |
| High-Speed refrigerated centrifuge | Hitachi | CR22N | for buffer exchange |
| Leptolyngbya sp. JSC-1 | from Dr. Donald A. Bryant at Pennsylvania State University, USA. | ||
| Low temperature measurement accessory | Hitachi | 5J0-0112 | The accessory includes a transparent Dewar container for 77K fluorescence spectra |
| Methanol | Merck | 1.07018,2511 | for Coomassie Blue staining |
| Microcentrifuge | Thermo Fisher | Pico 21 | for PBS extraction |
| Mini-Beadbeater-16 | BioSpec | Model 607 | for PBS extraction |
| Potassium phosphate dibasic | PanReac AppliChem | 121512.121 | for PBS extraction |
| Potassium phosphate monobasic | PanReac AppliChem | 141509.121 | for PBS extraction |
| Screw cap vial | BioSpec | 10832 | for PBS extraction |
| SmartView Pro Imager | Major Science | UVCI-2300 | for Znic staining signal detection |
| Sodium dodecyl sulfate | Zymeset | BSD101 | protein loading buffer |
| Sucrose | Zymeset | BSU101 | for PBS isolation |
| Synechocystis sp. PCC 6803 | glucose-tolerant strain from Dr. Chu, Hsiu-An at Academia Sinica, Taiwan | ||
| Tris | BioShop | TRS 011.1 | protein loading buffer |
| Triton X-100 | BioShop | TRX 506.500 | for PBS extraction |
| Ultra 10 K membrane centrifugal filter | Millipore | UFC901024 | for buffer exchange |
| Ultra 3 K membrane centrifugal filter | Millipore | UFC500324 | for buffer exchange |
| Ultracentrifuge | Hitachi | CP80WX | ultracentrifugation |
| UV/Vis spectrophotometer | Agilent | Cary 60 | Spectrophotometer |
| Zinc sulfate | PanReac AppliChem | 131787.121 | for Znic staining |
| β-Mercaptoethanol | BioBasic | MB0338 | protein loading buffer |
Odniesienia
- Bryant, D. A., Guglielmi, G., de Marsac, N. T., Castets, A. M., Cohen-Bazire, G. The structure of cyanobacterial phycobilisomes: A model. Archives of Microbiology. 123 (2), 113-127 (1979).
- Glazer, A. N. Phycobilisomes: Structure and dynamics. Annual Review of Microbiology. 36, 173-198 (1982).
- Glazer, A. N. Light harvesting by phycobilisomes. Annual Review of Biophysics and Biophysical Chemistry. 14 (1), 47-77 (1985).
- Liu, H., et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science. 342 (6162), 1104 (2013).
- Bryant, D. A., Canniffe, D. P. How nature designs light-harvesting antenna systems: Design principles and functional realization in chlorophototrophic prokaryotes. Journal of Physics B: Atomic, Molecular and Optical Physics. 51 (3), 033001 (2018).
- Hirose, Y., et al. Diverse chromatic acclimation processes regulating phycoerythrocyanin and rod-shaped phycobilisome in cyanobacteria. Molecular Plant. 12 (5), 715-725 (2019).
- Gan, F., et al. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science. 345 (6202), 1312-1317 (2014).
- Ho, M. Y., Gan, F., Shen, G., Bryant, D. A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynthesis Research. 131 (2), 187-202 (2017).
- Ho, M. Y., et al. Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1861 (4), 148064 (2020).
- Sanfilippo, J. E., Garczarek, L., Partensky, F., Kehoe, D. M. Chromatic Acclimation in Cyanobacteria: A diverse and widespread process for optimizing photosynthesis. Annual Review of Microbiology. 73, 407-433 (2019).
- Grossman, A. R., Schaefer, M. R., Chiang, G. G., Collier, J. L. The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiological Reviews. 57 (3), 725-749 (1993).
- Bryant, D. A., Glazer, A. N., Eiserling, F. A. Characterization and structural properties of the major biliproteins of Anabaena sp. Archives of Microbiology. 110 (1), 61-75 (1976).
- Soulier, N., Laremore, T. N., Bryant, D. A. Characterization of cyanobacterial allophycocyanins absorbing far-red light. Photosynthesis Research. 145 (3), 189-207 (2020).
- Guglielmi, G., Cohen-Bazire, G., Bryant, D. A. The structure of Gloeobacter violaceus and its phycobilisomes. Archives of Microbiology. 129 (3), 181-189 (1981).
- Zhang, J., et al. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature. 551 (7678), 57-63 (2017).
- Li, Y., et al. Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochimica et Biophysica Acta. 1857 (1), 107-114 (2016).
- Herrera-Salgado, P., Leyva-Castillo, L. E., Rios-Castro, E., Gomez-Lojero, C. Complementary chromatic and far-red photoacclimations in Synechococcus ATCC 29403 (PCC 7335). I: The phycobilisomes, a proteomic approach. Photosynthesis Research. 138 (1), 39-56 (2018).
- Yamanaka, G., Glazer, A. N., Williams, R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. Journal of Biological Chemistry. 253 (22), 8303-8310 (1978).
- Gantt, E., Lipschultz, C. A., Grabowski, J., Zimmerman, B. K. Phycobilisomes from blue-green and red algae: Isolation criteria and dissociation characteristics. Plant Physiology. 63 (4), 615-620 (1979).
- Patel, A., Mishra, S., Pawar, R., Ghosh, P. K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expression and Purification. 40 (2), 248-255 (2005).
- Zolla, L., Bianchetti, M. High-performance liquid chromatography coupled on-line with electrospray ionization mass spectrometry for the simultaneous separation and identification of the Synechocystis PCC 6803 phycobilisome proteins. Journal of Chromatography A. 912 (2), 269-279 (2001).
- Soni, B., Kalavadia, B., Trivedi, U., Madamwar, D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata-Isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochemistry. 41 (9), 2017-2023 (2006).
- Williams, J. G. K. . Methods in Enzymology. , 766-778 (1988).
- Brown Igor, I., et al. Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Applied and Environmental Microbiology. 76 (19), 6664-6672 (2010).
- Wang, L., et al. Isolation, purification and properties of an R-phycocyanin from the phycobilisomes of a marine red macroalga Polysiphonia urceolata. PLoS One. 9 (2), 87833 (2014).
- Berkelman, T. R., Lagarias, J. C. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Analytical Biochemistry. 156 (1), 194-201 (1986).
- Rigbi, M., Rosinski, J., Siegelman, H. W., Sutherland, J. C. Cyanobacterial phycobilisomes: Selective dissociation monitored by fluorescence and circular dichroism. Proceedings of the National Academy of Sciences. 77 (4), 1961-1965 (1980).
- Dubbs, J. M., Bryant, D. A. Molecular cloning and transcriptional analysis of the cpeBA operon of the cyanobacterium Pseudanabaena species PCC 7409. Molecular Microbiology. 5 (12), 3073-3085 (1991).
- Stevenson, K., McVey, A. F., Clark, I. B. N., Swain, P. S., Pilizota, T. General calibration of microbial growth in microplate readers. Scientific Reports. 6 (1), 38828 (2016).
- Zhang, S., Shen, G., Li, Z., Golbeck, J. H., Bryant, D. A. Vipp1 is essential for the biogenesis of Photosystem I but not thylakoid membranes in Synechococcus sp. PCC 7002. Journal Biological Chemistry. 289 (23), 15904-15914 (2014).
- Zhang, S., Bryant, D. A. Biochemical validation of the glyoxylate cycle in the cyanobacterium Chlorogloeopsis fritschii Strain PCC 9212. Journal Biological Chemistry. 290 (22), 14019-14030 (2015).
- Huang, J. Y., et al. Mutations of cytochrome b559 and Psbj on and near the QC site in photosystem II influence the regulation of short-term light response and photosynthetic growth of the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry. 55 (15), 2214-2226 (2016).
- Li, Y., Lin, Y., Loughlin, P., Chen, M. Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris - A filamentous cyanobacterium containing chlorophyll f. Frontiers in Plant Science. 5, 67 (2014).
- Bennett, A., Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. Journal of Cell Biology. 58 (2), 419-435 (1973).
- Su, X., Fraenkel, P. G., Bogorad, L. Excitation energy transfer from phycocyanin to chlorophyll in an apcA-defective mutant of Synechocystis sp. PCC 6803. Journal of Biological Chemistry. 267 (32), 22944-22950 (1992).
- Arteni, A. A., Ajlani, G., Boekema, E. J. Structural organisation of phycobilisomes from Synechocystis sp. strain PCC6803 and their interaction with the membrane. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1787 (4), 272-279 (2009).
- Kondo, K., Ochiai, Y., Katayama, M., Ikeuchi, M. The Membrane-associated CpcG2-phycobilisome in Synechocystis: A new photosystem I antenna. Plant Physiology. 144 (2), 1200-1210 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone