Method Article
Transfer of Manipulated Tumor-associated Neutrophils into Tumor-Bearing Mice to Study their Angiogenic Potential In Vivo
W tym Artykule
Podsumowanie
Here, we show therapeutic potential of anti-angiogenic tumor-associated neutrophils after their transfer into tumor-bearing mice. This protocol can be used to manipulate neutrophil activity ex vivo and to subsequently evaluate their functionality in vivo in developing tumors. It is an appropriate model for studying potential neutrophil-based immunotherapies.
Streszczenie
The contribution of neutrophils to the regulation of tumorigenesis is getting increased attention. These cells are heterogeneous, and depending on the tumor milieu can possess pro- or anti-tumor capacity. One of the important cytokines regulating neutrophil functions in a tumor context are type I interferons. In the presence of interferons, neutrophils gain anti-tumor properties, including cytotoxicity or stimulation of the immune system. Conversely, the absence of an interferon signaling results in prominent pro-tumor activity, characterized with strong stimulation of tumor angiogenesis. Recently, we could demonstrate that pro-angiogenic properties of neutrophils depend on the activation of nicotinamide phosphoribosyltransferase (NAMPT) signaling pathway in these cells. Inhibition of this pathway in tumor-associated neutrophils leads to their potent anti-angiogenic phenotype. Here, we demonstrate our newly established model allowing in vivo evaluation of tumorigenic potential of manipulated tumor-associated neutrophils (TANs). Shortly, pro-angiogenic tumor-associated neutrophils can be isolated from tumor-bearing interferon-deficient mice and repolarized into anti-angiogenic phenotype by blocking of NAMPT signaling. The angiogenic activity of these cells can be subsequently evaluated using an aortic ring assay. Anti-angiogenic TANs can be transferred into tumor-bearing wild type recipients and tumor growth should be monitored for 14 days. At day 14 mice are sacrificed, tumors removed and cut with their vascularization assessed. Overall, our protocol provides a novel tool to in vivo evaluate angiogenic capacity of primary cells, such as tumor-associated neutrophils, without a need to use artificial neutrophil cell line models. vc
Wprowadzenie
Type I Interferons (IFNs) play an important role in the stimulation of host responses to neoplasias, as the lack of type I IFN signaling results in significantly elevated tumor growth1. One of the mechanisms involved in this process is the regulation of tumorigenic activity of tumor-associated neutrophils, which is controlled by colony-stimulating factor 3 receptor (CSF3R) downstream signaling2. Colony-stimulating factor 3 (CSF3), or granulocyte colony-stimulating factor, was shown to activate signaling involving nicotinamide phosphoribosyltransferase (NAMPT)3,4. NAMPT is a rate-limiting enzyme for nicotinamide adenine dinucleotide synthesis, which enhances glycolysis and regulates DNA repair, gene expression, and stress response promoting cancer cells survival and proliferation5. NAMPT is overexpressed in multiple cancer types, including colorectal, ovarian, breast, gastric, prostate cancer and gliomas6. NAMPT is essential not only for tumor cells, but also for a wide variety of other cell types that are present in tumors, such as myeloid cells - it drives their differentiation4, inhibits apoptosis and stimulate expression of multiple cytokines or matrix-degrading enzymes in macrophages7.
Tumor-associated neutrophils represent important modulators of tumor growth. TAN functions are strongly dependent on the type I IFN availability, as these cytokines prime anti-tumor activity of neutrophils. To the contrary, the absence of IFNs supports tumorigenic activation of these cells, especially their pro-angiogenic properties. In agreement with this, mice deficient in IFNs develop significantly larger and better vascularized tumors, which are strongly infiltrated with pro-tumoral/pro-angiogenic neutrophils1,2,8,9,10. Importantly, such pro-angiogenic TANs show elevated activity of NAMPT, suggesting its essential role in pro-tumor polarization of neutrophils.
Depletion of neutrophils using Ly6G antibody or inhibition of their migration (CXCR2 antibody) results in decreased tumor angiogenesis, growth, and metastasis1,8. Nevertheless, generated monoclonal antibodies are immunogenic, and their administration is associated with a range of life-threatening side effects11. Treatment with small molecules, such as NAMPT inhibitor FK866, that modulate neutrophil tumoriogenicity, could help to avoid such complications. Unfortunately, pharmacological systemic inhibition of NAMPT, next to its therapeutic effect on tumor growth, leads to severe side effects including gastrointestinal toxicity and thrombocytopenia. Therefore, the systemic application of NAMPT inhibitors is not feasible12,13,14.
For this reason, we suggest here a protocol where NAMPT activity is blocked directly in isolated TANs. Such anti-tumor neutrophils are then adoptively transferred into a tumor-bearing host. This protocol will help avoid systemic toxic side-effects of the compounds, while its effect on the target cells will be sustained.
Protokół
All the procedures including animal subjects have been approved by the regulatory authorities: LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz NRW) and Regierungspräsidium Tübingen, Germany. All manipulations should be performed in sterile conditions (under laminar flow hood) using sterile reagents and instruments (syringes, scissors, forceps, disposable scalpels, Petri dishes).
NOTE: The overall scheme of the protocol is shown in the Figure 1.
1. Preparation of B16F10 melanoma cell line
- Prepare mycoplasma-negative cells grown to a 90% confluent monolayer (approximately 10 x 106 cells/T75 flask) in complete Iscove’s Modified Dulbecco’s Medium (IMDMc: IMDM + 10% Fetal Bovine Serum (FBS) + 1% penicillin-streptomycin).
- Remove the medium, and rinse the cells with phosphate buffered saline (PBS). Apply 6 mL of a cell detachment solution containing proteolytic and collagenolytic enzymes (see the Table of Materials), and incubate at 37 °C for 2 min.
- Knock the flask gently to mobilize remaining adherent cells from the bottom. Collect the cell suspension in 15 mL tubes and centrifuge at 300 x g for 7 min and 20 °C.
- Remove the supernatant, and resuspend the pellet well in 1 mL of PBS. Add 14 mL of PBS and centrifuge (300 x g and 20 °C for 7 min).
- Remove the supernatant and resuspend the pellet in 1 mL of PBS.
- Count the cells, and resuspend them to the concentration of 3 x 106/mL PBS (for the step 2) or 6 x 106/mL PBS (for the step 6) for injection. Keep cells on ice for a maximum of 30 min.
2. Allogenic tumor model in mice
- Use 10 female Ifnar1-/- mice 8-12 weeks old that are kept under specific-pathogen-free (SPF) conditions.
NOTE: Female mice are preferable in a subcutaneous model of tumor growth, since males are more aggressive and thus prone to infractions of the tumor site, which influences tumor growth. - Shave the skin of the mouse on the flank with an electrical shaver, and disinfect the skin with tissue wet with 70% ethanol.
- Collect the prepared B16F10 melanoma cells at a concentration of 3 x 106/mL PBS (see the step 1) in a 1 mL syringe and 0.4 x 19 mm needle. Inject 100 μL of the suspension subcutaneously.
- Mix the cells well before every injection. Use needles not less than 0.4 mm in diameter as to not disturb tumor cells.
- Place up to 5 mice in one cage, and control tumor size (length, width and depth) with a caliper for 14 days.
NOTE: According to the animal regulations, the tumor size should not exceed 15 mm in diameter, mice with bigger or necrotic/open tumors should be sacrificed beforehand. - At day 14, sacrifice the mice in the CO2 chamber.
- Disinfect the skin with 70% ethanol and remove tumors with scissors and forceps in a sterile Petri dish. Keep tumors in a 50 mL tube in complete Dulbecco's Modified Eagle Medium (DMEMc: DMEM + 10% FBS + 1% penicillin-streptomycin) on ice.
3. TAN isolation
- Place tumors into sterile 6-well plates, 5 tumors per well. Cut tumors into 2-3 mm pieces with sterile scissors.
- Digest with 1 mL of dispase/collagenase D/DNase I solution (0.2 mg/0.2 mg/100 mg in 1 mL of DMEMc) per tumor. Incubate at 37 °C, 5% CO2 in a humid incubator, and mix with a 10 mL syringe without a needle every 15 min 3 times.
- To remove undigested fibers, mesh cells through 100 µm filters into 15 mL tubes (one well per filter per tube). Add PBS to 15 mL, centrifuge tubes at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Lyse erythrocytes with a lysis buffer (NH4Cl 150 mM, KHCO3 10 mM, EDTA 0.1 mM, pH 7.3, 20 °C) by adding 1 mL into each tube. Mix well, and combine the solution from all tubes into one. Stop the reaction after 2 minutes with 11 mL of ice-cold (4 °C) DMEMc.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend the pellet with 15 mL of cold PBS. Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Resuspend the pellet in 1 mL of PBS. Add 3 μL of Fc-block antibodies (CD16/CD32, stock 0.5 mg/mL), and incubate on ice for 15 min.
- Add antibodies: 10 μL of Ly6G-PE (stock 0.2 mg/mL) and 10 μL of CD11b-APC (stock 0.2 mg/mL). Add 20 μL of 6-Diamidin-2-phenylindol viability dye (DAPI, stock 5 mg/mL) and incubate on ice in darkness for 30 min.
NOTE: Another combination of viability dyes and fluorescent conjugates of antibodies can be used. - Add PBS up to 15 mL, centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant.
- Resuspend the pellet in DMEMc to the concentration approximately 10 x 106/mL, and keep on ice.
- Sort CD11b+ Ly6Ghi alive (DAPI-negative) neutrophils with a fluorescence-activated cell sorter (gating strategy see Figure 2).
NOTE: Keep the tube with cell suspension and the tube with DMEMc for sorted cells at 4 °C. Use the following optimal sorting settings: a 70 μm nozzle, a threshold rate of maximal 22,000 events/second and a flow rate of 1-3. - Check the purity of the sorted neutrophils using a cytometer for a recommended purity of >95%.
- Centrifuge sorted neutrophils at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend the sorted cells in DMEMc to the concentration of 1 x 106/mL.
NOTE: The expected number of neutrophils in one 14-day B16F10 tumor (10 mm diameter) is approximately 3 x 104 cells.
4. NAMPT inhibition in TANs in vitro
- Prepare FK866 (NAMPT inhibitor) stock in dimethylsulfoxide (DMSO) at a final concentration of 100 mM.
- Seed sorted neutrophils (step 3.11) into 2 wells of a 96-well U-bottom plate (1.5 x 105 neutrophils/well). Add FK866 into the intervention well (final concentration of 100 nM), and an equal amount of DMEMc with DMSO into the control well. Incubate for 2 h at 37 °C, 5% CO2 in a humid incubator.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend in 200 μL of PBS in each well. Repeat 2 times.
- Centrifuge at 460 x g, 4 °C for 5 min, and remove the supernatant. Resuspend in commercial endothelial cell growth medium (supplemented with 4 µL/mL endothelial cell growth supplement, 0.1 ng/mL recombinant human epidermal growth factor, 1 ng/mL recombinant human basic fibroblast growth factor, 90 µg/mL heparin and 1 µg/mL hydrocortisone) to a final concentration of 0.2 x 106 cells/mL (in 0.75 mL) (for step 5) or in PBS to the final concentration of 0.6 x 106 cells/mL (in 0.25 mL) (for step 6).
5. Estimation of angiogenic properties of TANs using the aortic ring assay
- Dissect the thoracic aorta from a male C57BL/6J (WT) mouse. Clean and cut into 0.5 mm width rings. Place all rings in a well of a 24-well plate with 1 mL of supplemented endothelial cell growth medium. Incubate overnight at 37 °C, 5% CO2 in a humid incubator.
NOTE: The use of young (younger than 8 weeks) male mice for aorta dissection is preferable, since they give a more robust angiogenic response15. - Fill the wells of the 96-well flat-bottom plate with 50 µL of solubilized basement membrane matrix, let the gel set for 30 min in a 37 °C, 5% CO2 humid incubator to allow the matrix to polymerize. Prepare at least 3 wells per condition.
- Embed the rings in solubilized basement membrane matrix by placing an aortic ring on the top of the solid matrix layer, 1 ring in the center of each well. Add another 50 µL of solubilized basement membrane matrix to cover each ring.
- Place the plate in a 37 °C, 5% CO2 humid incubator for another 30 min to allow the polymerization of the second matrix layer.
- Add 150 μL/well of supplemented endothelial cell growth medium and 2x104 Ifnar1-/- TANs (control and FK866-treated) (step 4.4).
- Incubate the plate for 14 days at 37 °C, 5% CO2 in a humid incubator.
- Image using a standard phase-contrast microscope and estimate the endothelial branching. Quantitative assessment of vessel morphometric and spatial parameters including branching index can be performed automatically using the image processing program designed for scientific images.
NOTE: Representative results are depicted in the Figure 3.
6. Adoptive transfer of treated neutrophils in the allogenic tumor model
- Prepare B16F10 melanoma cells (step 1.6) in PBS at a concentration of 6 x 106 cells/mL.
- Prepare 2 types of neutrophils: FK866-treated neutrophils and control untreated neutrophils (step 4.4) in PBS at a concentration 6 x 105 cells/mL. Mix neutrophils with B16F10 melanoma cells (the final neutrophil to tumor cells ratio 1:10) to have 2 types of cell mixtures.
- Take 10 female WT mice 8-12 weeks old, 5 in each group. Shave the skin on the flank with an electrical shaver, and disinfect with 70% ethanol.
- Inject 100 µL of the cell suspension (step 6.2) subcutaneously with an insulin syringe and a 0.4 mm diameter needle, to both groups of mice. Place 1-5 mice from the same group in one cage.
- At day 2, prepare 2 types of neutrophils: FK866-treated neutrophils and control untreated neutrophils (step 4.4) in PBS at a concentration 6 x 105 cells/mL. Inject 100 µL of cell suspension (step 6.4) i.v. into the tail vein with an insulin syringe and a 0.4 mm diameter needle, to both groups of mice. Place mice back to the cage.
7. Tumor growth measurement, histological examination
- Monitor tumor growth every other day. Evaluate the tumor size with calipers and calculate the tumor volume with the formula V=4/3*π*(h*w2)/8 (h=height, w=width, depth= width).
- Sacrifice mice at the day 14 in the CO2 chamber. Remove tumors and measure the tumor weights.
- Freeze tumors in optimum cutting temperature compound in liquid nitrogen, and store at -80 °C.
- Thaw the samples to -20 °C and prepare 5 μm sections using a cryotome. Let the cryocuts dry for 30 min at 20 °C. Fix the sections in -20 °C cold acetone for 2 min and let them dry for 30 min at 20 °C.
- Block with Fc-block antibodies (CD16/CD32, stock 0.5 mg/mL 1:500) in PBS for 1 h at 20 °C.
- Stain with rabbit anti mouse Laminin gamma antibody (1:1500 in PBS, 200 µL) for 1 h at 20 °C. Wash with PBS three times.
- Stain with secondary goat anti-rabbit antibody (stock 0.5 mg/mL, 1:400 in PBS,), anti-mouse αSMA (1:500 in PBS) and 2 µL of DAPI (stock 5 mg/mL, 1:100 in PBS) in a final volume of 200 µL of antibody solution. Incubate for 1 h at 20 °C in darkness. Wash with PBS three times.
- Dry slides for 20 minutes at 20 °C in darkness. Mount with anhydrous mounting medium for microscopy and cover with a coverslip. Let it dry 1 h in 37 °C.
- Perform microscopical examination. Quantify the vascularization by counting the total number (optionally area) of Laminin+ vessels and the number (area) of SMA+ developed vessels.
NOTE: To perform image analysis, take all images under the same conditions (light, contrast, magnification). In this case, processing parameters are fixed, and image processing becomes completely automatic. Representative results are depicted in the Figure 4 and Figure 5.
Wyniki
Using the procedure described here, Ifnar1-/- neutrophils were isolated from tumors and treated with NAMPT inhibitor FK866 for 2 h. Untreated Ifnar1-/- neutrophils were used as a control. The effectivity of the treatment was evaluated using the aortic ring assay, which reflects the key steps involved in angiogenesis (matrix degradation, migration, proliferation, reorganization). We could demonstrate that FK866-treated neutrophils have a significantly decreased capacity to stimulate aortic branch formation, as compared to untreated cells (Figure 3A, 3B). FK866-treated anti-angiogenic neutrophils were injected subcutaneously into tumor-bearing mice (at day 0 flank and day 2 i.v.). We could observe significantly impaired tumor growth, as compared to mice injected with untreated Ifnar1-/- neutrophils (Figure 4A, 4B). Histological examination of the extracted tumors proved the significant suppression of angiogenesis in tumors isolated from mice treated with FK866-treated TANs, as compared to those injected with untreated Ifnar1-/- neutrophils (Figure 5A,B).
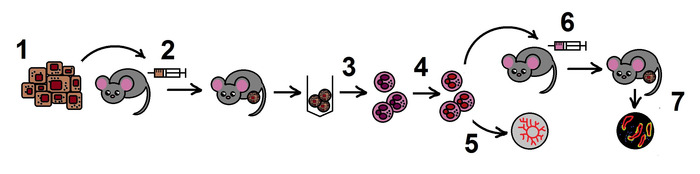
Figure 1. The scheme of the protocol. Step 1. Preparation of B16F10 melanoma cell line; 2. Allogenic tumor model in mice; 3. Isolation of TANs from the tumors; 4. Inhibition of NAMPT in TANs in vitro; 5. Estimation of angiogenic properties of TANs in the aortic ring assay; 6. Adoptive transfer of treated neutrophils in the allogenic tumor model; 7. Tumor growth monitoring, histological examination. Please click here to view a larger version of this figure.
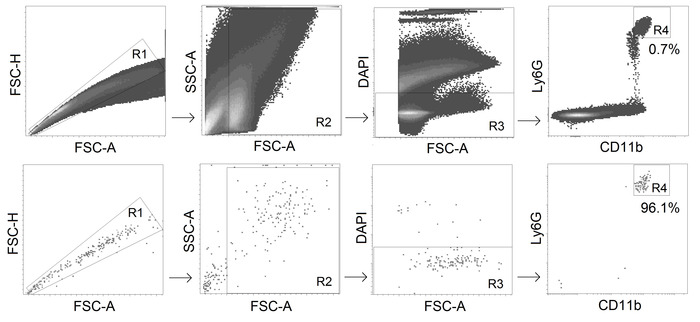
Figure 2. Gating strategy for TANs sorting. CD11b+ Ly6Ghi alive neutrophils are sorted from tumors with the purity ≥95%. Please click here to view a larger version of this figure.

Figure 3. Suppression of angiogenic properties of TANs after FK866 treatment. Angiogenic properties of sorted Ifnar1-/- TANs treated with FK866 or with medium were estimated using aorta ring assay. Branch formation was monitored during 14 days, representative results at the day 14 are presented (A). Treatment with FK866 significantly decreased the number of endothelial branches (B). Data are shown as median, interquartile range and min-max, *p<0.05. Please click here to view a larger version of this figure.

Figure 4. Retardation of tumor growth after adoptive transfer of FK866-treated neutrophils. The influence of TANs on the tumor growth was assessed. TANs were isolated, treated with FK866 and injected into tumor-bearing mice as described above. At day 14 mice were sacrificed, tumors removed and analyzed. Ifnar1-/- TANs treated with FK866 versus controls were compared. (A) Tumor growth was measured, (B) tumor mass and (C) size were estimated. Data are shown as median, interquartile range and min-max, *p<0.05. Please click here to view a larger version of this figure.
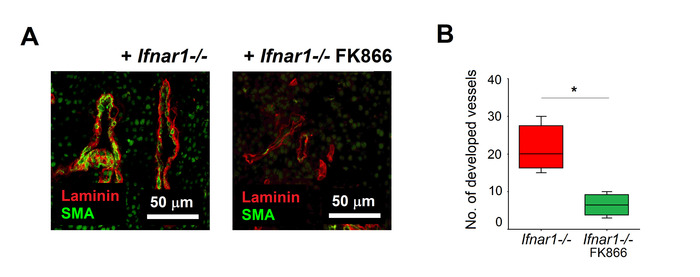
Figure 5. Suppressed tumor vascularization after adoptive transfer of FK866-treated neutrophils. Tumors were isolated as described above (Fig 4). Vessel maturation was assessed using anti-SMA antibodies (mature vessels) and anti-gamma laminin (endothelial cells). (A) Representative staining of tumors are shown: SMA (green), laminin (red). Scale bars: 50 μm. (B) Quantification of tumor vascularization after adoptive transfer of TANs cultivated with FK866 (green) or medium (red) Data are shown as median, interquartile range and min-max, *p<0.05. Please click here to view a larger version of this figure.
Dyskusje
Despite progress in surgical and pharmacological cancer treatment, successful therapy remains a challenge. Since immune cells are known to play an important role in the regulation of tumor growth, novel methods inhibiting tumorigenicity of such cells should be established. Here we demonstrate a novel approach to suppress tumor growth via adoptive transfer of anti-angiogenic tumor-associated neutrophils. Selective targeting of pro-angiogenic NAMPT signaling in TANs, using FK866 inhibitor, prevents side effects, which are observed upon systemic FK866 treatment.
The most critical part of the protocol is the need to use freshly isolated primary neutrophils. Neutrophils are short-living cells, undergoing apoptosis or activated during the procedure of isolation. Murine neutrophils should be kept in 4 °C media during all steps of isolation, including cell sorting. Isolation of neutrophils should be performed as soon as possible and the experiment should not be paused. Usage of Fc-block allows reducing the unspecific staining of the cells with high Fc-receptor expression, like NK cells. We also recommend to minimize the number of fluorescent-conjugated antibodies to simplify the gating strategy and to avoid the activation of neutrophils due to antibody binding.
The limiting step of the protocol is the isolation of alive neutrophils from tumors due to a relatively low amount of these cells in tumors (not more than 1% of single alive cells in melanoma). This could only be possible using flow cytometry-based sorting. At the same time, the usage of blood neutrophils for this protocol should be avoided due to only minor regulation of NAMPT expression and their low functionality, which is altered upon tumor tissue arrival16. Possibly, in order to use blood neutrophils, they should be previously activated using tumor-derived growth factors.
To avoid neutrophil apoptosis, short treatment with FK866 (2-4 h) is suggested, as it has no influence on the viability of TANs, while prolonged treatment induces neutrophil apoptosis16. In sum, the protocol demonstrates the potential of ex vitro manipulated anti-angiogenic neutrophils to functionally suppress tumor growth in mouse melanoma tumor model.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Our work was supported by grants from Deutsche Krebshilfe, Grant Number: 111647, and German Research Council (DFG), Grant Number: JA 2461/2-1.
Materiały
| Name | Company | Catalog Number | Comments |
| 15 ml tubes | Sarstedt AG & Co., Nümbrecht, Germany | 62,554,502 | |
| 50 ml tubes | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 227261 | |
| 5ml / 10ml / 25ml sterile tipps for the automatic pipette | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 6006180 / 607180 / 760180 | |
| 6 well flat-bottom cell culture plates | Sarstedt AG & Co., Nümbrecht, Germany | 833,920 | |
| 96 well flat-bottom cell culture plates | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 655180 | |
| 96 well U-bottom cell culture plates | Cellstar, Greiner Bio One International GmbH, Frickenhausen, Germany | 65018 | |
| AMG EVOS fl digital inverted microscope | AMG, Bothel, U.S. | ||
| anti-mouse CD11b | BD Pharmigen, Becton Dickinson, Franklin Lakes, U.S. | 553312 | clone M1/70, APC-conjugated, 0.2mg/mL |
| anti-mouse Ly6G | BioLegend, California, U.S. | 127608 | clone 1A8, PE-conjugated, 0.2mg/mL |
| BD FACS AriaII | BD Biosciences, Becton Dickinson, Franklin Lakes, U.S. | cell sorter | |
| Caliper | Vogel Germany, Kevelaer, Germany | ||
| Casy cell counter | Innovatis, Roche Innovatis AG, Bielefeld, Germany | ||
| Cell Trics 50µm / 100 µm sterile filters | Sysmex Partec GmbH, Goerlitz, Germany | 04-004-2327 / 04-004-2328 | |
| Centrifuge Rotina 420 R | Andreas Hettich, Tuttlingen, Germany | 4706 | |
| Collagenase D | Sigma-Aldrich/Merck, Darmstadt, Germany | 11088858001 | |
| DAPI (4',6-Diamidino-2-Phenylindole, Dilactate) | BioLegend, California, U.S. | 422801 | Stock: 5mg/ml |
| Dispase I | Sigma-Aldrich/Merck, Darmstadt, Germany | D4818-2MG | |
| DMEM | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 41966-029 | DMEM complete: DMEM + 10% FBS + 1% penicillin-streptomycin |
| DMSO (Dimethylsufoxide) | WAK-Chemie Medical GmbH, Steinbach, Germany | WAK-DMSO-10 | CryoSure-DMSO |
| DNase I | Sigma-Aldrich/Merck, Darmstadt, Germany | DN25-100MG | |
| DPBS | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 14190-094 | |
| Endothelial cell growth medium | PromoCell, Heidelberg, Germany | c-22010 | |
| FBS (Fetal Bovine Serum) | Biochrom, Berlin, Germany | S0115 | |
| Fc-block (Anti-mouse CD16/32) | BD Pharmingen, Becton Dickinson,Becton Dickinson, Franklin Lakes, U.S. | 553142 | clone 2.4G2, Stock: 0.5mg/mL |
| FK 866 hydrochloride | Axon Medchem, Groningen, Netherlands | Axon 1546 | Stock: 100 mM |
| Goat Anti-Rabbit IgG H&L | Abcam, Cambridge, U.K. | ab97075 | Cy3-conjugated, Stock: 0.5 mg/mL |
| Heracell 240i CO2 Incubator | Thermo Fisher Scientific, Waltham, U.S. | 51026334 | |
| IMDM | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 12440-053 | IMDM complete: IMDM + 10% FBS + 1% penicillin-streptomycin |
| Isis GT420 shaver | B. Braun Asculap, Suhl, Germany | 90200714 | |
| Matrigel Matrix basement membrane | Corning Life Sciences, Amsterdam, Netherlands | 7205011 | |
| Microtome Cryostat Microm HM 505 N | Microm International GmbH, Walldorf, Germany | ||
| Monoclonal Anti-Actin, α-Smooth Muscle | Sigma-Aldrich/Merck, Darmstadt, Germany | F3777 | FITC-conjugated, no information about stock concentration |
| Needles 0.4 mm x 16 mm | BD Microlance, Becton Dicson, Becton Dickinson, Franklin Lakes, U.S. | 302200 | |
| Neomount | Merck, Darmstadt, Germany | HX67590916 | |
| Normal goat serum | Jackson ImmunoResearch Laboratories, West Grove, U.S. | 005-000-121 | |
| Penicillin Streptomycin | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | 15140-122 | |
| Pipetus automatic pipette | Hirschmann Laborgeräte, Eberstadt, Germany | 9907200 | |
| ProLong Gold Antifade Mountant with DAPI | Invitrogen, Thermo Fisher Scientific, Massachusetts, U.S. | P36935 | |
| rabbit anti mouse Laminin gamma 1 chain | Immundiagnostik, Bensheim, Germany | AP1001.1 | No information about stock concentration |
| StemPro Accutase | Gibco, Life Technologies/Thermo Fisher Scientific, Massachusetts, U.S. | A1105-01 | |
| Sterile disposal scalpel (no. 15) | MedWare, Naples, U.S. | 120920 | |
| Syringes 1 ml | BD Plastipak, Becton Dickinson, Franklin Lakes, U.S. | 303172 | |
| Syringes 10 ml | BD Discardit II, Becton Dickinson, Franklin Lakes, U.S. | 309110 | |
| T75 sterile cell culture flasks | Sarstedt AG & Co., Nümbrecht, Germany | 833,911,302 | |
| Tissue-Tek O.C.T. Compound | Sakura Finetek, Torrance, U.S. | 4583 | |
| Zeiss AxioObserver.Z1 Inverted Microscope with ApoTome Optical Sectioning | Carl Zeiss, Oberkochen, Germany |
Odniesienia
- Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S., Weiss, S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. Journal of Clinical Investigation. 120 (4), 1151-1164 (2010).
- Andzinski, L., Wu, C. F., Lienenklaus, S., Kröger, A., Weiss, S., Jablonska, J. Delayed apoptosis of tumor associated neutrophils in the absence of endogenous IFN-β. International Journal of Cancer. 136, 572-583 (2015).
- Rongvaux, A., et al. Pre-B-cell colony-enhancing factor, whose expression is upregulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. European Journal of Immunology. 32, 3225-3234 (2002).
- Skokowa, J., et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nature Medicine. 15, 151-158 (2009).
- Yaku, K., Okabe, K., Hikosaka, K., Nakagawa, T. NAD Metabolism in Cancer Therapeutics. Frontiers in Oncology. 8, 622(2018).
- Audrito, V., et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 125, 111-123 (2015).
- Brentano, F., et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix degrading activities. Arthritis & Rheumatology. 56, 2829-2839 (2007).
- Jablonska, J., Wu, C. -F., Andzinski, L., Leschner, S., Weiss, S. CXCR2-mediated tumor associated neutrophilrecruitment is regulated by IFN-beta. International Journal of Cancer. 134, 1346-1358 (2014).
- Andzinski, L., et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. International Journal of Cancer. 138, 1982-1993 (2016).
- Wu, C. -F., et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. International Journal of Cancer. 137, 837-847 (2015).
- Hansel, T. T., Kropshofer, H., Singer, T., Mitchell, J. A., George, A. J. The safety and side effects of monoclonal antibodies. Nature Reviews Drug Discovery. 9 (4), 325-338 (2010).
- Hasmann, M., Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Research. 63, 7436-7442 (2003).
- Holen, K., Saltz, L. B., Hollywood, E., Burk, K., Hanauske, A. R. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Investigational New Drugs. 26, 45-51 (2008).
- von Heideman, A., Berglund, A., Larsson, R., Nygren, P. Safety and efficacy of NAD depleting cancer drugs: results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Cancer Chemotherapy and Pharmacology. 65, 1165-1172 (2010).
- De Rossi, G., Scotland, R., Whiteford, J. Critical Factors in Measuring Angiogenesis Using the Aortic Ring Model. Journal of Genetic Syndromes and Gene Therapy. 4 (5), (2013).
- Pylaeva, E., et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. International Journal of Cancer. 144 (1), 136-149 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone