Method Article
Design and Development of a Model to Study the Effect of Supplemental Oxygen on the Cystic Fibrosis Airway Microbiome
W tym Artykule
Podsumowanie
The goal of this protocol is to develop a model system for the effect of hyperoxia on cystic fibrosis airway microbial communities. Artificial sputum medium emulates the composition of sputum, and hyperoxic culture conditions model the effects of supplemental oxygen on lung microbial communities.
Streszczenie
Airway microbial communities are thought to play an important role in the progression of cystic fibrosis (CF) and other chronic pulmonary diseases. Microbes have traditionally been classified based on their ability to use or tolerate oxygen. Supplemental oxygen is a common medical therapy administered to people with cystic fibrosis (pwCF); however, existing studies on oxygen and the airway microbiome have focused on how hypoxia (low oxygen) rather than hyperoxia (high oxygen) affects the predominantly aerobic and facultative anaerobic lung microbial communities. To address this critical knowledge gap, this protocol was developed using an artificial sputum medium that mimics the composition of sputum from pwCF. The use of filter sterilization, which yields a transparent medium, allows optical methods to follow the growth of single-celled microbes in suspension cultures. To create hyperoxic conditions, this model system takes advantage of established anaerobic culturing techniques to study hyperoxic conditions; instead of removing oxygen, oxygen is added to cultures by daily sparging of serum bottles with a mixture of compressed oxygen and air. Sputum from 50 pwCF underwent daily sparging for a 72-h period to verify the ability of this model to maintain differential oxygen conditions. Shotgun metagenomic sequencing was performed on cultured and uncultured sputum samples from 11 pwCF to verify the ability of this medium to support the growth of commensal and pathogenic microbes commonly found in cystic fibrosis sputum. Growth curves were obtained from 112 isolates obtained from pwCF to verify the ability of this artificial sputum medium to support the growth of common cystic fibrosis pathogens. We find that this model can culture a wide variety of pathogens and commensals in CF sputum, recovers a community highly similar to uncultured sputum under normoxic conditions, and creates different culture phenotypes under varying oxygen conditions. This new approach might lead to a better understanding of unanticipated effects induced by the use of oxygen in pwCF on airway microbial communities and common respiratory pathogens.
Wprowadzenie
Cystic fibrosis (CF) is a genetic disease characterized by an inability to clear thick mucus from the lungs, leading to repeated infections and progressive lung function decline that often results in the need for lung transplantation or death. The airway microbiome of people with cystic fibrosis (pwCF) appears to track disease activity1, with a reduction in microbial diversity associated with adverse long-term outcomes2,3. In clinical studies of pwCF, supplemental oxygen therapy has been associated with more advanced disease4,5, though traditionally, the use of oxygen therapy has been viewed as simply a marker for disease severity6. Recent studies from a clinical trial of patients with respiratory failure have shown that higher patient oxygen levels are paradoxically associated with an increase in serious bacterial infections and higher mortality7, suggesting that supplemental oxygen may contribute to disease pathogenesis. The effect of supplemental oxygen on the cystic fibrosis lung microbiome and associated lung and airway microbial communities has not been well studied.
Mechanistic studies often cannot be performed directly on human subjects due to logistical difficulties and potential ethical issues associated with interventions of unknown medical benefit or harm. Translational approaches that integrate human biospecimens into model systems can offer important biological insights in these cases. While the ability to use or tolerate oxygen has traditionally been an important component of microbial classification, little is known about how the therapeutic introduction of supplemental oxygen to the environment might perturb airway microbial communities. To shed light on the unknown effects of supplemental oxygen on the airway microbiomes of pwCF, we needed to address two major challenges; first, the creation of a culture medium that physiologically approximates the composition of CF sputum; second, the creation of a model system that allows the maintenance of elevated oxygen concentrations in culture over extended periods of time.
Artificial sputum media (ASM) are widely used to emulate lung sputum ex vivo8,9,10, but there is no clear consensus on a specific recipe. This protocol describes an artificial sputum medium recipe and preparation strategy carefully designed to physiologically approximate sputum from pwCF. Table 1 outlines the chosen recipe values based on published literature. Basic chemical components and pH were matched to values identified by studies of human CF sputum11,12,13. Low concentration physiological nutrients were added using egg yolk, which was included as 0.25% of the final volume10, as well as vitamin and trace metal mixes14,15. Mucin, the key component of sputum16, was included at 1% w/v14. Although more labor-intensive, filter sterilization was chosen over the more conventional practice of heat sterilization to reduce potential problems from heat-induced denaturation of essential media components10. An additional benefit of filter sterilization is that it generates media that are transparent (heat-sterilization can create turbid media due to precipitation and coagulation of salts and proteins), allowing this artificial sputum media to be used to follow microbial growth based on increases in turbidity.
This model system for the hyperoxic culture is based on anaerobic culturing techniques where oxygen is added rather than removed, creating a model for the effect of supplemental oxygen use for pwCF. Figure 1 and the associated oxygen sparging protocol outlines the components of an oxygen sparging system, which can be obtained at low cost from general laboratory and hospital suppliers. This system enables the mixing of compressed oxygen and air to fixed concentrations ranging from 21%-100% oxygen. The integration of an oxygen sensor allows for the verification of the concentration of the output gas mixture, as well as checking the outflow gas composition of previously sparged serum bottles to verify that the oxygen conditions have been maintained within the desired range.
This protocol outlines procedures to create an artificial sputum medium, the construction and use of an oxygen sparging system, and the application of both to culture CF sputum under differential oxygen conditions.
Protokół
This study received approval from the Partners Institutional Review Board (Protocol # 2018P002934). Inclusion criterion included adult patients with cystic fibrosis who provided written informed consent for the study. There was no exclusion criterion. According to protocol guidelines, all sputum samples were collected from patients with cystic fibrosis during a scheduled outpatient visit with their clinical provider.
1. Artificial Sputum Medium Preparation
NOTE: Quantities listed here are for the production of 1 L of final artificial sputum medium, and assume the specific reagents listed in the Materials Table. Numbers must be adjusted for other volumes or for the use of different reagents to ensure the same final product. See Table 1 for target concentrations.
- Artificial sputum chemical mix (ASCM)
NOTE: ASCM makes up 25% of the final medium volume. It is shelf-stable and can be prepared in bulk or in advance. If being prepared for later use, autoclave the chemical mix and safely store it at room temperature.- Mix the constituent chemical stock solutions.
- Prepare 1 M NaCl stock: Add 58.44 g of NaCl per liter of sterile water.
- Prepare 1 M KCl stock: Add 74.55 g of KCl per liter of sterile water.
- Prepare 1 M MgSO4 stock: Add 246.47 g of MgSO4·7H2O per liter of sterile water, or 120.37 g of anhydrous MgSO4 per liter of sterile water.
- Prepare 1 M glucose stock: Add 180.16 g of glucose per liter of sterile water.
- Autoclave sterilize the chemical stock solutions, as well as an empty 250 mL bottle. Perform the autoclaving steps to at least standard values of 121 °C and 15 PSI for 30 min.
- Add 80.59 mL of sterile water to the empty 250 mL bottle.
- Add 152.30 mL of 1 M NaCl stock to the mix.
- Add 15.8 mL of 1 M KCl stock to the mix.
- Add 610 µL of 1 M MgSO4 stock to the mix.
- Add 700 µL of 1 M glucose stock to the mix.
- Mix the constituent chemical stock solutions.
- Artificial sputum mucin mix (ASMM)
NOTE: ASMM makes up 50% of the final medium volume. Ensure that it is prepared on the same day as the final medium batch.- Add 450 mL of sterile water to an empty 1 L bottle.
- Add 50 mL of 10x Phosphate-Buffered Saline (PBS) to the bottle.
- Add a disposable magnetic stir bar to the bottle.
- Autoclave the bottle containing PBS and the stir bar.
- Measure out 10 g of mucin powder and add it to the PBS.
- Shake the bottle vigorously for preliminary mixing.
- Place the bottle onto a hot plate with a magnetic stirrer. Set heat to medium-high targeting 50 °C and stirring speed to 1100 rpm. Ramp up the speed gradually so that the bar does not fly off the magnet.
- Allow to heat and stir for 15 min.
- Pick up the bottle with heat-resistant gloves. Observe to check if mucin powder settles out of the solution.
- If mucin powder is not fully dissolved, return the bottle to heat/stirrer for 5-min intervals until it is completely dissolved.
- Allow the mucin mix to cool to room temperature.
- Artificial sputum biological mix (ASBM)
NOTE: ASBM is 25% of the final medium volume. Prepare it on the same day as the final medium batch, and unlike the other mixes, do not expose its components to any heat.- Thaw the 100x vitamin stock in a 4 °C fridge or on ice.
NOTE: Pre-portion the vitamin stock into 10 mL aliquots to minimize the number of freeze/thaw cycles. - Add 124.24 mL of sterile water to the empty autoclaved 250 mL bottle.
- Add 25.76 mL of 50x essential amino acid stock to the mix.
- Add 80.14 mL of 100x non-essential amino acid stock to the mix.
- Add 10 mL of (thawed) 100x vitamin stock to the mix.
- Add 1 mL of 1000x trace metals stock to the mix.
- Add 8.33 mL of 30% egg yolk emulsion to the mix.
- Add 400 µL of 10 g/L ferritin stock to the mix.
- Mix the solution well via manual shaking.
- Thaw the 100x vitamin stock in a 4 °C fridge or on ice.
- Artificial sputum medium (ASM)
- Add 250 mL of ASCM to the 1 L bottle containing ASMM.
- Add 250 mL of ASBM to the medium bottle.
- Titrate the medium with basic MOPS buffer (1 M) to reach a pH of 6.3 on a narrow range pH paper. Prior to titration, the medium mix will be too acidic.
- Refrigerate the resulting artificial sputum medium at 4 °C until it is ready for filtration.
- To start the filtration process, transfer 200 mL of unfiltered artificial sputum medium to a vacuum filtration system with a 0.22 µm pore size filter.
- Connect the filtration system to the vacuum pump, turn on the vacuum pump, set it to 70 mbar, and then place the chamber on an orbital shaker shaking at 90 rpm in a cold room at 4 °C.
- Top off with an additional 150 mL of the medium as an appreciable amount is filtered. It takes 1-2 days to filter 1 L of medium completely.
- Repeat with additional chambers until all the media is filtered.
NOTE: Try not to filter more than 350 mL of the medium through the same 0.22 µm filter since mucin will plug the filter over time.
- Refrigerate filtered artificial sputum medium at 4 °C until ready for use. Use ASM within one month of preparation for best results.
2. Oxygen Sparging
- Sparging station setup
NOTE: This protocol should only need to be done in full once, after which point the setup can be maintained through simple maintenance as necessary. See Figure 1 for a visual schematic of the oxygen sparging system.- Obtain and properly secure the compressed air and oxygen tanks.
CAUTION: High pressure makes the tanks extremely dangerous when mishandled. Ensure that the tanks are completely sealed and secured, there are no leaks when the tank is closed, and that all handling personnel is fully trained in their use. - Attach an air regulator to the compressed air tank with a wrench. For optimal flow reading on the regulator, attach the regulator as close as possible to an upright position.
- Attach an oxygen regulator to the compressed oxygen tank, attaching as close as possible to an upright position. Depending on the oxygen tank, one may need to invert the direction to tighten.
- Connect the tubing from the regulators to a Y-connector to combine the gas flow from the two tanks.
- Connect the output of the Y-connector to the central T-junction valve.
- Connect one side of the central T-junction valve to a gas pressure gauge.
- Connect the other side of the gas pressure gauge to a 25 mm diameter sterile syringe filter with a 0.22 µm pore size.
- Attach a second 25 mm diameter syringe filter to a syringe without a plunger to be used as a gas release during sparging.
- Connect the final side of the central T-junction valve to a second T-junction valve for the oxygen monitor.
- Connect a 25 mm diameter syringe filter to one side of this second T-junction valve, along with tubing to attach 18 G needles.
- Connect the final side of the second T-junction valve to the oxygen monitoring apparatus.
- Connect a cut-off tube to the other side of the oxygen monitoring apparatus to be used as a gas release during monitoring.
CAUTION: When testing/using the oxygen sparging system, take careful note of the position of the T-junctions and ensure it matches the intended path through the system. Failure to do so will result in pressure buildup inside the system and cause components to fail and come apart . - For the maintenance of the system and to keep it working at optimal performance, the following practices are beneficial.
- Reinforce the connections with liberal amounts of Teflon tape to greatly improve their seal and reduce the chance of components coming apart from the internal pressure.
- Keep combined flow rate under 10 L/min to mitigate maximum pressure and prevent failures.
- If a leak is suspected, use a detergent solution such as commercially available liquid leak detectors to identify its location easily, as it will bubble above any gas leaks. Patch leaks using a Polytetrafluoroethylene tape (e.g., Teflon).
- Replace the 25 mm diameter syringe filters in the oxygen sparging system bi-weekly, but this varies with use frequency. Over time, particles caught in the filter reduce the gas flow rate and cause pressure buildups.
- Calibrate the oxygen monitor to 21% oxygen compressed air prior to carrying out measurements.
- Upon completion of the system use, turn off the tanks and bleed the excess gas from the regulators until the flow completely stops.
- Obtain and properly secure the compressed air and oxygen tanks.
- Serum bottle culture sparging
- Label 500 mL autoclaved serum bottles with sample identifiers, date/time of inoculation, and target oxygen percentage.
- In a biological hood, add 24 mL of the artificial sputum medium to each serum bottle being set up.
- Add 1 mL of the patient sputum homogenized with an 18-G needle (diluted with sterile saline if necessary to obtain sufficient volume of sample for each culture condition) to each serum bottle.
- Using sterile tweezers, place the autoclaved rubber stoppers onto the top of each serum bottle.
- Press down the rubber stoppers, take care not to touch the underside of the stopper with hands.
- Remove the bottles from the hood, apply and crimp the aluminum seals. Remove the center piece from the seals.
- Wipe down the top of bottles with an alcohol wipe and pass them through a Bunsen burner flame.
- Affix a sterile 18-G needle to a plunger-less syringe with a filter. Insert this gas release into the bottle first.
- Affix a sterile 18-G needle to the gas output from the system and insert the gas output needle into the bottle as well.
- Route the T-junctions from the tanks through the oxygen monitor. Verify that the target oxygen concentration is flowing through the system. Target approximately 5 L/min of gas flow.
- Reroute the T-junctions from the tanks through to the gas output. The gas starts to flow through the serum bottle.
CAUTION: Pay close attention to the pressure gauge during oxygen sparging. If pressure increases unexpectedly, shut the system off immediately. - Run oxygen sparge through the serum bottle for 1 min. At 5 L/min, this allows for 10 air exchanges and ensures the internal atmosphere reaches the desired partial pressure.
- Remove the gas release 18-G needle.
- Allow the pressure in the serum bottle to build to +1 atmosphere (2 atmospheres at sea level) and then immediately remove the gas output needle.
NOTE: Maintaining pressure aids retention of hyperoxic conditions over time. - Place the serum bottle into a 37 °C incubator shaker at 150 RPM. Incubate the samples for three 24-h intervals. At each 24-h interval, take an aliquot for downstream analysis, re-sparge the samples and return them to incubation for a total incubation time of 72 h.
- Outflow oxygen measurement
- Calibrate the oxygen meter to 21% compressed air, and then turn off the tank.
- Route the serum bottle intake through the oxygen monitor and affix a sterile needle to the end.
- Insert the needle through the rubber stopper into the serum bottle.
- Wait for the outflow reading to stabilize. A low flow rate out of the serum bottles means this may take up to 2 min. Report the peak difference from room air (number furthest from 21%).
- If performing multiple readings, flush the system with compressed air between readings.
Wyniki
These protocols were applied to 50 expectorated sputum samples from pwCF presenting for routine care to an outpatient cystic fibrosis clinic at Massachusetts General Hospital in Boston, Massachusetts. Each patient's sputum was cultured under 21%, 50%, and 100% oxygen conditions using the artificial sputum medium, with 0.5 mL aliquots taken from each culture at 24 h, 48 h, and 72 h of culture time for testing. Cultures were photographed when extractions were made to track visual changes. In addition, a 0.5 mL aliquot of each primary sputum sample was taken prior to culturing. This resulted in 10 discrete samples per patient and a final N of 500 samples. Of these, sputum from 11 patients (11 uncultured sputa, 11 cultured sputa from 21% oxygen at 48 h of incubation) underwent nucleic acid extraction17, sequencing libraries were generated using a commercial DNA library preparation kit, and metagenomic sequencing was performed on a whole genome sequencing platform targeting ~ 5 Gb of sequence per sample with 150 base pair, paired-end reads. Raw reads were processed using the bioBakery suite of tools18, which includes quality control and removal of human "contaminant" sequences and taxonomic profiling with the MetaPhlAn3 profiler19. At the time of nucleic acid extraction, 10 million cells of Imtechella halotolerans, a halotolerant species normally found in estuary ecosystems and not in human microbial communities, were spiked into each sample, allowing the quantification of absolute microbial load for each sample20.
Figure 2 shows individual and average outflow oxygen measurements and pH levels over the course of the culture process for 50 sputum samples cultured under each oxygen condition and an example of a visual differential culture phenotype. Cultures were maintained at 37 °C except during brief periods when sparging and removal of sample aliquots was performed. With both sparging intervals of 12 h and 24 h, elevated oxygen concentrations were maintained, although a drop over time was observed for all three oxygen conditions, with 100% oxygen falling to approximately 85%, 50% oxygen falling to 40%, and 21% oxygen falling to 18%. Oxygen conditions remained distinct, and importantly, elevated oxygen concentrations were maintained throughout the process for hyperoxic samples. pH measurements showed a greater degree of variability but stayed well within a physiologically normal range with no statistically significant changes over time. These measurements indicate that these methods maintain discrete differential oxygen conditions throughout the culture process. Lastly, an example of one of many visual culture phenotypes that differentiated across oxygen concentration is shown. This sample had marked turbidity differences after 72 h of culture, with higher oxygen associated with lower visual turbidity. Differential culture phenotypes support the presence of hyperoxia-induced effects on culture communities.
Figure 3 compares microbial load, microbial diversity, and microbial community composition between uncultured sputum and cultured sputum (21% oxygen condition for a period of 48 h). Measurements reveal the only major difference introduced by the act of culturing to be an approximately 20-fold increase in microbial load as compared to uncultured sputum. The immune system and the typical mechanical sputum clearance mechanisms such as coughing normally serve as a regulatory process limiting the microbial load in the lung, even in cases of dysfunction and infection like those seen in pwCF. Ex vivo culture has no such regulatory mechanisms, and microbial communities are instead free to proceed toward cellular saturation. Alpha and beta diversity metrics indicate that despite this difference in microbial load, the underlying community composition remains well-preserved, with minimal global differences introduced by the culture process.
Figure 4 expands on the comparison between uncultured and cultured sputum samples, looking at the binary presence/absence of the 120 microbial species conclusively identified by shotgun metagenomics sequencing from cultured and uncultured sputum obtained from 11 patients. Microbes are clustered based on phylogenetic similarities. 46 (38.3%) of these species were identified in both uncultured and cultured samples (cyan color), while 35 (29.2%) were exclusively identified in uncultured samples (yellow) and 39 (32.5%) were exclusively identified in cultured samples (blue). It is likely that there is greater parity than what we identified using sequencing in terms of what is present and what is absent, but some taxa fall below the sequencing detection threshold in some cases. The differences do indicate that the culture process introduces some bias in cultured compared to uncultured sputum. Most notably, culturing increases the presence of fungi such as Candida and Aspergillus, as well as Enterobacterales members including Escherichia, Serratia, and Streptococcus members. Conversely, Bacteroidetes members such as Prevotella and Clostridiales, which are anaerobes, were present in uncultured samples but not present in cultured samples. This may be attributed to the lack of an anaerobic condition in our experimental model.
Figure 5 shows absorbance-based growth curves of common CF lung pathogens isolated from sputum obtained from 50 different pwCF. These isolates represent phenotypically different clinical isolates obtained using enrichment culture procedures from the Massachusetts General Hospital Clinical Microbiology Laboratory, and include Pseudomonas aeruginosa (N = 53), Staphylococcus aureus (N = 37), Stenotrophomonas maltophilia (N = 12), Klebsiella pneumoniae (N = 3), and Achromobacter sp (N = 7). Growth curves were obtained by culturing each isolate in artificial sputum media at 37 °C in the dark, with ASM sans bacterial inoculation serving as a negative control. The transparent quality of ASM (which is due to filter rather than heat sterilization) allows for conducting optical measures to estimate growth curves. Optical readings at 600 nm (OD600) were taken every 10 min, and the first 24 h of each curve are shown. The absence of changes in optical readings in the ASM-only negative control indicates cultures free of contamination. The demonstrative curves shown here follow typical growth curve patterns indicating the viability of this ASM recipe as a medium for the absorbance-based generation of growth curves.
| Column 1 | Column 2 | Column 3 | Column 4 | Column 5 | Column 6 | Column 7 | Column 8 | Column 9 | |
| Value | Comstock | Kirchner | Sriramulu | Palmer | Flynn | Gallagher | Lai | Source | |
| Mucin | 2% w/v | 0.5% w/v | 0.5% w/v | - | 1% w/v | 2% w/v | 1% w/v | Flynn | |
| Sodium Chloride | 85.5 mM | 85.5 mM | 85.5 mM | 66.6 mM | 89.8 mM | 85.5 mM | 152.3 mM | Lapierre | |
| Potassium Chloride | 29.5 mM | 29.5 mM | 29.5 mM | 15.8 mM | - | 2.95 mM | 15.8 mM | Palmer | |
| Magnesium Sulfate | - | - | - | 0.6 mM | 1 mM | 1 mM | 0.61 mM | Palmer | |
| Iron Sulfate | - | - | - | 0.0036 mM | - | - | - | - | |
| Ammonium Chloride | - | - | - | 2.3 mM | 60 mM | - | - | - | |
| Monopotassium Phosphate | - | - | - | 2.5 mM | 60 mM | - | - | - | |
| Glucose | - | - | - | 3.2 mM | 13 mM | 40 mM | 0.7 mM | Sambeek | |
| Lactate | - | - | - | 9 mM | - | - | - | - | |
| Essential Amino Acids | 14.45x | 0.25 g/L | 0.25 g/L | Per Acid | 0.5x | 0.375x | 1.29x | Palmer | |
| Non-Essential Amino Acids | 28.9x | 0.25 g/L | 0.25 g/L | Per Acid | 0.25x | 0.5x | 8.01x | Palmer | |
| Vitamins | - | - | - | - | - | 1x | 1x | Gallagher | |
| Trace Metals | - | - | - | - | 1x | 1x | 1x | Flynn | |
| Egg Yolk | 0.25% | 0.25% | 0.25% | - | - | 0.25% | 0.25% | Kirchner | |
| Ferritin | 0.0003 g/L | - | - | - | - | 0.0004 g/L | 0.0004 g/L | Gallagher | |
| Salmon Sperm DNA | 1.4 g/L | 4 g/L | 4 g/L | - | - | 1.4 g/L | - | - | |
| DPTA | - | - | 0.0059 g/L | - | - | - | - | - | |
| pH | - | 6.9 | - | 6.8 | - | - | 6.3 | Lapierre | |
| Storage | 0 | 4° | - | - | 4° | 4° | 4° | Kirchner | |
| Sterilization | Autoclave | Filter | Autoclave | - | Autoclave | Autoclave | Filter | Kirchner | |
Table 1: Artificial sputum medium recipe derived from review of literature. (Column 1) Reagents and key values in the formulation of artificial sputum medium. (Columns 2-7) Comparison of recipes from extant literature8,9,10,12,14,15. (Columns 8-9) Artificial sputum medium recipe detailed in this protocol and the corresponding sources that informed each selected value10,11,12,13,14,15.
| Column 1 | Column 2 | Column 3 |
| CF Sputum | ASM | |
| Total Amino Acids | 10.25 mM | 10.76 mM |
| Alanine | 0.96 mM | 0.80 mM |
| Arginine | 0.17 mM | 0.94 mM |
| Asparagine | 0.91 mM | |
| Aspartic Acid | 0.45 mM | 0.80 mM |
| Cysteine | 0.09 mM | 0.33 mM |
| Glutamic Acid | 0.84 mM | 0.80 mM |
| Glycine | 0.65 mM | 0.80 mM |
| Histidine | 0.28 mM | 0.35 mM |
| Isoleucine | 0.60 mM | 0.52 mM |
| Leucine | 0.87 mM | 0.52 mM |
| Lysine | 1.15 mM | 0.64 mM |
| Methionine | 0.34 mM | 0.13 mM |
| Ornithine | 0.36 mM | |
| Phenylalanine | 0.29 mM | 0.26 mM |
| Proline | 0.90 mM | 0.80 mM |
| Serine | 0.78 mM | 0.80 mM |
| Threonine | 0.58 mM | 0.52 mM |
| Tryptophan | 0.07 mM | 0.06 mM |
| Tyrosine | 0.43 mM | 0.26 mM |
| Valine | 0.60 mM | 0.52 mM |
Table 2: Amino acid concentrations previously described in cystic fibrosis sputum and in artificial sputum medium recipe detailed in this protocol. (Column 1) Key amino acids. (Column 2) Amino acid concentrations of sputum from people with cystic fibrosis12. (Column 3) Amino acid concentrations in artificial sputum medium detailed in this protocol

Figure 1: Connection schematic of oxygen sparging system components. Flow diagram of the connections between components of the system used to sparge serum bottles to desired oxygen concentrations between 21% and 100%. The system has 3 modes of use determined by the position of the two T-junction valves. The system can route gas from the tanks through the gas output or through the oxygen percentage monitor, as well as route outflow gas from previously sparged serum bottles through the monitor to check concentration after time has elapsed. Please click here to view a larger version of this figure.
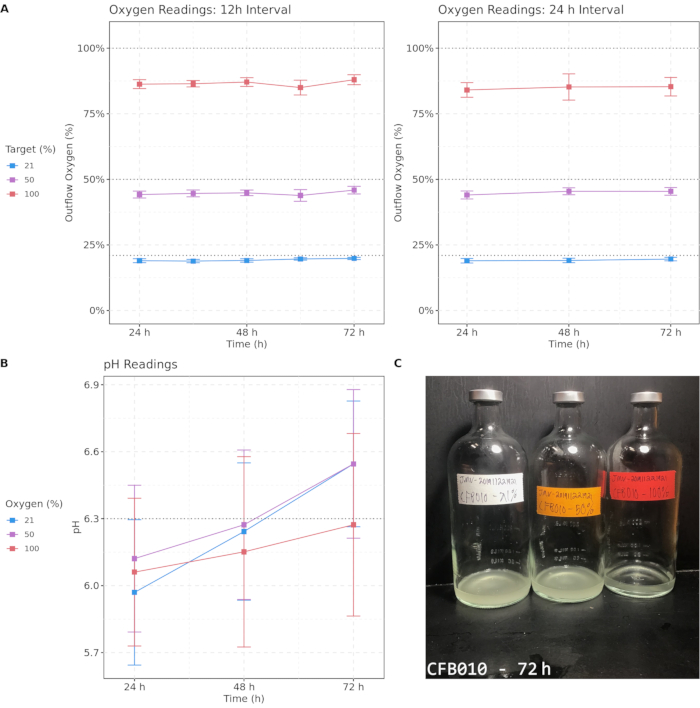
Figure 2: Target oxygen concentrations are approximately maintained with both 12 h and 24 h sparging intervals, and pH remains in the physiological range during culture. (A) Outflow oxygen readings from 12 h and 24 h oxygen sparging intervals over a 72-h period. (B) pH readings for samples measured every 24 h. (C) An example image of cultured sample CFB010 after 72 h, displaying differential turbidity across oxygen concentrations. The color indicates target oxygen percentage; error bars denote 95% confidence intervals. Critical thresholds are emphasized with dashed lines. Please click here to view a larger version of this figure.

Figure 3: Culturing increases microbial load, but underlying community composition is preserved. Uncultured sputum (yellow) and cultured sputum (blue) using artificial sputum medium at 21% oxygen for 48 h. Aliquots underwent nucleic acid extraction and shotgun metagenomics sequencing to detect possible bias introduced from culture conditions. (A) Absolute microbial load (determined by spike-in controls) and alpha diversity metrics. Using linear mixed effects models, uncultured vs. cultured sputum predicted microbial load but not alpha diversity. (B) Ordination of first two components of beta diversity metrics, controlling for difference in microbial load. No significant difference in either metric after PERMANOVA. Please click here to view a larger version of this figure.

Figure 4: The majority of identified taxa are present in both source sputum and culture, while others only appear in source sputum or culture. Shotgun metagenomics sequencing used to compare differences in microbial community composition between uncultured and cultured sputum samples. Phylogenetic tree of all identified microbial species in sequenced samples (N = 120). Species marked with yellow (N = 35, 29.2%) were only seen in uncultured sputum samples. Species marked with blue (N = 39, 32.5%) were only seen in artificial sputum medium culture samples. Species marked with cyan (N = 46, 38.3%) were seen in both uncultured and cultured samples. Please click here to view a larger version of this figure.

Figure 5: Artificial sputum medium is sufficiently transparent to be used as a growth curve medium for culturing clinical isolates. Optimal density readings at 600 nm were taken every 10 min, and the first 24 h of each curve are shown. Gray lines represent individual readings, and orange lines represent the mean absorbance for each taxon. Artificial sputum medium blank included as a control. Please click here to view a larger version of this figure.
Dyskusje
In this study, an in vitro model was developed to study the effect of hyperoxia on lung microbial communities. This model, based on artificial sputum medium and daily sparging of serum bottles, maintains elevated oxygen concentrations and supports the growth of microbes identified in sputum from pwCF.
There are several critical steps of this approach. First is the choice to use filter-sterilization rather than heat-sterilization of the artificial sputum medium. Filter-sterilization prevents denaturing of mucin and other heat-sensitive components of the medium and yields a clear medium that can be used for optical measures of microbial growth. While filter-sterilization has been proposed in other protocols10, we have found that the addition of orbital shaking during the filtration process was essential to prevent clogging of the filter that otherwise occurred when a minimum amount of the prepared medium had been filtered. While the filtered artificial sputum medium may have a lower-than-intended final mucin concentration due to mucin impaction in the filter, sputum from pwCF has been shown to have lower mucin concentrations than sputum from people without cystic fibrosis21. The starting mucin concentration of 1% used in this protocol is higher than in other approaches that have used filter-sterilization, with one group using a starting mucin concentration of 0.5%10, while typical recipes use mucin concentrations ranging from 0.5%-2% (Table 1). Thus, even with a loss of mucin in the filter-sterilization process, the final medium prepared using this protocol is likely to have mucin concentrations within the physiological range22.
The second is the composition of artificial sputum medium. The recipe for artificial sputum medium was chosen based on existing physiologic studies of sputum from pwCF (Table 1). Using shotgun metagenomics sequencing, we were able to verify that sputum cultured with this artificial sputum medium broadly recapitulates the microbial community composition of uncultured sputum (Figure 3). At normoxic conditions, this medium also supported the growth of 112 different clinical isolates representing common pathogens isolated from the sputum of cystic fibrosis patients. Thus, these data demonstrate that this formulation of artificial sputum medium supports the growth of airway microbiota from pwCF. Salmon sperm DNA, a common addition to existing recipes (Table 1), was omitted. One intended application of this model is metagenomics sequencing, and thus salmon sperm DNA was not included in order to reduce the addition of non-microbial nucleic acids as these reads would be filtered out after sequencing, thus decreasing our effective sequencing depth. While sputum from pwCF has high concentrations of extracellular DNA23, a significant proportion of it is microbial in origin24, and it is unclear if the addition of salmon sperm DNA to the artificial sputum medium makes it more physiologic or whether the culture approach described in this protocol leads to high levels of microbially-derived extracellular DNA; we did not distinguish between extra- and intra-cellular DNA concentrations in our studies. Future studies may wish to verify the concentration of extracellular DNA generated by this culturing method.
Third, to our knowledge, no published studies on cystic fibrosis lung microbial communities have addressed hyperoxic conditions. This model uses inexpensive and commonly available equipment from general laboratory or hospital suppliers to build an oxygen sparging system. Important considerations for the maintenance of hyperoxic conditions include the volume of the culture medium relative to the available headspace in the serum bottles. In initial attempts during protocol development, 125 mL serum bottles were used. However, the use of 500 mL serum bottles (allowing for a 475 mL headspace to 25 mL culture ratio) allowed the maintenance of the desired concentration for up to 24 h, thus reducing the frequency of oxygen sparging. This approach generates discrete oxygen conditions, thus allowing the simultaneous culture of different patient samples across multiple oxygen conditions. Other tools from anaerobic culture can be leveraged for hyperoxic culture, including the use of anaerobic jars or Balch-type tubes sparged with oxygen. Analyses of lung microbial communities using metagenomics sequencing indicate that overall alpha and beta diversity are comparable between cultured and uncultured sputum. When evaluating the differential abundance at the species level, culturing at 21% oxygen, enriched for the growth of aerobes and facultative anaerobes, including Enterobacterale, Streptococcus, and fungi. This is likely due to the exclusion of an anaerobic condition which has been observed in the airways of pwCF25,26. Future studies could consider the inclusion of nitrogen gas into this model to study of a range of anoxic and oxic conditions and corresponding aerobic and anaerobic microbiota found in airway microbial communities.
The key principles outlined in these protocols may be instructive for the conduct of similar studies related to the influence of oxygen on complex microbial communities or common lung pathogens. Oxygen is the most common therapy used in treating all advanced lung diseases, and a better understanding of how it might lead to collateral unanticipated effects on airway microbial communities and common respiratory pathogens will be important for the care of pwCF and other chronic lung diseases.
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
Part of this work was performed at the Marine Biological Lab with support from the Marine Biological Lab, DOE (DE-SC0016127), NSF (MCB1822263), HHMI (grant number 5600373), and a gift from the Simons Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| BME Vitamins (100x) Solution | MilliporeSigma | B6891 | Concentrated solution of supplemental vitamins. |
| Crimper, 30 mm | DWK Life Sciences | 224307 | Crimper for attaching aluminum seals to serum bottles. |
| D-(+)-Glucose | MilliporeSigma | G7021 | Solid glucose powder (dextrorotatory isomer). |
| Diaphragm Pump ME 2 NT | VACUUBRAND | 20730003 | Vacuum pump for vacuum filtration. |
| Egg Yolk Emulsion | HiMedia | FD045 | Sterile emulsion of 30% egg yolk in saline. |
| Ferritin, Cationized from Horse Spleen | MilliporeSigma | F7879 | Ferritin (iron-storage protein) solution. |
| FIREBOY plus Safety Bunsen Burner | Integra Biosciences | 144000 | Bunsen burner with user interface and safety features. |
| Hydrion pH Paper (1.0–14.0) | Micro Essential Laboratory | 94 | pH testing paper for the range of 1.0–14.0. |
| Hydrion pH Paper (4.0–9.0) | Micro Essential Laboratory | 55 | pH testing paper for the range of 4.0–9.0. |
| Hydrion pH Paper (6.0–8.0) | Micro Essential Laboratory | 345 | pH testing paper for the range of 6.0–8.0. |
| Hypodermic Needle-Pro EDGE Safety Device, 18 G | Smiths Medical | 401815 | 18 G needles with safety caps. |
| In-Line Pressure Gauge | MilliporeSigma | 20469 | Gas pressure gauge for monitoring bottle pressure. |
| Innova 42 Incubated Shaker | Eppendorf | 2231000756 | Combination incubator/orbital shaker. |
| Luer-Lok Syringe with Attached Needle | Becton Dickinson | 309580 | Combination 3 mL syringe and 18 G needle. |
| Luer Valve Assortment | World Precision Instruments | 14011 | Valves for gas flow tubing. |
| LSE Orbital Shaker | ThermoFisher Scientific | 6780-NP | Orbital shaker to agitate media during filtration. |
| Magnesium Sulfate Heptahydrate | MilliporeSigma | M2773 | Solid epsom salt (magnesium sulfate heptahydrate). |
| Medical Air Single Stage Regulator with Flowmeter | Western Enterprises | M1-346-15FM | Air flow rate regulator with 15 L/min meter. |
| MEM Amino Acids (50x) Solution | MilliporeSigma | M5550 | Concentrated solution of essential amino acids. |
| MEM Non-Essential Amino Acids (100x) Solution | MilliporeSigma | M7145 | Concentrated solution of non-essential amino acids. |
| Millex-GP Filter, 0.22 µm | MilliporeSigma | SLMP25SS | 0.22 µm polyethersulfone membrane sterile syringe filter. |
| Milli-Q Academic | MilliporeSigma | ZMQS60E01 | Milli-Q sterile water filtration system. |
| MiniOX 3000 Oxygen Monitor | MSA | 814365 | Gas flow oxygen percentage monitor. |
| MOPS Buffer (1 M, pH 9.0) | Boston BioProducts | BBM-90 | MOPS buffer for adjusting media pH. |
| Mucin from Porcine Stomach | MilliporeSigma | M2378 | Mucin (glycosylated gel-forming protein) powder. |
| Natural Polypropylene Barbed Fitting Kit | Harvard Apparatus | 72-1413 | Connectors for gas flow tubing. |
| Nextera XT DNA Library Preparation Kit | Illumina | FC-131-1096 | Library preparation for identification during sequencing. |
| NovaSeq 6000 Sequencing System | Illumina | 770-2016-025-N | Shotgun sequencing platform for generating sample reads. |
| Oxygen Single Stage Regulator with Flowmeter | Western Enterprises | M1-540-15FM | Oxygen flow rate regulator with 15 L/min meter. |
| Oxygen Tubing with 2 Standard Connectors | SunMed | 2001-01 | Tubing for connecting gas system components. |
| Phosphate buffered saline, 10x, pH 7.4 | Molecular Biologicals International | MRGF-6235 | Concentrated phosphate-buffered saline solution. |
| PC 420 Hot Plate/Stirrer | Marshall Scientific | CO-PC420 | Combination hot plate/stirrer. |
| Potassium Chloride | MilliporeSigma | P9541 | Solid potassium chloride salt. |
| PTFE Disposable Stir Bars | ThermoFisher Scientific | 14-513-95 | Disposable magnetic stir bars. |
| PTFE Thread Seal Teflon Tape | VWR | 470042-938 | Teflon tape for reinforcing gas system connections. |
| Q-Gard 2 Purification Cartridge | MilliporeSigma | QGARD00D2 | Purification cartridge for Milli-Q system. |
| Reusable Media Storage Bottles | ThermoFisher Scientific | 06-423A | Bottles for mixing and storing culture media. |
| Rubber Stopper, 30 mm, Gray Bromobutyl | DWK Life Sciences | 224100-331 | Rubber stoppers for serum bottles. |
| Serum Bottle with Molded Graduations, 500 mL | DWK Life Sciences | 223952 | Glass serum bottles for sealed culturing. |
| Small Bore Extension Set | Braun Medical | 471960 | Tubing extension with luer lock connectors. |
| Sodium Chloride | MilliporeSigma | S3014 | Solid sodium chloride salt. |
| Spike-in Control I (High Microbial Load) | ZymoBIOMICS | D6320 | Spike-in microbes (I. halotolerans and A. halotolerans) for absolute microbial load calculations |
| Stericup Quick Release Sterile Vacuum Filtration System | MilliporeSigma | S2GPU02RE | 250 mL 0.22 µm vacuum filtration chamber. |
| Super Sani-Cloth Germicidal Disposable Wipes | Professional Disposables International | H04082 | Disposable germicidal wipes for sterilization. |
| Trace Metals Mixture, 1000x | ThermoFisher Scientific | NC0112668 | Concentrated solution of physiological trace metals. |
| Unlined Aluminum Seal, 30 mm | DWK Life Sciences | 224187-01 | Aluminum seals crimped over top of rubber stoppers. |
| USP Medical Grade Air Tank | Airgas | AI USP200 | Compressed air tank for input to sparging system. |
| USP Medical Grade Oxygen Tank | Airgas | OX USP200 | Compressed oxygen tank for input to sparging system. |
Odniesienia
- Carmody, L. A., et al. Fluctuations in airway bacterial communities associated with clinical states and disease stages in cystic fibrosis. PLoS One. 13 (3), 0194060 (2018).
- Acosta, N., et al. Sputum microbiota is predictive of long-term clinical outcomes in young adults with cystic fibrosis. Thorax. 73 (11), 1016-1025 (2018).
- Muhlebach, M. S., et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathogens. 14 (1), 1006798 (2018).
- Zolin, A., Bossi, A., Cirilli, N., Kashirskaya, N., Padoan, R. Cystic fibrosis mortality in childhood. Data from European cystic fibrosis society patient registry. International Journal of Environmental Research and Public Health. 15 (9), (2018).
- Ramos, K. J., et al. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 30% of predicted in the United States. Chest. 30 (6), 1320-1328 (2017).
- Ramos, K. J., et al. Predictors of non-referral of patients with cystic fibrosis for lung transplant evaluation in the United States. Journal of Cystic Fibrosis. 15 (2), 196-203 (2016).
- Girardis, M., et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The Oxygen-ICU randomized clinical trial. JAMA. 316 (15), 1583-1589 (2016).
- Comstock, W. J., et al. The WinCF model - An inexpensive and tractable microcosm of a mucus plugged bronchiole to study the microbiology of lung infections. Journal of Visualized Experiments: JoVE. (123), e55532 (2017).
- Diraviam Dinesh, S. Artificial sputum medium. Protocol Exchange. , (2010).
- Kirchner, S., et al. Use of artificial sputum medium to test antibiotic efficacy against Pseudomonas aeruginosa in conditions more relevant to the cystic fibrosis lung. Journal of Visualized Experiments: JoVE. (64), e3857 (2012).
- Grandjean Lapierre, S., et al. Cystic fibrosis respiratory tract salt concentration: An Exploratory Cohort Study. Medicine. 96 (47), 8423 (2017).
- Palmer, K. L., Aye, L. M., Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. Journal of Bacteriology. 189 (22), 8079-8087 (2007).
- Van Sambeek, L., Cowley, E. S., Newman, D. K., Kato, R. Sputum glucose and glycemic control in cystic fibrosis-related diabetes: a cross-sectional study. PLoS One. 10 (3), 0119938 (2015).
- Flynn, J. M., Niccum, D., Dunitz, J. M., Hunter, R. C. Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathogens. 12 (8), 1005846 (2016).
- Gallagher, T., et al. Liquid chromatography mass spectrometry detection of antibiotic agents in sputum from persons with cystic fibrosis. Antimicrobial Agents and Chemotherapy. 65 (2), (2021).
- Voynow, J. A., Rubin, B. K. Mucins, mucus, and sputum. Chest. 135 (2), 505-512 (2009).
- Sui, H. Y., et al. Impact of DNA extraction method on variation in human and built environment microbial community and functional profiles assessed by shotgun metagenomics sequencing. Frontiers in Microbiology. 11, 953 (2020).
- McIver, L. J., et al. bioBakery: a meta'omic analysis environment. Bioinformatics. 34 (7), 1235-1237 (2018).
- Truong, D. T., et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nature Methods. 12 (10), 902-903 (2015).
- Stammler, F., et al. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome. 4 (1), 28 (2016).
- Henke, M. O., Renner, A., Huber, R. M., Seeds, M. C., Rubin, B. K. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. American Journal of Respiratory Cell and Molecular Biology. 31 (1), 86-91 (2004).
- Henderson, A. G., et al. Cystic fibrosis airway secretions exhibit mucin hyper concentration and increased osmotic pressure. Journal of Clinical Investigation. 124 (7), 3047-3060 (2014).
- Matthews, L. W., Spector, S., Lemm, J., Potter, J. L. Studies on pulmonary secretions. I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. American Review of Respiratory Disease. 88, 199-204 (1963).
- Ibanez de Aldecoa, A. L., Zafra, O., Gonzalez-Pastor, J. E. Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Frontiers in Microbiology. 8, 1390 (2017).
- Tunney, M. M., et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 177 (9), 995-1001 (2008).
- Worlitzsch, D., et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. Journal of Clinical Investigation. 109 (3), 317-325 (2002).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone