Method Article
A Two-Step Strategy that Combines Epigenetic Modification and Biomechanical Cues to Generate Mammalian Pluripotent Cells
W tym Artykule
Podsumowanie
We here present a method that combines the use of chemical epigenetic erasing with mechanosensing-related cues to efficiently generate mammalian pluripotent cells, without the need of gene transfection or retroviral vectors. This strategy is, therefore, promising for translational medicine and represents a notable advancement in stem cell organoid technology.
Streszczenie
Cell phenotype can be reversed or modified with different methods, with advantages and limitations that are specific for each technique. Here we describe a new strategy that combines the use of chemical epigenetic erasing with mechanosensing-related cues, to generate mammalian pluripotent cells. Two main steps are required. In the first step, adult mature (terminally differentiated) cells are exposed to the epigenetic eraser 5-aza-cytidine to drive them into a pluripotent state. This part of the protocol was developed, based on the increasing understanding of the epigenetic mechanisms controlling cell fate and differentiation, and involves the use of the epigenetic modifier to erase cell differentiated state and then drive into a transient high plasticity window.
In the second step, erased cells are encapsulated in polytetrafluoroethylene (PTFE) micro-bioreactors, also known as Liquid Marbles, to promote 3D cell rearrangement to extend and stably maintain the acquired high plasticity. PTFE is a non-reactive hydrophobic synthetic compound and its use permits the creation of a cellular microenvironment, which cannot be achieved in traditional 2D culture systems. This system encourages and boosts the maintenance of pluripotency though bio-mechanosensing-related cues.
The technical procedures described here are simple strategies to allow for the induction and maintenance of a high plasticity state in adult somatic cells. The protocol allowed the derivation of high plasticity cells in all mammalian species tested. Since it does not involve the use of gene transfection and is free of viral vectors, it may represent a notable technological advance for translational medicine applications. Furthermore, the micro-bioreactor system provides a notable advancement in stem cell organoid technology by in vitro re-creating a specific micro-environment that allows for the long-term culture of high plasticity cells, namely as ESCs, iPSCs, epigenetically erased cells and MSCs.
Wprowadzenie
During the last decades, the widely accepted concept of unidirectional progression towards cell commitment and differentiation was completely revised. It has been demonstrated that cell specification can be reversed, and a terminally differentiated cell can be pushed towards a less committed and higher permissive state, using different methods.
Among the several methods proposed, one of the most promising method involves the use of chemical compounds to induce cells into a so called chemically induced pluripotency. The small molecules used in this approach are able to interact and modify the epigenetic signature of an adult mature cell, avoiding the need of any transgenic and/or viral vector1,2,3,4,5,6,7,8,9,10. Numerous studies have recently shown that it is possible to switch cells from one phenotype to another by providing specific biochemical and biological stimuli that induce the reactivation of hypermethylated genes11,12,13,14,15. These demethylating events allow for the conversion of terminally differentiated cells into a primitive progenitor, a multipotent or a high plasticity/pluripotent cell1,2,3,4,5,6,7,8,9,10.
In parallel, many studies have been recently focussing on the understanding of mechanosensing-related cues and, more specifically, on the possibility to use mechanical forces to directly influence cell plasticity and/or differentiation16,17,18,19. Indeed, it has been clearly demonstrated that the extracellular matrix (ECM) plays a key role in the control of cell fate. In particular, the biomechanical and biophysical signals produced by ECM directly regulate molecular mechanisms and signaling pathways, influencing cell behavior and functions20,21. These recent data have paved the way to the development of novel 3D culture systems that more closely mimic the in vivo cell microenvironment, replicating mechanical and physical stimuli driving cell behaviour.
We here describe a two-step protocol that combines the use of chemical epigenetic erasing with mechanosensing-related cues, to generate mammalian pluripotent cells. In the first step, cells are incubated with the demethylating molecule 5-aza-cytidine (5-aza-CR). This agent is able to induce a significant global DNA demethylation through a combined effect of the direct ten-eleven translocation 2 (TET2)-mediated action8,10 and the indirect inhibition of the DNA methyltransferases (DNMT)22,23. This step induces the removal of the epigenetic blocks with a subsequent re-activation of pluripotency-related gene expression and, therefore, the generation of high plasticity cells1,2,3,8,10, hereinafter referred as “epigenetically erased cells”. In the second step, cells are encapsulated in a 3D culture system. To this end, the non-reactive hydrophobic synthetic compound polytetrafluoroethylene (PTFE; with particle size of 1 μm) is used as micro-bioreactor, that permits the creation of a cellular microenvironment unachievable through the use of traditional 2D culture systems10. The PTFE powder particles adhere to the surface of the liquid drop in which cells are re-suspended and isolate the liquid core from the supporting surface, while allowing gas exchange between the interior liquid and the surrounding environment24. The “PTFE micro-bioreactor” thus obtained, also known as “Liquid Marble”, encourages cells to freely interact with each other, promoting 3D cell rearrangement25,26,27, and extends and stably maintains the acquired high plasticity state though bio-mechanosensing-related cues10.
Protokół
All studies were reviewed and approved by the Ethical Committee of the University of Milan. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH). Human cells isolation from healthy adult individuals was approved by the Ethical Committee of the Ospedale Maggiore Policlinico, Milano. All the methods in our study were carried out in accordance with the approved guidelines.
1. Skin fibroblast isolation
NOTE: All the procedures described below can be applied to fibroblasts isolated from different mammalian species, including mouse, porcine, and human. Murine cells were isolated from 7-week-old male mice and porcine skin tissue were collected at local slaughterhouse. Human cells were isolated from adult patients, after written informed consent.
- Prepare 0.1% porcine gelatin solution.
- Weigh 0.1 g of porcine gelatin and dissolve it in 100 mL of water. Sterilize gelatin solution by autoclaving before use.
- Coat 35 mm Petri dish with 0.1% porcine gelatin by adding 1.5 mL of the prepared solution. Incubate for 2 h at room temperature.
- Cut mammalian (mouse, porcine, and human) skin biopsies of approximately 2-5 cm in length and place them in Dulbecco's Phosphate Buffered Saline (PBS) containing 2% antibiotic antimycotic solution. Store at + 4 °C until use.
NOTE: Biopsy collections must be carried out in agreement and after the Ethical Committee’s approval, in accordance with the established guidelines. - Extensively wash the collected biopsies three times in fresh sterile PBS containing 2% antibiotic antimycotic solution.
- Collect biopsies from the last wash and place them into a sterile 100 mm Petri dish. Use a sterile scalpel to cut them into pieces of approximately 2 mm3 size.
- At the end of 2 h incubation, remove the excess of gelatin solution from the 35 mm Petri dish (described in step 1.1.2) and, using a sterile surgical tweezer, immediately place 5-6 skin fragments into each pre-coated culture dish.
- Wet the fragments by adding 100 µL droplets of fibroblast isolation medium (Table 1) over each of them. Culture at 37 °C in 5% CO2 incubator.
NOTE: To prevent the medium from evaporation, place the 35 mm Petri dish within a 100 mm or bigger Petri dish containing sterile water. Ensure to cap both Petri dishes. - After 24 h of culture, check the quantity of the medium in the 35 mm culture Petri dish. If needed, add 500 µL of fibroblast isolation medium to keep wet the fragments.
- Carefully remove the medium and refresh it at least every 2 days of culture using a pipette.
- When fibroblasts start to grow out of the skin fragments placed in the 35 mm Petri dish (described in step 1.5.) and begin to form a cell monolayer (usually 6 days). Remove skin pieces using a sterile surgical tweezer and culture in 2 mL of fibroblast isolation medium.
- Continue to culture the cell monolayer at 37 °C in 5% CO2 incubator until 80% confluence and refresh medium every other day.
2. Fibroblast primary cell line culture
- When fibroblasts reach 80% confluence, carefully remove fibroblast isolation medium and wash cells three times with 3 mL of PBS containing 1% antibiotic antimycotic solution.
- For cell detaching, add 600 µL of 0.25% trypsin-EDTA solution in the culture dish and incubate at 37 °C for 3-5 min.
- Add 5.4 mL of fibroblast culture medium to neutralize trypsin when cells start to detach from the culture dish (Table 1).
- Dislodge cells by repeated and gentle pipetting. Plate cells in new culture dishes (without gelatin), keeping the passage ratio between 1:2 and 1:4 (depending on growth rate).
NOTE: Centrifugation is not necessary. - Maintain cells in culture and change medium every 2 days, until they have reached 80% confluency and passage them.
NOTE: Propagate fibroblasts twice a week to maintain vigorous growth.
3. Fibroblast exposure to 5-aza-CR
- Prepare fresh 1 mM 5-aza-CR stock solution.
- Weigh 2.44 mg of 5-aza-CR and dissolve it in 10 mL of DMEM high glucose. Resuspend the powder by vortexing. Sterilize the solution with 0.22 µm filter.
NOTE: 5-aza-CR stock solution must be prepared immediately before use. - Prepare 5-aza-CR working solution by diluting 1 µL of 5-aza-CR stock solution (3.1.1.) in 1 mL of fibroblast culture medium.
NOTE: The concentration of 5-aza-CR working solution is 1 μM1,2,3,8,9.
- Weigh 2.44 mg of 5-aza-CR and dissolve it in 10 mL of DMEM high glucose. Resuspend the powder by vortexing. Sterilize the solution with 0.22 µm filter.
- Trypsinize cells as previously described (2.1.-2.3.) and dislodge cells by repeatedly and gently pipetting.
- Collect the cell suspension and transfer it into a conical tube.
- Count cells using a counting chamber under an optical microscope at room temperature. Calculate the volume of medium needed to re-suspend cells to obtain 4 x 104 cells in 30 μL of fibroblast culture medium supplemented with 1 μM 5-aza-CR (see step 3.1.2.).
NOTE: The formula to be used depends on the specific type of chamber.
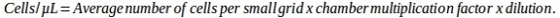
- Centrifuge the cell suspension at 150 x g for 5 min at room temperature. Remove the supernatant and resuspend pellet with the fibroblast culture medium supplemented with 1 μM 5-aza-CR (see step 3.1.2.). For the volume of the fibroblast culture medium to be used see step 3.4.
NOTE: As a negative control, resuspend cells ad the same concentration in fibroblast culture medium without 5-aza-CR and proceed with cell encapsulation in PTFE powder (step 4.1.-4.13.).
4. Fibroblast encapsulation in PTFE micro-bioreactors
- Fill a 35 mm Petri dish with polytetrafluoroethylene (PTFE) powder to produce a bed (Figure 1A).
NOTE: Use 35 mm bacteriology Petri dishes to avoid liquid marble adhesion. In order to obtain a thin hydrophobic and porous shell, use a PTFE powder with an average particle size of 1 μm and produced with a maximum grind of 2.0 NPIRI. This allows for the creation of gas-permeable liquid marbles. Furthermore, the translucent coating facilitates the observation of cell aggregation processes in real-time Larger particle size leads to high polydispersity that can cause elevated evaporation, deformity and loss of the spherical shape, and the premature dissolution of the micro-bioreactors. - Dispense 30 μL single droplet containing 4 x 104 cells (see steps 3.4.- 3.5.) onto the powder bed (Figure 1B).
- Gently rotate the 35 mm Petri dish in a circular motion to ensure that PFTE powder entirely cover the surface of the liquid drop to form a liquid marble micro-bioreactor (Figure 1C).
- Pick up the liquid marble micro-bioreactor using a 1,000 μL pipette tip, cut at the edge, to accommodate the diameter of the marble (Figure 1D,E). Plate the liquid marble micro-bioreactor onto a clean bacteriology Petri dish to stabilize it (Figure 1F).
NOTE: To create a friction to grip the marble inside the tip, cut the pipette tips with a diameter approximately slightly less than the liquid marble diameter. - Transfer the liquid marble micro-bioreactor from the Petri dish into a 96 well plate (one marble/well) (Figure 1G).
- Slowly add 100 μL of media from the margin of the well. The micro-bioreactor starts to float on top of the media (Figure 1H).
NOTE: The micro-bioreactor breaks in direct liquid contact, due to the disruption of PTFE hydrophobicity. As an alternative approach, the liquid marble micro-bioreactors can be individually placed in a 35 mm bacteriology culture dish. In this case, in order to prevent liquid marble evaporation, the 35 mm Petri dish containing the micro-bioreactor must be inserted in a 100 mm Petri dish, previously aliquoted with sterile water - Incubate liquid marble micro-bioreactor for 18 h at 37 °C in 5% CO2 incubator1,2,3,8,9.
NOTE: The PTFE particle size of 1 μm can ensure an optimal gas exchange between the interior liquid and the surrounding environment. - After 5-aza-CR incubation for 18 h, collect the liquid marble micro-bioreactor using a 1,000 μL pipette tip cut at the edge (see step 4.5).
- Place the micro-bioreactor in a new 35 mm bacteriology Petri dish (Figure 1D-F).
- Use a needle to puncture the liquid marble and break it.
- Recover formed spheroids with a 200 μL pipette tip, cut at the edge, under a stereomicroscope (Figure 1I,J).
NOTE: Epigenetically erased cells encapsulated in PTFE form a 3D spherical structure (one aggregate in each liquid marble). - To assess the acquisition of pluripotent state in response to 5-aza-CR, check the onset of the pluripotency- related gene expression, OCT4, NANOG, REX1, and SOX2, by qualitative PCR (Table 2).
- Proceed with the second step of the protocol as described below.
5. Culture in PTFE micro-bioreactors of epigenetically erased cells
- Prepare fresh ESC culture medium (Table 1).
- Transfer organoids in a Petri dish containing ESC medium for washing 5-aza-CR residuals (see steps 5.1.-5.2.).
- Prepare a new 35 mm bacteriology Petri dish containing PTFE powder bed (see also step 4.1.).
- Dispense a single organoid in a droplet of 30 μL ESC culture medium onto the powder bed using a 200 μL pipette tip, cut at the edge (see steps 4.9.; 5.3.).
- Gently rotate the 35 mm Petri dish in a circular motion to form a new liquid marble micro-bioreactor, pick up it using a 1,000 μL pipette tip, cut at the edge, and place the newly formed micro-bioreactor into a well of 96-well plate (one marble/well) (see steps 4.3.-4.6.).
- To float the micro-bioreactors, add 100 μL of media from the margin of the well to slowly bathe the marble (see note 4.7.).
- Culture liquid marble micro-bioreactors at 37 °C in 5% CO2 incubator for as long as required. Change medium every other day, following the procedure described in 5.3.-5.7.
NOTE: In the present manuscript, results obtained with organoids culture for 28 days are provided. However, if needed longer culture period can be performed.
Wyniki
The present protocol describes all the steps to be performed to generate and stably maintain mammalian pluripotent cells from adult somatic cells. This method has been successful with fibroblasts isolated from different mammalian species, namely mouse, porcine and human. The representative results here reported are obtained from all cell lines, irrespectively of the species of origin.
Morphological analyses show that, after 18 h incubation with the demethylating agent 5-aza-CR, fibroblasts encapsulated in PTFE micro-bioreactors (3D Post 5-aza-CR) aggregate and formed 3D spherical structures displaying a uniform size geometry, in all the three species considered. (Figure 2A-C, 3D Post 5-aza-CR). 86.31 ± 4.13% of encapsulated cells remarkably modified their phenotype, showing features typically related to a high plasticity phenotype8. In contrast, post 5-aza-CR cells cultured into 2D standard conditions, although replaced the typical fibroblast elongated shape with a round or oval one, were considerably smaller in size with larger and granulated nuclei, retain a monolayer distribution (Figure 2). The morphological changes were accompanied by the onset of pluripotency-related gene expression both in 3D and 2D Post 5-aza-CR cells. Transcription for POU class 5 homeobox 1 (OCT4), Nanog homeobox (NANOG), ZFP42 zinc finger protein (REX1), and sex determining region Y-box 2 (SOX2) was also observed, which is absent in untreated fibroblasts (T0), was detected (Figure 3, Figure 4, Figure 5). Furthermore, quantitative PCR analysis demonstrated a significant up-regulation of the above mentioned genes, as well as of the ten-eleven translocation-2 (TET2), epithelial cell adhesion molecule (EPCAM), and cadherin 1 (CDH1) genes in 3D Post 5-aza-CR cells (Figure 3, Figure 4, Figure 5, blue bars) compared to cells cultured in 2D standard plastic dishes (2D Post 5-aza-CR) (Figure 3, Figure 4, Figure 5, orange bars). In parallel, a significant downregulation of the fibroblast specific marker Thy-1 cell surface antigen (THY1) was clearly detectable in 3D and 2D Post 5-aza-CR cells (Figure 3, Figure 4, Figure 5).
The achievement of a high plasticity state was also confirmed by ELISA analysis of DNA global methylation, that demonstrates a significant decrease of methylation levels in both 3D and 2D Post 5-aza-CR cells (Figure 6A-C). Moreover, in agreement with the gene expression results, DNA methylation levels were significantly lower in 3D Post 5-aza-CR cells (Figure 6A-C, blue bars), compared to 2D Post 5-aza-CR ones (Figure 6A-C, orange bars).
Even more interestingly, 3D Post 5-aza-CR cells retain the acquired 3D spherical structure (Figure 2A, 3D 28d) and maintain high expression levels of pluripotency-related genes (Figure 3, Figure 4, and Figure 5 blue bars) as well as low DNA methylation levels (Figure 6A-C, blue bars), during all the subsequent culture period and, specifically, until 28 day, when culture was arrested. In contrast, although 2D Post 5-aza-CR cells transcribe for the same pluripotency genes after treatment with the demethylating agent, they turned down such expression by day 7 (Figure 3, Figure 4, Figure 5, nd). Similarly, the decrease in methylation levels was maintained for the first 72 hours; then methylation slowly increased, returning comparable to untreated fibroblasts (Figure 6A-C, T0, white bars) by day 7 of culture (Figure 6A-C, orange bars).

Figure 1: Cell encapsulation in PTFE micro-bioreactor and organoid recovery. (A) A bacteriology Petri dish was filled with PTFE to prepare a powder bed. (B) A single droplet of medium containing cells was dispensed on top of the PTFE bed. (C) The Petri dish was gently rotated with circular movements to coat the droplet and produce the micro-bioreactor. (D) A 1000 μL pipette tip was cut at the end (red arrow) and (E) used to collect the micro-bioreactor. (F) The liquid marble was transferred to a clean Petri dish to stabilize it, (G) placed into a 96 well plate (one marble/well) and (H) floated onto the media. (I) To collect newly formed organoid, the micro-bioreactor was punctured with a needle and (J) the obtained cell aggregates were recovered under a stereomicroscope. Please click here to view a larger version of this figure.
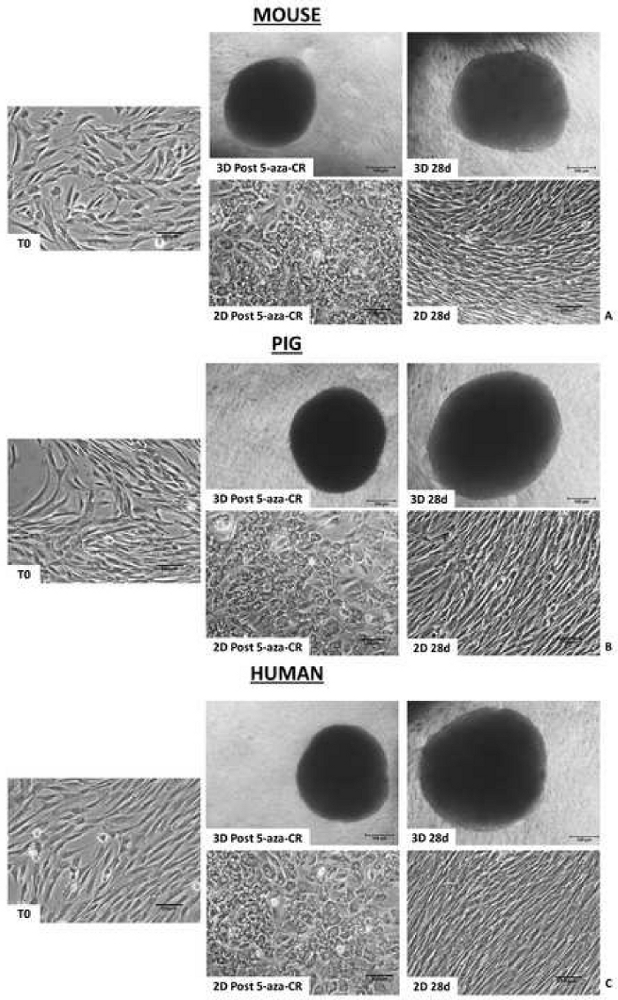
Figure 2: Mammalian epigenetically erased cells encapsulated in PTFE micro-bioreactors form 3D spherical structures. Murine (A), porcine (B) and human (C) cells encapsulated in PTFE and treated with 5-aza-CR form 3D spherical structures (3D Post 5-aza-CR), that were stably maintained during all the subsequent culture period (3D 28d; Scale bar, 100 μm). In contrast, murine (A), porcine (B) and human (C) cells plated onto plastic dishes and treated with 5-aza-CR replaced the typical fibroblast elongated shape (T0) into a round epithelioid phenotype and retained a monolayer distribution (2D Post 5-aza-CR). By day 7 of culture, 2D cells reverted to their original elongated shape which was stably maintained for the subsequent culture period (2D 28 d; Scale bar, 100 μm). Please click here to view a larger version of this figure.

Figure 3: Murine epigenetically erased cells encapsulated in PTFE micro-bioreactors show high level and long-term maintenance of pluripotency-related gene expression. Transcription levels for Oct4, Nanog, Rex1, Sox2, Tet2, Epcam, Cdh1 and Thy1 genes in murine untreated fibroblasts (T0, white bars), fibroblasts exposed to 5-aza-CR (Post 5-aza-CR), and at different time points of culture for PTFE encapsulated (blue bars) and standard plastic dish (orange bars) cultured cells. Gene expression values are reported with the highest expression set to 1 and all others relative to this. Different superscripts denote significant differences (P < 0.05). Please click here to view a larger version of this figure.
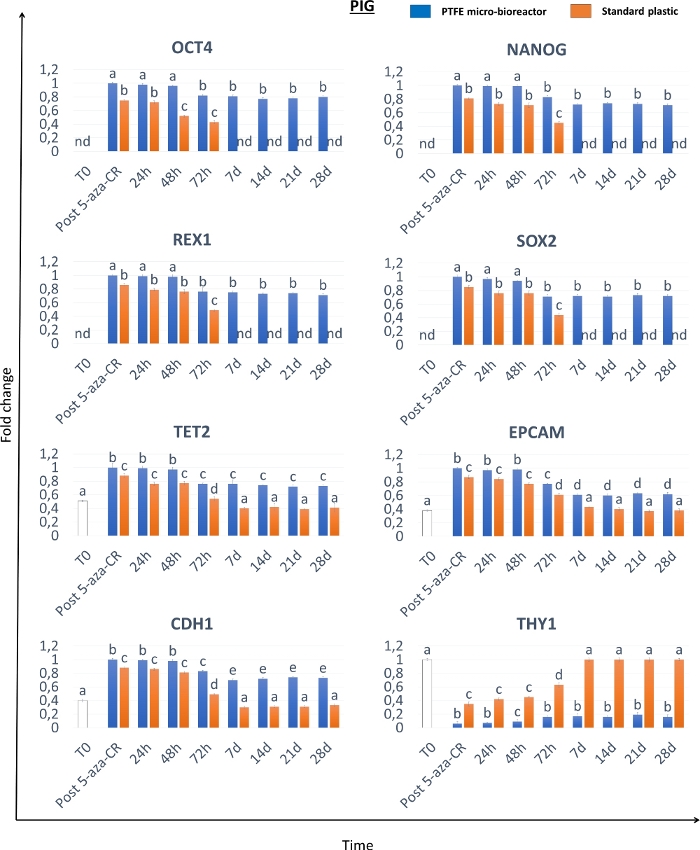
Figure 4: Porcine epigenetically erased cells encapsulated in PTFE micro-bioreactors show high level and long-term maintenance of pluripotency-related gene expression. Transcription levels for OCT4, NANOG, REX1, SOX2, TET2, EPCAM, CDH1 and THY1 genes in porcine untreated fibroblasts (T0, white bars), fibroblasts exposed to 5-aza-CR (Post 5-aza-CR), and at different time points of culture for PTFE encapsulated (blue bars) and standard plastic dish (orange bars) cultured cells. Gene expression values are reported with the highest expression set to 1 and all others relative to this. Different superscripts denote significant differences (P < 0.05). Please click here to view a larger version of this figure.

Figure 5: Human epigenetically erased cells encapsulated in PTFE micro-bioreactors show high level and long-term maintenance of pluripotency-related gene expression. Transcription levels for OCT4, NANOG, REX1, SOX2, TET2, EPCAM, CDH1 and THY1 genes in human untreated fibroblasts (T0, white bars), fibroblasts exposed to 5-aza-CR (Post 5-aza-CR), and at different time points of culture for PTFE encapsulated (blue bars) and standard plastic dish (orange bars) cultured cells. Gene expression values are reported with the highest expression set to 1 and all others relative to this. Different superscripts denote significant differences (P < 0.05). Please click here to view a larger version of this figure.
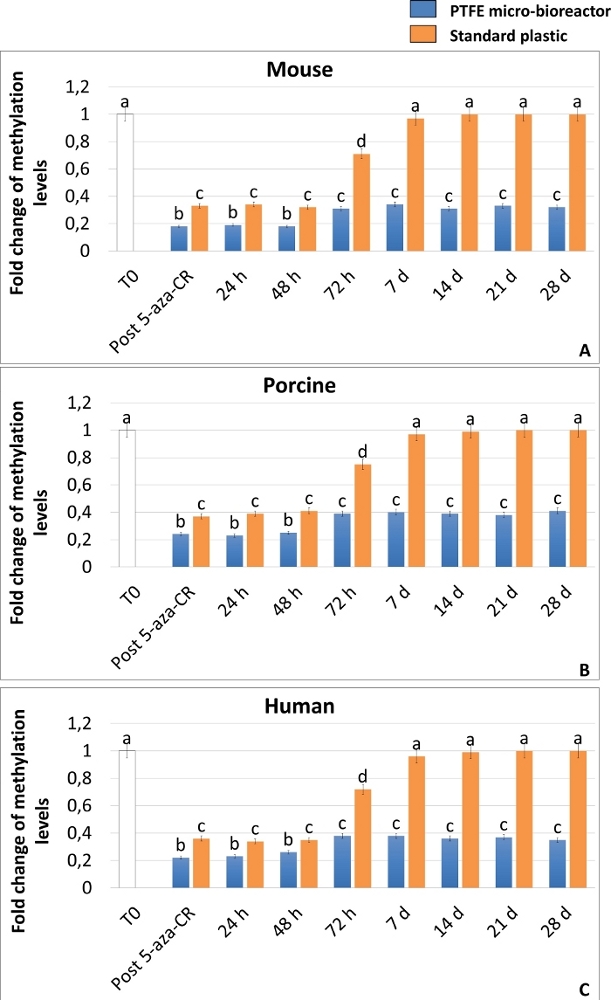
Figure 6: The PTFE micro-bioreactor enhances 5-aza-CR demethylating effect and maintains long-term DNA hypomethylation in mammalian epigenetically erased fibroblasts. Global DNA methylation levels of murine (A), porcine (B) and human (C) cells encapsulated in PTFE micro-bioreactors (blue bars) or plated on standard plastic (orange bars) exposed to 5-aza-CR (Post 5-aza-CR) and cultured in ESC medium for 28 days. Untreated fibroblasts (T0; white bars). Results represent the mean ± SD of three independent experiments with five independent biological replicates. Different superscripts denote significant differences (P < 0.05). Please click here to view a larger version of this figure.
| Fibroblast Isolation Medium (100 mL) | |
| DMEM, high glucose, pyruvate | 77 mL |
| Fetal Bovine Serum, qualified, heat inactivated | 20 mL |
| L-Glutamine solution | 1 mL |
| Antibiotic Antimycotic Solution (100×) | 2 mL |
| Fibroblast Culture Medium (100 mL) | |
| DMEM, high glucose, pyruvate | 88 mL |
| Fetal Bovine Serum, qualified, heat inactivated | 10 mL |
| L-Glutamine solution | 1 mL |
| Antibiotic Antimycotic Solution (100×) | 1 mL |
| ESC culture medium (10 mL) | |
| Ham's F-10 Nutrient Mix | 3,99 mL |
| DMEM, low glucose, pyruvate | 3,99 mL |
| KnockOut Serum Replacement | 1 mL |
| Fetal Bovine Serum, qualified, heat inactivated | 500 µL |
| Antibiotic Antimycotic Solution (100×) | 100 µL |
| L-Glutamine solution | 100 µL |
| MEM Non-Essential Amino Acids Solution (100X) | 100 µL |
| 2-Mercaptoethanol (10 mM) | 100 µL (0.1 mM) |
| Nucleoside mix (0.3 M Guanosine, 0.3 M Adenosine, 0.3 M Cytidine, 0.3 M Uridine and 0.1 M Timidine) | 100 µL (3 mM Guanosine, 3 mM Adenosine, 3 mM Cytidine, 3 mM Uridine and 1 mM Timidine) |
| ESGRO Recombinant Mouse LIF Protein (1000 units/mL) | 10 µL (1 unit/mL) |
| Recombinant Human FGF basic (bFGF) (5 µg/mL) | 10 µL (5 ng/mL) |
Table 1: Composition of fibroblast isolation medium, fibroblast culture medium and ESC culture medium.
| Target | PCR Primer Sets | Species | |
| Cdh1 | Forward: GAGGAACCCACAGCCTCATA | Mouse | |
| Reverse: GTTGACCGTCCCTTCACAGT | Mouse | ||
| Epcam | Forward: TGGCAACAAGTTGCTCTCTG | Mouse | |
| Reverse: CTTGTCGGTTCTTCGGACTC | Mouse | ||
| Nanog | Forward: AGGCTGATTTGGTTGGTGTC | Mouse | |
| Reverse: CCAGGAAGACCCACACTCAT | Mouse | ||
| Oct4 | Forward: ACACCTGGCTTCAGACTTCG | Mouse | |
| Reverse: AGTTGCTTTCCACTCGTGCT | Mouse | ||
| Rex1 | Forward: CAGGTTCTGGAAGCGAGTTC | Mouse | |
| Reverse: GACAAGCATGTGCTTCCTCA | Mouse | ||
| Sox2 | Forward: AGAACCCCAAGATGCACAAC | Mouse | |
| Reverse: CTCCGGGAAGCGTGTACTTA | Mouse | ||
| Tet2 | Forward: GCACAGGGAGCAAGAGATTC | Mouse | |
| Reverse: ATGTTGACATTGCCAGTGGA | Mouse | ||
| Thy1 | Forward: AACTCTTGGCACCATGAACC | Mouse | |
| Reverse: GCACGTGCTTCCTCTTCTCT | Mouse | ||
| CDH1 | Forward: GAATGACAATGGCCCCATAC | Porcine | |
| Reverse: AGGTGGTCACCTGGTCTTTG | Porcine | ||
| EPCAM | Forward: GCTTGGTCAGTGCCAGTGTA | Porcine | |
| Reverse: CTTCTGACCCCAGCAGTGTT | Porcine | ||
| NANOG | Forward: ATCCAGCTTGTCCCCAAAG | Porcine | |
| Reverse: ATTTCATTCGCTGGTTCTGG | Porcine | ||
| OCT4 | Forward: GTTCAGCCAAACGACCATCT | Porcine | |
| Reverse: CTCCAGGTTGCCTCTCACTC | Porcine | ||
| REX1 | Forward: CTTCAAGGAGAGCGCAAAAC | Porcine | |
| Reverse: TGTCCCCAATCAAAAATGCT | Porcine | ||
| SOX2 | Forward: GCCCTGCAGTACAACTCCAT | Porcine | |
| Reverse: GCTGATCATGTCCCGTAGGT | Porcine | ||
| TET2 | Forward: CAGAAAACAATGCAGCCAGA | Porcine | |
| Reverse: GAATGGCTCGGTCTCTGAAG | Porcine | ||
| THY1 | Forward: GGCATCGCTCTCTTGCTAAC | Porcine | |
| Reverse: GCTGGAGAAGTTGGTTCGAG | Porcine | ||
| CDH1 | Forward: TGCCCAGAAAATGAAAAAGG | Human | |
| Reverse: GTGTATGTGGCAATGCGTTC | Human | ||
| EPCAM | Forward: GCTGGTGTGTGAACACTGCT | Human | |
| Reverse: ACGCGTTGTGATCTCCTTCT | Human | ||
| NANOG | Forward: AGAAAAACAACTGGCCGAAG | Human | |
| Reverse: TGCTCCAGGACTGGATGTTC | Human | ||
| OCT4 | Forward: AATTTGCCAAGCTCCTGAAG | Human | |
| Reverse: GTTGCCTCTCACTCGGTTCT | Human | ||
| REX1 | Forward: ACGTTTCGTGTGTCCCTTTC | Human | |
| Reverse: TATAACCGCTTTTGGGGTTG | Human | ||
| SOX2 | Forward: ACACCAATCCCATCCACACT | Human | |
| Reverse: GCAAACTTCCTGCAAAGCTC | Human | ||
| TET2 | Forward: CCCACTTACCTGCGTTTCAT | Human | |
| Reverse: ACTGTGACCTTTCCCCACTG | Human | ||
| THY1 | Forward: AGATCCCAGAACCATGAACC | Human | |
| Reverse: GCACGTGCTTCTTTGTCTCA | Human | ||
| Target | TaqMan Assays Catalog Number | Species | |
| Actb | Mm02619580_g1 | Mouse | |
| Cdh1 | Mm01247357_m1 | Mouse | |
| Epcam | Mm00493214_m1 | Mouse | |
| Gapdh | Mm99999915_g1 | Mouse | |
| Nanog | Mm02019550_s1 | Mouse | |
| Oct4 | Mm03053917_g1 | Mouse | |
| Rex1 | Mm03053975_g1 | Mouse | |
| Sox2 | Mm03053810_s1 | Mouse | |
| Tet2 | Mm00524395_m1 | Mouse | |
| Thy1 | Mm00493681_m1 | Mouse | |
| ACTB | Ss03376563_uH | Porcine | |
| CDH1 | Ss06942341_m1 | Porcine | |
| EPCAM | Ss03384752_u1 | Porcine | |
| GAPDH | Ss03375629_u1 | Porcine | |
| NANOG | Ss04245375_s1 | Porcine | |
| OCT4 | Ss03389800_m1 | Porcine | |
| REX1 | Ss03373622_g1 | Porcine | |
| SOX2 | Ss03388002_u1 | Porcine | |
| TET2 | Ss06880359_m1 | Porcine | |
| THY1 | Ss03376963_u1 | Porcine | |
| ACTB | Hs01060665_g1 | Human | |
| CDH1 | Hs01023895_m1 | Human | |
| EPCAM | Hs00901885_m1 | Human | |
| GAPDH | Hs02786624_g1 | Human | |
| NANOG | Hs02387400_g1 | Human | |
| OCT4 | Hs00999632_g1 | Human | |
| REX1 | Hs00399279_m1 | Human | |
| SOX2 | Hs01053049_s1 | Human | |
| TET2 | Hs00325999_m1 | Human | |
| THY1 | Hs00174816_m1 | Human | |
Table 2: Primer information.
Dyskusje
During the last decades, several studies focused on the development of strategies to revert a terminally differentiated cell towards a less committed and higher permissive state. The protocol here described allow the generation and long-term maintenance of pluripotent cells starting from adult mature terminally differentiated cells. The method combines two independent steps that involve the induction of a high permissive state which is achieved through chemical epigenetic erasing and its subsequent maintenance ensured using a 3D culture system.
The formation of 3D spheroid structures observed in PTFE encapsulated cells (Figure 2) is consistent with other studies demonstrating PTFE ability to efficiently encourage cell aggregation, facilitating the establishment of olfactory ensheathing cell (OEC) spheroid structures25 or the formation of 3D toroidal aggregates26. These morphological changes are paralleled by the onset of pluripotency-related gene expression (Figure 3, Figure 4, Figure 5) that shows significantly higher levels in 3D Post 5-aza-CR cells, when compared to 2D Post 5-aza-CR cells (Figure 3, Figure 4, Figure 5). Consistently, 3D Post 5-aza-CR cells display a higher DNA hypomethylation than 2D Post 5-aza-CR ones (Figure 6). Overall, these results indicate 5-aza-CR ability to induce a high plasticity state, regardless to the cell culture system used. However, the chemically induced pluripotency state achieved by the cells, is significantly promoted using a PTFE micro-bioreactor which boosts pluripotency gene transcription and increases 5-aza-CR demethylating effects. Even more interestingly, only 3D Post 5-aza-CR cells stably retain the acquired 3D spherical structure (Figure 2) and maintain high expression levels of pluripotency-related genes (Figure 3, Figure 4, Figure 5) as well as low DNA methylation levels (Figure 6), during all the subsequent period of culture. Altogether, the representative results here reported demonstrate that this two-step strategy is highly efficient and robust, and the use of a PTFE micro-bioreactor not only boosts high plasticity, but also allows its stable, long-term maintenance in the mammalian species considered. We recently demonstrated that these beneficial effects are related to the activation of the Hippo-signaling pathway and its mechanotransduction-related cues10, that have been previously shown to have a key role in the active regulation of cell pluripotency28,29,30.
The two most critical steps for a successful procedure are the rigorous maintenance of cells at 37 °C, at all times, including their handling under the sterile laminar flow and the microscope and the use of a correct cell number/liquid volume rate during micro-bioreactor production, that may vary according to the specific cell type used. In our experience, it is also highly recommended to prepare reagents freshly, prior to their use in culture (this is absolutely crucial for 5-aza-CR stock solution). Furthermore, medium refreshing must be carried out under a stereomicroscope since the 3D spherical organoids may be lost during medium changes.
The main strengths of this method are no transgenic and/or viral vector requirement; the robustness and reproducibility in different mammalian species; low costs; and high flexibility to different cell types. On the other hand, a possible limitation could be represented by the restricted number of data obtained, due to the small volumes of the micro-bioreactors. In addition, the use of high cell density may cause low oxygen transfer rates and limited growth in suspension. To overcome these problems, further work on scale-up and/or scale-down strategies remains necessary.
It is important to highlight that the key aspects common to all 3D spheroid-based applications are the reproducibility, the production efficiency, the organoid size uniformity and the influence on cellular physiology. These features are strictly correlated to the mechanical forces generated within the culture system and vary according to the different methods used. For instance, multicellular organoids can be cultured using non-adherent dishes in stationary systems. This approach is primarily based on diffusion-limited conditions and, usually, results in the formation of loose-aggregated clusters. The hanging-drop technique shows the same limitation. Indeed, the deposition of cell suspension drops onto the underside of the lid of a tissue culture dish leads to the creation of microgravity environment that concentrates cells at the free liquid-air interface, inducing the generation of low-aggregated multicellular spheres. A possible alternative is represented by the spinner flask technique. However, this method is highly expensive since it requires elevated quantities of culture medium. Furthermore, the formed organoids need to be transferred to stationary culture system when used for characterization or further in vitro tests. All these issues can be overcome by using liquid marble micro-bioreactors. Indeed, they provide a non-adherent liquid surface that combines the advantages of both stationary and spinning methods, inducing a rapid cell aggregation and the generation of compact spheroids. In parallel, the concave bottom, the spherical shape, and the internal liquid flow of each marble allow cells to settle onto the bottom of the micro-bioreactor, resulting in the formation of organoids uniform in size and shape. Another significant advantage in the use of the liquid marbles is represented by the optimal gas exchanges that, thanks to their spherical shape, can occur through the entire surface.
In conclusion, the protocol here described allows for an efficient and simple generation of mammalian pluripotent cells. Since this strategy is viral vector free and does not involve the use of any gene transfection, it is highly promising for translational medicine applications and may be considered a step forward in patient-specific cell therapy. Furthermore, the use of 3D micro-bioreactor culture systems may represent a notable breakthrough in stem cell organoid technology and may constitute an advantageous micro-environment for long-term culture of different cell types, such as ESCs, iPSCs, and MSCs. An additional advantage is represented by the small volume that allows to study the effect of paracrine/autocrine signalling of the rich environment established within the micro-bioreactor.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was funded by Carraresi Foundation and MiND FoodS Hub ID: 1176436. All the authors are members of the COST Action CA16119 In vitro 3-D total cell guidance and fitness (CellFit).
Materiały
| Name | Company | Catalog Number | Comments |
| 2-Mercaptoethanol | Sigma-Aldrich | M7522 | Component of ESC medium |
| 5-Azacytidine | Sigma-Aldrich | A2385 | 5-aza-CR, for fibroblast epigenetic erasing |
| Adenosine | Sigma-Aldrich | A4036 | Component of nucleoside mix for ESC medium |
| Antibiotic Antimycotic Solution (100×) | Sigma-Aldrich | A5955 | Component of fibroblast and ESC media |
| CFX96 Real-Time PCR | Bio-Rad Laboratories | NA | Thermal cycler for quantitative PCR |
| Cytidine | Sigma-Aldrich | C4654 | Component of nucleoside mix for ESC medium |
| DMEM, high glucose, pyruvate | Thermo Fisher Scientific | 41966052 | For fibroblast isolation and culture medium |
| DMEM, low glucose, pyruvate | Thermo Fisher Scientific | 31885023 | For ESC medium |
| Dulbecco’s Phosphate Buffered Saline | Sigma-Aldrich | D5652 | PBS; for biopsy and cell wash and for solution preparation |
| Dynabeads mRNA DIRECT Micro Purification Kit | Thermo Fisher Scientific | 61021 | mRNA estraction |
| ESGRO Recombinant Mouse LIF Protein | Sigma-Aldrich | ESG1106 | Component of ESC medium |
| Fetal Bovine Serum, qualified, heat inactivated | Thermo Fisher Scientific | 10500064 | Component of fibroblast and ESC media |
| FGF-Basic (AA10-155) Recombinant Human Protein | Thermo Fisher Scientific | PHG0024 | Component of ESC medium |
| Gelatin from porcine skin | Sigma-Aldrich | G1890 | For dish coating |
| GeneAmp PCR System 2700 | Applied Biosystems | NA | Thermal cycler for qualitative PCR |
| Global DNA Methylation ELISA Kit | CELL BIOLABS | STA-380 | Methylation study |
| GoTaq G2 Flexi DNA Polymerase | Promega | M7801 | Qualitative PCR |
| Guanosine | Sigma-Aldrich | G6264 | Component of nucleoside mix for ESC medium |
| Ham's F-10 Nutrient Mix | Thermo Fisher Scientific | 31550031 | For ESC medium |
| KnockOut Serum Replacement | Thermo Fisher Scientific | 10828028 | Component of ESC medium |
| KOVA glasstic slide 10 with grids | Hycor Biomedical | 87144 | For cell counting |
| Leica MZ APO Stereo Microscope | Leica | NA | For organoid observation |
| L-Glutamine solution | Sigma-Aldrich | G7513 | Component of fibroblast and ESC media |
| MEM Non-Essential Amino Acids Solution (100X) | Thermo Fisher Scientific | 11140035 | Component of ESC medium |
| Millex-GS 0.22 µm pore filters | Millipore | SLGS033SB | For solution sterilization |
| M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant | Promega | M3681 | mRNA reverse transcription |
| Multiskan FC Microplate Photometer | Thermo Fisher Scientific | 51119000 | For ELISA plate reading |
| Nikon Eclipse TE300 Inverted Phase Contrast Microscope | Nikon | NA | For cell observation |
| Perkin Elmer Thermal Cycler 480 | Perkin Elmer | NA | Thermal cycler for reverse transcription |
| Poly(tetrafluoroethylene) 1 μm particle size | Sigma-Aldrich | 430935 | For generating micro-bioreactor |
| PureLink Genomic DNA Mini Kit | Thermo Fisher Scientific | K182001 | Genomic DNA estraction |
| TaqMan Gene Expression Cells-to-CT Kit | Thermo Fisher Scientific | AM1728 | Quantitative PCR |
| Thymidine | Sigma-Aldrich | T1895 | Component of nucleoside mix for ESC medium |
| Tissue Culture Dish 100X20 mm, Standard | Sarstedt | 833902 | For fibroblast isolation |
| Tissue Culture Dish 35X10 mm, Standard | Sarstedt | 833900 | For Fibroblast isolation |
| Tissue Culture Dish 35X10 mm, Suspension | Sarstedt | 833900500 | Bacteriology petri dish for liquid marble culture |
| Tissue Culture Plate 96 Well,Standard,F | Sarstedt | 833924005 | For liquid marble culture |
| Trypsin-EDTA solution | Sigma-Aldrich | T3924 | For fibroblast dissociation |
| Tube 15ml, 120x17mm, PS | Sarstedt | 62553041 | For cell suspension centrifugation |
| Uridine | Sigma-Aldrich | U3003 | Component of nucleoside mix for ESC medium |
Odniesienia
- Pennarossa, G., et al. Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proceedings of the National Academy of Sciences of the United States of America. 110 (22), 8948-8953 (2013).
- Pennarossa, G., et al. Reprogramming of pig dermal fibroblast into insulin secreting cells by a brief exposure to 5-aza-cytidine. Stem Cell Reviews and Reports. 10 (1), 31-43 (2014).
- Brevini, T. A. L., et al. Morphological and molecular changes of human granulosa cells exposed to 5-azacytidine and addressed toward muscular differentiation. Stem Cell Reviews and Reports. 10 (5), 633-642 (2014).
- Mirakhori, F., Zeynali, B., Kiani, S., Baharvand, H. Brief azacytidine step allows the conversion of suspension human fibroblasts into neural progenitor-like cells. Cell Journal. 17 (1), 153-158 (2015).
- Tan, S. J., et al. Muscle tissue engineering and regeneration through epigenetic reprogramming and scaffold manipulation. Scientific Reports. 5, 16333 (2015).
- Brevini, T. A. L., Pennarossa, G., Acocella, F., Brizzola, S., Zenobi, A., Gandolfi, F. Epigenetic conversion of adult dog skin fibroblasts into insulin-secreting cells. Veterinary Journal. 211, 52-56 (2016).
- Chandrakanthan, V., et al. PDGF-AB and 5-Azacytidine induce conversion of somatic cells into tissue-regenerative multipotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 113 (16), 2306-2315 (2016).
- Manzoni, E. F. M., et al. 5-azacytidine affects TET2 and histone transcription and reshapes morphology of human skin fibroblasts. Scientific Reports. 6, 37017 (2016).
- Pennarossa, G., et al. Epigenetic erasing and pancreatic differentiation of dermal fibroblasts into insulin-producing cells are boosted by the use of low-stiffness substrate. Stem Cell reviews and reports. 14 (3), 398-411 (2018).
- Pennarossa, G., et al. Use of a PTFE micro-bioreactor to promote 3D cell rearrangement and maintain high plasticity in epigenetically erased fibroblasts. Stem Cell Reviews and Reports. 15 (1), 82-92 (2019).
- Constantinides, P. G., Jones, P. A., Gevers, W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 267 (5609), 364-366 (1977).
- Taylor, S. M., Jones, P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 17 (4), 771-779 (1979).
- Taylor, S. M., Constantinides, P. A., Jones, P. A. 5-Azacytidine, DNA methylation, and differentiation. Current Topics in Microbiology and Immunology. 108, 115-127 (1984).
- Jones, P. A. Effects of 5-azacytidine and its 2'-deoxyderivative on cell differentiation and DNA methylation. Pharmacology & Therapeutics. 28 (1), 17-27 (1985).
- Palii, S. S., Van Emburgh, B. O., Sankpal, U. T., Brown, K. D., Robertson, K. D. DNA methylation inhibitor 5-Aza-2'-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Molecular and Cellular Biology. 28 (2), 752-771 (2008).
- Vining, K. H., Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews. Molecular Cell Biology. 18 (12), 728 (2017).
- Matamoro-Vidal, A., Levayer, R. Multiple influences of mechanical forces on cell competition. Current Biology. 29 (15), 762-774 (2019).
- Yim, E. K., Sheetz, M. P. Force-dependent cell signaling in stem cell differentiation. Stem Cell Research & Therapy. 3 (5), 41 (2012).
- Kumar, A., Placone, J. K., Engler, A. J. Understanding the extracellular forces that determine cell fate and maintenance. Development. 144 (23), 4261-4270 (2017).
- Bissell, M. J., Rizki, A., Mian, I. S. Tissue architecture: the ultimate regulator of breast epithelial function. Current Opinion in Cell Biology. 15 (6), 753-762 (2003).
- Streuli, C. H., et al. Laminin mediates tissue-specific gene expression in mammary epithelia. The Journal of Cell Biology. 129 (3), 591-603 (1995).
- Christman, J. K. 5-Azacytidine and 5-aza-2[prime]-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 21, 5483-5495 (2002).
- Stresemann, C., Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. International Journal of Cancer. 123 (1), 8-13 (2008).
- Ledda, S., Idda, A., Kelly, J., Ariu, F., Bogliolo, L., Bebbere, D. A novel technique for in vitro maturation of sheep oocytes in a liquid marble microbioreactor. Journal of Assisted Reproduction and Genetics. 33 (4), 513-518 (2016).
- Vadivelu, R. K., et al. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Scientific Reports. 5, 15083 (2015).
- Vadivelu, R. K., Kamble, H., Munaz, A., Nguyen, N. T. Liquid Marble as bioreactor for engineering three-dimensional toroid tissues. Scientific Reports. 7 (1), 12388 (2017).
- Vadivelu, R. K., Kamble, H., Munaz, A., Nguyen, N. T. Liquid Marbles as bioreactors for the study of three-dimensional cell interactions. Biomedical Microdevices. 19 (2), 31 (2017).
- Varelas, X., et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biology. 10 (7), 837-848 (2008).
- Panciera, T., et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell. 19 (6), 725-737 (2016).
- Pennarossa, G., Paffoni, A., Ragni, G., Gandolfi, F., Brevini, T. A. L. Rho signaling-directed YAP/TAZ regulation encourages 3D spheroid colony formation and boosts plasticity of parthenogenetic stem cells. Advances in Experimental Medicine and Biology. 1237, 49-60 (2020).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone