Method Article
Preparation of Rat Skeletal Muscle Homogenates for Nitrate and Nitrite Measurements
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
We present protocols for three different methods for the homogenization of four different muscle groups of rat skeletal muscle tissue to measure and compare the levels of nitrate and nitrite. Furthermore, we compare different sample weights to investigate whether tissue sample size affects the results of homogenization.
Streszczenie
Nitrate ions (NO3-) were once thought to be inert end products of nitric oxide (NO) metabolism. However, previous studies demonstrated that nitrate ions can be converted back to NO in mammals through a two-step reduction mechanism: nitrate being reduced to nitrite (NO2-) mostly by oral commensal bacteria, then nitrite being reduced to NO by several mechanisms including via heme- or molybdenum-containing proteins. This reductive nitrate pathway contributes to enhancing NO-mediated signaling pathways, particularly in the cardiovascular system and during muscular exercise. The levels of nitrate in the body before such utilization are determined by two different sources: endogenous NO oxidation and dietary nitrate intake, principally from plants. To elucidate the complex NO cycle in physiological circumstances, we have examined further the dynamics of its metabolites, nitrate and nitrite ions, which are relatively stable compared to NO. In previous studies skeletal muscle was identified as a major storage organ for nitrate ions in mammals, as well as a direct source of NO during exercise. Therefore, establishing a reliable methodology to measure nitrate and nitrite levels in skeletal muscle is important and should be helpful in extending its application to other tissue samples. This paper explains in detail the preparation of skeletal muscle samples, using three different homogenization methods, for nitrate and nitrite measurements and discusses important issues related to homogenization processes, including the size of the samples. Nitrate and nitrite concentrations have also been compared across four different muscle groups.
Wprowadzenie
Nitric oxide (NO), a small gaseous signaling molecule, plays a critical role in physiological and pathophysiological processes1. NO can be produced from L-arginine by endogenous enzymes of the nitric oxide synthase (NOS) family before undergoing rapid oxidation to nitrate (NO3-) and, possibly, nitrite (NO2-) in blood and tissues2,3. Recently, these anions have been shown to be reduced back to NO in mammalian systems4. Nitrate is converted to nitrite, mainly by commensal bacterial nitrate reductases in the oral cavity acting on ions secreted by the salivary glands and directly ingested 5, and to some extent, by mammalian enzymes such as xanthine oxidoreductase6,7. Nitrite can be further reduced to NO by several mechanisms including deoxyhemoglobin8, deoxymyoglobin9, molybdenum-containing enzymes10, and non-enzymatic reduction in the presence of protons11,12.
This nitrate-nitrite-NO pathway is enhanced under hypoxic conditions wherein NOS activity is diminished because NOS requires oxygen for NO generation4. Many recent studies have reported beneficial effects of dietary nitrate on blood pressure regulation and exercise performance, suggesting that nitrate reduction pathways contribute to the augmentation of NO signaling13,14,15. Previous studies have shown that some skeletal muscles are likely the major nitrate storage places in the body16. Compared to blood or other internal organs such as liver, skeletal muscle (gluteus maximus) contains significantly higher levels of nitrate and has a substantial mass in the mammalian body. Treadmill exercise was shown to enhance nitrate reduction to nitrite and to NO in gluteus in a rat model7. These results imply that some skeletal muscles could be important sources for NO through nitrate reduction pathways in physiological situations. More recent studies suggest that these findings, including changes in muscle nitrate levels during exercise, also occurs in humans17.
Two of the current authors had previously established a method to measure nitrate and nitrite levels in blood and other liquid samples18. However, when the levels of these anions in tissue homogenates were initially analyzed, detailed protocols were not available. To understand the nitrate-nitrite-NO dynamics in several different organs, our goal was to develop an accurate and efficient method to measure nitrate and nitrite levels in mammalian tissues including skeletal muscle. In earlier studies, rodent tissues were used to develop reliable homogenization processes and then analyze nitrate and nitrite contents in those homogenates7,16,19. The usage of this homogenization method was extended to human skeletal muscle biopsy samples, whereby the values were confirmed, and importantly, the values observed for muscle compared to blood/plasma were in similar ranges and ratios to those observed in rodents17. In recent years, other groups also started measuring nitrate and nitrite levels in skeletal muscle homogenates, reporting comparable values to the ones reported by our group20,21.
The aim of this protocol paper is to describe in detail the preparation of skeletal muscle homogenates using three different homogenization methods for subsequent measurement of nitrate and nitrite levels. Additionally, the effects of tissue weight used for homogenization on values of nitrate and nitrite in skeletal muscle samples were examined. We believe that these methods can be easily applied to other types of mammalian tissues. Recently, especially in the field of exercise physiology, attention had been paid to the possible differences in nitrate/nitrite/NO physiology according to muscle groups. We also report the amounts of nitrate and nitrite in four different rodent muscles and find a nonuniform distribution of both ions among these different muscles; an observation which requires further study.
Protokół
Animal protocol was approved by NIDDK Animal Care and Use Committee (ASP K049-MMB-20). Animals were handled and treated according the current Guide for the Care and Use of Laboratory Animals freely available on AAALAC website.
1. Rat skeletal muscle collection
- While a rat is under deep anesthesia (5% isoflurane, confirmed by absent reaction to tail/leg pinch), start perfusion with saline containing heparin by placing a 19 G needle into an apex of the left ventricle and making a nick on the right atrium. Allow saline with heparin to perfuse through the internal organs until at least 1.5 liter/kg have flowed through the tissue. At this point, animals are dead from exsanguination and carcasses are ready to be processed for sample collection.
NOTE: Achieving good perfusion is critical, especially for accurate measurements of nitrite, because nitrite is oxidized into nitrate by any remaining hemoglobin. - Identify target muscle tissues and excise them from the hind legs22 using clean surgical instruments. Remove as much fat and connective tissues from the muscle tissues as possible.
- Place the desired amount of muscle into a microcentrifuge tube and then place on dry ice. Store the tubes filled with tissue in the -80 °C freezer.
NOTE: In case of human biopsy samples, thoroughly blot them immediately upon collection with clean gauze to remove excess blood.
2. Preparation for homogenization
- Preparation of nitrite-preserving solution (stop solution)
- Prepare a clear yellow solution containing 890 mM potassium ferricyanide (K3Fe(CN)6) and 118 mM NEM (N-ethylmaleimide) in distilled water, ensuring no crystals. Add non-ionic surfactant (detergent) in a 1:9 ratio (v/v, Table of Materials), and mix gently to avoid foaming.
- Dilute the stop solution in a 1:9 ratio with distilled water. Place the diluted stop solution (1:5 ratio of muscle tissue to diluted stop solution) in the homogenization tube.
NOTE: Twenty milligrams of tissue will require 100 μL of stop solution, and 200 mg of tissue will require 1000 μL of stop solution in the tube. Presence of detergent in added solution is critical for disruption of cell membranes. Any non-ionic detergent can be used, but care must be taken to verify that it does not interfere with chemiluminescence method.
- Tissue preparation
- Take the tissue out of the -80 °C freezer and slowly thaw in ice. Remove the remaining fat and connective tissue from the skeletal muscle. Cut off pieces of skeletal muscle and blot on gauze to dry.
- Weigh out the amount of tissue (20, 50, and 200 mg). Place the pre-weighed skeletal muscle in the stop-solution in the homogenization tubes or place the pre-weighed tissue in a clean microcentrifuge tube for later use.
3. Homogenization
- Rotary homogenizer (Figure 1)
- Place the M-type tube containing the pre-weighed skeletal muscle and pre-measured stop solution into the machine. Homogenize each sample twice (setting on the most destructive homogenization cycle) and place the tube on ice immediately after each homogenization for 5 min to cool down.
- Centrifuge briefly at 2,000 × g and 4 °C for 5 min. Place the full tube back on ice and add the appropriate volume of methanol (≥ 99.9 %, 10x of tissue weight). Vortex thoroughly for 15 s.
NOTE: For 20 mg of tissue, use 200 μL of methanol. Methanol is used to precipitate proteins from tissue homogenate and does not interfere with chemiluminescence method. If other protein precipitation method is used, test its compatibility with chemiluminescence. - Homogenize once more and incubate on ice for 30 min. Centrifuge the samples for 35 min at 4 °C and 3,500 × g. Aspirate the supernatant, and measure nitrite/nitrate levels, or store at -80 °C for later use.
- Bead homogenizer (Figure 2)
- Place the skeletal muscle tissue in a bead-containing tube (1:5 ratio for tissue:diluted stop solution) and homogenize twice for 45 s at the highest speedavailable on the instrument used. Place the tube on ice immediately after each homogenization for 5 min to cool down.
- Briefly centrifuge using a small desktop centrifuge (2,000 x g) for 5 sec. Place the tube back on ice and add an appropriate volume of methanol (purity ≥ 99.9 %, 10x of tissue weight). Vortex thoroughly for 15 s.
NOTE 1: For 20 mg of tissue, use 200 μL of methanol.
NOTE 2: Methanol is used to precipitate proteins from tissue homogenate and does not interfere with chemiluminescence method. If other protein precipitation method is used, test its compatibility with chemiluminescence. - Homogenize once more for 45 s at the highest speed available on the instrument used. Incubate on ice for 30 min. Centrifuge at 17,000 x g, 4 °C, 30 min. Aspirate the supernatant, and measure nitrite/nitrate levels, or store at -80 °C for later use.
- Pulverizer (Figure 3)
- Prepare tubes containing diluted stop solution (5x of tissue weight) and weigh them. Record the weight (tube + stop solution).
- Place the liquid nitrogen pulverizer tool on dry ice and wait for approximately 30 min for it to reach the desired temperature.
- Using tweezers chilled in liquid nitrogen, transfer one sample (tissue weight measured already) to the pulverizer. Add a small spoonful of liquid nitrogen to ensure the tissue is at liquid nitrogen temperature.
- After 95% of the liquid nitrogen has vaporized, place the crushing tool on top of the tissue, and press firmly. You should feel the sample crush. Using the mallet, strike the crushing tool 3-5x.
- Check the sample for any remaining chunks using a spoon cooled in liquid nitrogen. After cooling in liquid nitrogen, use a piece of tissue paper to wipe away any frozen water before touching the tissue. If a chunk is present, move it into the center, and then strike 5-6 more times with the mallet.
- When the whole sample has been pulverized, use the liquid nitrogen-cooled spoon to directly transfer the crushed tissue into the pre-weighed tube containing the diluted stop solution (step 3.3.1). Be sure to perform this step quickly as when the crushed skeletal muscle heats up, it gets sticky and difficult to transfer.
- Vortex for 15 s. Check that no tissue is stuck at the top of the tube by opening the tube. If there is, try to dislodge it and then vortex again.
- Centrifuge the sample for 2-3 s using a small desktop centrifuge. Weigh the tube again. Calculate the tissue weight by deducting the original tube weight (step 3.3.1) from this new weight. Place the tube on ice.
NOTE: The exact weights will help determine the exact tissue weight and how much tissue was lost during pulverization. - Once all samples have been processed up to step 3.3.8, add an appropriate volume of methanol (≥ 99.9 %, 10x of tissue weight). Vortex thoroughly for 15 s, and incubate on ice for 30 min.
NOTE: For 20 mg of tissue, use 200 μL of methanol. Methanol is used to precipitate proteins from tissue homogenate and does not interfere with chemiluminescence method. If other protein precipitation method is used, test its compatibility with chemiluminescence. - Centrifuge at 17,000 × g, 4 °C, 30 min. Aspirate the supernatant and measure nitrite/nitrate levels, or store at -80 °C for later use.
4. Nitrite/nitrate measurement with nitric oxide analyzer (NOA)
- Prepare all samples by either of three different homogenization methods described above and inject them into an NOA for nitrate and nitrite measurement.
NOTE: Detailed protocols for NOA use were published previously19.
Wyniki
To obtain representative results, skeletal muscle tissues from 8 Wistar rats (males and females, weight 250 ± 50 g) were used. Rat skeletal muscle homogenates (50 mg of gluteus maximus muscle for each method) were prepared by three different homogenization tools (rotary homogenizer, bead homogenizer, and pulverizer). The nitrate and nitrite contents of these homogenates were then determined using a nitric oxide analyzer (NOA) (Figure 4). Nitrate levels (Figure 4A) in those three homogenate samples were very similar to each other, ranging from 39.6 to 45.2 nmol/g.
Interestingly, nitrite levels (Figure 4B) in a homogenate sample prepared by a pulverizer showed the highest value (0.99 ± 0.15 nmol/g), and this was statistically different from the two other sample values. To test whether the size of tissues would affect the homogenization efficiency and the resulting nitrate and nitrite values, three different skeletal muscle tissue sizes (gluteus muscle tissue of 20, 50, and 200 mg) were used for homogenization by a bead homogenizer (Figure 5). Neither nitrate (Figure 5A) nor nitrite (Figure 5B) levels were significantly different between muscle sample sizes. However, slightly lower nitrate concentrations were observed when sample size was increased, in multiple experiments for reasons that are unclear.
Next, nitrate and nitrite levels in different kinds of rodent leg skeletal muscle tissues were measured (Figure 6). In addition to the gluteus maximus muscle, tibialis anterior (TA), extensor digitorum longus (EDL), and gastrocnemius muscles (50 mg each) were isolated from rat legs and homogenized using a bead homogenizer. Surprisingly, the nitrate levels of gluteus muscle (40.4 nmol/g) were approximately two-fold higher than those of the other three muscle tissues although in this particular experiment these differences did not reach statistical significance (Figure 6A). The nitrite concentration in the gluteus muscle was also higher than that of the other three muscle tissues and was significantly higher than that in the gastrocnemius sample (Figure 6B).
We determined the coefficient of variation for all three methods used and results are in attached table. For comparison, the table also shows coefficients of variation determined by Troutman et al21.
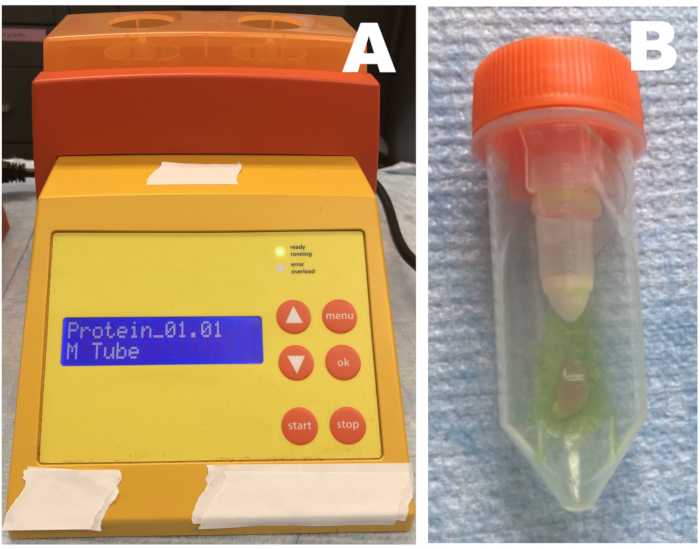
Figure 1: Rotary homogenizer. (A) Homogenizer; (B) tube containing tissue and stop solution. Skeletal muscle tissue samples are placed in a tube with diluted stop solution and homogenized three times. Samples should be immediately placed on ice after each homogenization step. Please click here to view a larger version of this figure.

Figure 2: Bead homogenizer. (A) Homogenizer; (B) beads containing tube. Skeletal muscle tissue samples are placed in a bead-containing tube (5 ceramic beads) with diluted stop solution and homogenized three times in total (45 s at highest speed for each time). Samples should be immediately placed on ice after each homogenization step. Please click here to view a larger version of this figure.

Figure 3: Pulverizer. (A) pulverizer parts (B) pulverizer body - mortar and pestle assembled. (C) detail of mortar and pestle assembly. Pulverizer parts are cooled on dry ice for 30 minutes. Liquid nitrogen is put into the mortar and then pre-weighed skeletal muscle tissue is added. After 90 percent of the liquid nitrogen is gone, the top portion - the pestle - is inserted into the basin. The pestle is pounded with a mallet 5-6 times until sample is powdery. The powdered tissue is then transferred to a tube with stop solution. Please click here to view a larger version of this figure.
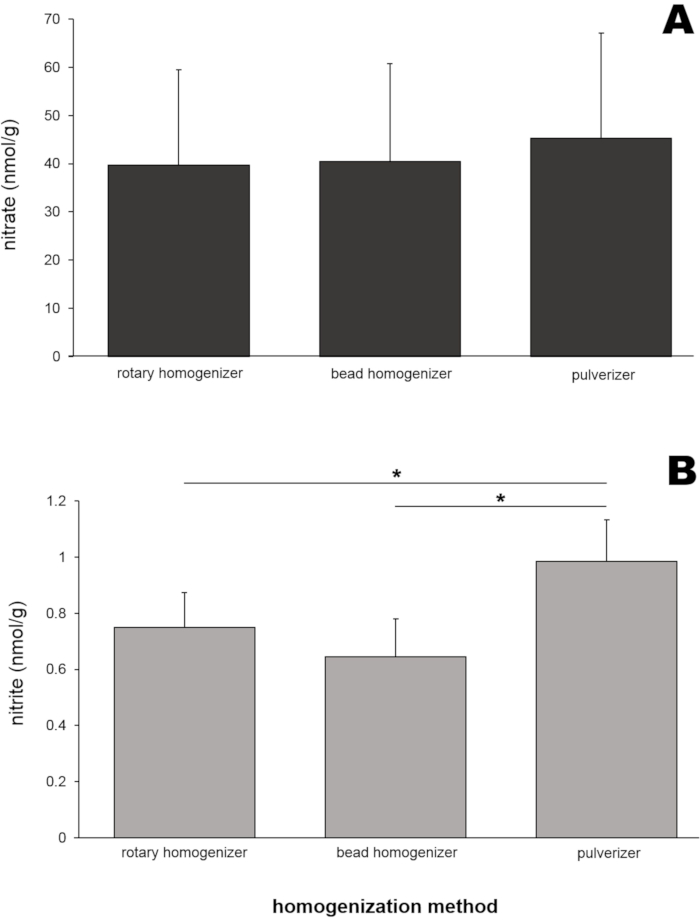
Figure 4: (A) Nitrate and (B) nitrite contents in gluteus muscle homogenates prepared by three different homogenization methods. Fifty milligrams of gluteus muscle were homogenized by three different homogenization methods. Methanol (500 μL) was added to the homogenates to precipitate proteins. A standard chemiluminescence method with vanadium chloride or tri-iodide solution was used to measure nitrate and nitrite levels, respectively, in the clear supernatant after centrifugation; n=7 (nitrate); n=8 (nitrite); data are presented as averages ± SD, * p < 0.05 using one-way ANOVA. Please click here to view a larger version of this figure.

Figure 5: (A) Nitrate and (B) nitrite contents in different sample sizes of gluteus muscle. Three different sizes of gluteus muscle homogenates were prepared by using a bead homogenizer. A standard chemiluminescence method with vanadium chloride or tri-iodide solution was used to measure nitrate and nitrite levels, respectively, in the clear supernatant after centrifugation; n=7 (nitrate); n=8 (nitrite); data are presented as averages ± SD, Please click here to view a larger version of this figure.
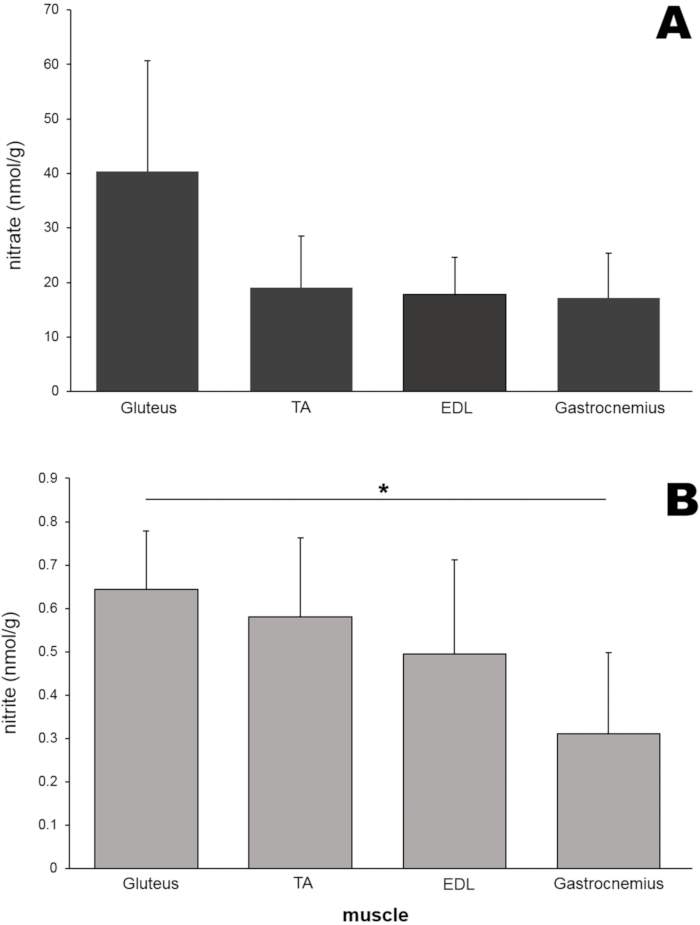
Figure 6: (A) Nitrate and (B) nitrite contents in four different muscles. Each muscle (50 mg) was homogenized by using a bead homogenizer, and a standard chemiluminescence method with vanadium chloride or tri-iodide solution was used to measure nitrate and nitrite levels, respectively; n=4-7 (nitrate); n=3-8 (nitrite); data are presented as averages ± SD, * p < 0.05 using one-way ANOVA. Please click here to view a larger version of this figure.
Supplemental File. Please click here to download this File.
Dyskusje
To monitor changes in the NO metabolites, nitrate and nitrite, as a function of physiological interventions, it is imperative to measure the levels of these ions in the different organs that are critical in their metabolism. As hemoglobin in blood will react with NO and its metabolites, it is also important to remove blood quickly from tissue samples as much as possible. Thus animals were perfused with saline before collecting skeletal muscle tissues (gluteus, TA, EDL, gastrocnemius muscle), and connective tissue and fat around the target muscle were immediately removed. For the homogenization solution, a 'stop solution' was specially formulated with ferricyanide, NEM, and non-ionic surfactant to preserve nitrite in various tissues19. The presence of non-ionic detergent is critical for full disruption of cell membranes. In the case of the two homogenization machines used in this study, the rotary homogenizer and bead homogenizer, the volume of stop solution required for homogenization was 5x the tissue weight so that the whole tissue was fully submerged in the solution and processed homogeneously.
The homogenization process was performed three times in total (twice before adding methanol and one more time after adding methanol to precipitate proteins), each time using a programmed setting determined by the instrument manufacturers. It is important to place samples on ice immediately after each homogenization to prevent sample deterioration by the mild heating, which may be generated during homogenization. For the pulverization method, tissue samples were initially crushed into powder while keeping them frozen using liquid nitrogen, then samples were mixed with stop solution (5x tissue weight). In all three methods, methanol (10x tissue weight) was added to the samples, and the mixture was incubated on ice for 30 min to precipitate proteins and fully extract nitrate and nitrite from the tissues. The presence of proteins in sample can cause excessive foaming of the reaction solution in the chamber when using chemiluminescence, so it is necessary to precipitate proteins from the samples. Any precipitation agent can be used, but it is necessary to confirm that such agents will not interfere either with the chemiluminescence method or cause any changes in nitrite/nitrate concentrations by reacting with these ions.
Nitrate levels in all gluteus samples prepared by the three methods were very similar (39.6-45.2 nmol/g, Figure 4A), suggesting these three homogenization methods are equally applicable to preparing muscle samples for nitrate analysis. Although nitrite levels were slightly higher in samples prepared by pulverization compared to the two other methods (Figure 4B), all measured values were in a limited range (0.64-0.99 nmol/g). The effect of tissue sample size on nitrate and nitrite values in skeletal muscle samples was examined because researchers often deal with very small samples, particularly from small animals or human muscle biopsies. Neither nitrate nor nitrite levels were significantly different in the range from 20 to 200 mg of skeletal muscle tissue in these experiments (Figure 5).
However, it is noteworthy that, although not statistically significant, nitrate levels tend to be slightly lower when measured in larger samples (Figure 5). We have noticed this phenomenon over the course of our published and unpublished studies, but we do not understand the reasons for it. Therefore, it is probably important to consider the sample weights and ideally keep them consistent throughout a study when preparing homogenates from multiple tissue samples. Another consideration when working with smaller samples (20 mg) is that the final total volume of processed sample is only ~300 µL, which significantly reduces the possibility to measure the same sample several times. However, especially for human studies, 20 mg is the usual acceptable size for muscle biopsies.
To examine whether different muscles in rat legs would have different nitrate/nitrite contents, the concentrations of those ions were compared in four different leg muscles (Figure 6). Both nitrate and nitrite levels were highest in the gluteus muscle compared to three other muscle tissues, although the differences did not reach statistical significance in this study, suggesting that nitrate metabolism in various muscle types may be governed differently. This phenomenon is being investigated in more detail currently.
Other research groups have also reported skeletal muscle nitrate concentrations, revealing significant variability in those values. The Coggan group showed that nitrate levels in Sprague Dawley rat soleus muscle vary from 62 to 124 nmol/g depending on muscle preparation methods21. The same group reported nitrate values in SD rat vastus lateralis (approximately 60 nmol/g) and soleus (approximately 215 nmol/g) in another study23. Ohtake et al. measured nitrate concentrations in mouse gastrocnemius muscle of >300 nmol/g; however, precise methods for muscle homogenate preparation were not described24. The Verdijk group reported nitrate contents in human muscle biopsies (vastus lateralis) ranging from 54 to 80 nmol/g depending on participant age20; in a similar study, Wylie et al. report 226 nmol/g for the same muscle group17. These results suggest that the concentrations of nitrate/nitrite in skeletal muscle samples reflect many factors-physiological (e.g., exercise status), environmental (e.g., diet), and technical (assay details)-all of which presumably contribute to measurement variability.
We determined the coefficient of variability (CV) for all three methods presented here - see attached supplemental file. Even if CV are far from ideal, and vary among the methods used, our CV values are comparable with those published by Troutman et al. 21 using similar methods with different treatment of samples. Unfortunately, there is still no clear explanation why such high variability persists; this paper is the only published detailed protocol we know of for processing muscle samples for nitrate and nitrite determination.
We also measured linearity and degree of nitrate recovery from nitrate-spiked muscle samples (see attached supplemental file). When we added three different concentrations of nitrate into gluteus samples for homogenization, we obtained a good linear response in increase of nitrate concentrations with ~80% recovery of added nitrate for both bead and rotary homogenizers. These results demonstrate that both homogenization methods can be used for determination of nitrate and nitrite levels in biological samples with good confidence. The loss of 20% of added nitrate is likely happening during the deproteinization and centrifugation of muscle tissue homogenate when some ions might co-precipitate with charged proteins or other cell parts.
In general, we hope that our proposed method of muscle sample preparation for measurements of nitrate and nitrite will prove useful not only into the exercise research field, but also into clinical studies. There are neuromuscular and/or metabolic disorders affecting large populations that might relate to nitric oxide cycle malfunctioning and could benefit from nitrate supply. However, one has first to establish if NO deficiency, in fact, exists and if it can be corrected by dietary intervention. We hope that the method described here will enable the fate of nitrate and other metabolites of the NO cycle to be monitored in skeletal muscle and other organs
We are aware that, at this point of its development, methods of skeletal muscle preparation for nitrate and nitrite measurements using chemiluminescence (the most quantitative of all determinations of the nitrogen oxides themselves) still has unknown variables, some of which are discussed above. However, even with its limitations, the described methods allow reasonably accurate comparison of nitrate/nitrite levels of different organs, including different skeletal muscles, and should allow formulation of hypotheses that can be then tested with reasonable accuracy.
Ujawnienia
The authors declare they have no conflicts of interest. Alan N. Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. These arrangements do not affect his adherence to JoVE journal policies.
Podziękowania
This work was supported by intramural NIH/NIDDK grant ZIA DK 0251041-14 to Alan N Schechter, MD.
Materiały
| Name | Company | Catalog Number | Comments |
| gentleMACS dissociator | Miltenyi Biotec | 130-093-235 | |
| gentle MACS M tube | Miltenyi Biotec | 130-093-236 | Length: 87 mm; Diameter: 30 mm |
| Heparin Sodium | Hospira | NDC-0409-7620-13 | |
| Isoflurane | Baxter | NDC-10019-360-60 | |
| Methanol | Sigma | 646377 | |
| Minilys bead homogenizer | Bertin Instruments | P000673-MLYS0-A | |
| NEM; N-ethylmaleimide | Sigma | 4260 | |
| Nitric Oxide analyzer | GE | Sievers NOA 280i | |
| NP-40; 4-Nonylphenylpolyethylene glycol | Sigma | 74385 | |
| Potassium ferricyanide; K3Fe(CN)6 | Sigma | 702587 | |
| Precellys lysing kit | Bertin Instruments | P000911-LYSK0-A | contains 2 mL tubes with 2.8 mm ceramic (zirconium oxide) beads for homogenization |
| Pulverizer kit | Cellcrusher | Cellcrusher kit |
Odniesienia
- Ignarro, L. J. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. Journal of Physiology and Pharmacology. 53 (4), 503-514 (2002).
- Moncada, S., Higgs, A. The L-arginine-nitric oxide pathway. New England Journal of Medicine. 329 (27), 2002-2012 (1993).
- Thomas, D. D., Liu, X., Kantrow, S. P., Lancaster, J. R. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proceedings of the National Academy of Sciences of the United States of America. 98 (1), 355-360 (2001).
- Lundberg, J. O., Weitzberg, E., Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 7 (2), 156-167 (2008).
- Govoni, M., Jansson, E. A., Weitzberg, E., Lundberg, J. O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 19 (4), 333-337 (2008).
- Jansson, E. A., et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nature Chemical Biology. 4 (7), 411-417 (2008).
- Piknova, B., Park, J. W., Kwan Jeff Lam, K., Schechter, A. N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 55-56, 54-61 (2016).
- Cosby, K., et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 9 (12), 1498-1505 (2003).
- Shiva, S., et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 100 (5), 654-661 (2007).
- Millar, T. M., et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Letter. 427 (2), 225-228 (1998).
- Benjamin, N., et al. Stomach NO synthesis. Nature. 368 (6471), 502 (1994).
- Lundberg, J. O., Weitzberg, E., Lundberg, J. M., Alving, K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 35 (11), 1543-1546 (1994).
- Larsen, F. J., Ekblom, B., Sahlin, K., Lundberg, J. O., Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. New England Journal of Medicine. 355 (26), 2792-2793 (2006).
- Kapil, V., et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 56 (2), 274-281 (2010).
- Jones, A. M. Dietary nitrate supplementation and exercise performance. Sports Medicine. 44, 35-45 (2014).
- Piknova, B., et al. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide. 47, 10-16 (2015).
- Wylie, L. J., et al. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. Journal of Physiology. 597 (23), 5565-5576 (2019).
- Piknova, B., Schechter, A. N. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods in Molecular Biology. 704, 39-56 (2011).
- Piknova, B., Park, J. W., Cassel, K. S., Gilliard, C. N., Schechter, A. N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. Journal of Visualized Experiments. (118), e54879 (2016).
- Nyakayiru, J., et al. Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. Journal of Applied Physiology. 123 (3), 637-644 (2017).
- Troutman, A. D., Gallardo, E. J., Brown, M. B., Coggan, A. R. Measurement of nitrate and nitrite in biopsy-sized muscle samples using HPLC. Journal of Applied Physiology. 125 (5), 1475-1481 (2018).
- Shinin, V., Gayraud-Morel, B., Tajbakhsh, S. Template DNA-strand co-segregation and asymmetric cell division in skeletal muscle stem cells. Methods in Molecular Biology. 482, 295-317 (2009).
- Long, G. M., Troutman, A. D., Fisher, A., Brown, M. B., Coggan, A. R. Muscle fiber type differences in nitrate and nitrite storage and nitric oxide signaling in rats. bioRxiv. , (2020).
- Ohtake, K., et al. Dietary nitrite supplementation improves insulin resistance in type 2 diabetic KKA(y) mice. Nitric Oxide. 44, 31-38 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone