Method Article
Heterotopic Auxiliary Rat Liver Transplantation With Flow-regulated Portal Vein Arterialization in Acute Hepatic Failure
W tym Artykule
Podsumowanie
Auxiliary liver transplantation provides a temporary support in acute hepatic failure, until regeneration of the failing liver. The heterotopic auxiliary liver transplantation (HALT) with portal vein arterialization (PVA) renders sufficient liver function. We developed an analogous technique in the rat, to examine the influence of the portal vein arterialization on the morphology and function of the graft.
Streszczenie
In acute hepatic failure auxiliary liver transplantation is an interesting alternative approach. The aim is to provide a temporary support until the failing native liver has regenerated.1-3 The APOLT-method, the orthotopic implantation of auxiliary segments- averts most of the technical problems. However this method necessitates extensive resections of both the native liver and the graft.4 In 1998, Erhard developed the heterotopic auxiliary liver transplantation (HALT) utilizing portal vein arterialization (PVA) (Figure 1). This technique showed promising initial clinical results.5-6 We developed a HALT-technique with flow-regulated PVA in the rat to examine the influence of flow-regulated PVA on graft morphology and function (Figure 2).
A liver graft reduced to 30 % of its original size, was heterotopically implanted in the right renal region of the recipient after explantation of the right kidney. The infra-hepatic caval vein of the graft was anastomosed with the infrahepatic caval vein of the recipient. The arterialization of the donor’s portal vein was carried out via the recipient’s right renal artery with the stent technique. The blood-flow regulation of the arterialized portal vein was achieved with the use of a stent with an internal diameter of 0.3 mm. The celiac trunk of the graft was end-to-side anastomosed with the recipient’s aorta and the bile duct was implanted into the duodenum. A subtotal resection of the native liver was performed to induce acute hepatic failure. 7
In this manner 112 transplantations were performed. The perioperative survival rate was 90% and the 6-week survival rate was 80%. Six weeks after operation, the native liver regenerated, showing an increase in weight from 2.3±0.8 g to 9.8±1 g. At this time, the graft’s weight decreased from 3.3±0.8 g to 2.3±0.8 g.
We were able to obtain promising long-term results in terms of graft morphology and function. HALT with flow-regulated PVA reliably bridges acute hepatic failure until the native liver regenerates.
Wprowadzenie
Acute hepatic failure poses a severe problem in clinical medicine leading to a mortality rate of 60-80 %.8 In acute hepatic failure, auxiliary liver transplantation is a promising alternative therapy to bridge the acute phase. The native liver is left to regenerate and following to the regeneration the immunosuppression drugs can be discontinued. As a consequence the graft will become atrophic. However the operative technique for auxiliary liver transplantation is still discussed.1-3 In this context, Terpstra reported the lack of space for the graft, the portal blood steal phenomenon and the problem of venous congestion and drainage.9-10 The donor’s portal vein was anastomosed end-to-side to the recipient’s portal vein. This resulted in portal flow competition between the graft and the native liver and in portal vein thrombosis. Most of those technical problems can be avoided with the use of the APOLT-method (orthotopic implantation of auxiliary segments).4 However this method necessitates extensive resections of both the native liver and the graft.4 In 1998, Erhard and his team developed the heterotopic auxiliary liver transplantation (HALT) utilizing portal vein arterialization (PVA) (Figure 1). The hilum and the portal vein of the native liver are respected with the use of this method. That spares the recipient from unwanted side-effects such as portal vein thrombosis and portal blood steal phenomenon, which could disturb native liver recovery. Additionally, no extensive liver resections of the native liver and graft are required for this method, with consequently less bleeding and more remaining total liver parenchymal mass for bridging (graft) and regeneration (native liver). This technique showed promising initial clinical results.5-6 The goal of this experimental work was the development of a HALT technique with portal vein arterialization in the rat for research purposes (Figure 2). In the preliminary experiments we didn’t take into consideration the blood flow regulation of the arterialized portal vein, which resulted in congestion of the transplanted graft after reperfusion and massive bleeding from the liver capsule.11 Since then the blood flow in the arterialized portal vein was regulated by a stent with an inner diameter of 0.3 mm and a length of 8 mm. Pilot experiments had shown that a stent diameter of 0.3 mm yields an average blood flow in the arterialized portal vein that is within the upper physiological range (1.7 +/- 0.4 ml/min/g liver weight).12 Our results without blood flow regulation correlate with the results of Hong, who performed HALT with PVA via the iliac artery of the recipient.13 In his experiments without blood flow regulation massive portal and sinusoid congestions occurred.
The aim of our video is to demonstrate step by step this ne.w experimental surgical technique in the rat. In the long term the proposed technique can be used in studies on liver regeneration and inter-liver competition and portal vein arterialization, portal vein hyperperfusion and portal blood flow regulation.
Protokół
1. Experimental Animals
- Obtain a Local Ethics Committee approval for all experiments which will be performed, in accordance with the animal protection FELASA guidelines (K 40, 17/99).
- Use male Lewis rats available to purchase from any commercial breeder (e.g., Charles River Wiga GmbH, Sulzfeld, Germany). Here, 224 animals (112 donors, 112 recipients) were used to perform 112 transplantations. The recipients were sacrificed at 6 weeks postoperatively. At the time of operation, the animals should weigh 250-350 g.
- Keep the animals in climate-controlled rooms with free access to food and water.
2. Anesthesia and Operative Preparation
- Place the rat in a plexiglas box connected to the anesthesia machine with an oxygen flow rate of 1 L/min, and an isofluorane vaporizer with 5 % anesthetic gas to induce anesthesia. Maintain anesthesia at 1.5 % isofluorane.
- Place the rats in supine position on a warming plate.
- Remove the fur of the abdomen with a depilation cream.
- Prepare a sterile surgical field as following: Use a disinfectant to clean the benchtop. For the disinfection of the incision site use Chlorhexidine first, alcohol second and, finally, a scrub solution (e.g., Chlorhexidine and 70% isopropyl alcohol). Wear a disposable surgical gown and surgical gloves.
- Use sterile microsurgery instruments.
- Use an operation microscope in 12-20-fold magnification.
3. Surgical Procedure
- Donor operation
Preparation- Perform a median laparotomy and resect the left lateral lobe and the median lobe of the graft as described by Higgins.14
- Then prepare the hepatoduodenal ligament. First ligate the bile duct in order to induce congestion and dilation of the small bile duct. Then ligate and transect the gastroduodenal vein and artery. Afterwards, isolate the celiac trunk and the common hepatic artery and transect the left gastric artery and the splenic artery. Incise the congested bile duct, and introduce a 20-G stent into it. The positioning of the stent must ensure the drainage of the entire hilar bile duct system. Transect the bile duct distally to the stent.
- Isolate the infrahepatic segment of the caval vein right above the right renal vein.
Perfusion - After ligation of the aorta above the celiac trunk, clamp the portal vein and incise it. Introduce the perfusion catheter (14 G) into the portal vein, and perform the perfusion with 30 ml of histidine-tryptophan-ketoglutarate (HTK)-solution (12 cm H2O, 4°C).
- Cut the diaphragm and the suprahepatic caval vein to allow the perfusate to flow out.
- Excise the celiac trunk along with an aortic patch and rinse it with histidine-tryptophan-ketoglutarate solution.
Explantation - Transect the infrahepatic caval vein right above the right renal vein.
- Then, place a stent with a diameter of 0.3 mm in the portal vein and fix it with a ligature. During the following operation, the portal vein is arterialized via the right renal artery. Transect the portal vein distally to the stent.
- After ligation of the suprahepatic caval vein, transect it together with a cuff of diaphragm.
- Explant the graft and store it in histidine-tryptophan-ketoglutarate (HTK)-solution at 4 ° Celsius in a plastic bag on ice. Place corner sutures (8-0 Ethilon) in the infrahepatic caval vein.
- Recipient operation
- Perform a median laparotomy and prepare the infrahepatic caval vein. Then isolate the right renal artery and the ureter, ligate the ureter and transect it. Clamp the right renal artery at its origin, then ligate the right renal vein. Then after transection of both renal vessels, perform the nephrectomy. Subsequently, shorten the right renal artery, rinse it with heparin saline solution (40 UI heparin/ml saline solution).
- Transect the right renal vein proximally to the ligature after proximal and distal clamping of the infrahepatic caval vein to create an oval lumen in the infrahepatic caval vein. Rinse the caval vein with heparin saline solution. Implant the graft in the right renal region of the recipient’s abdomen.
- Anastomose the donor’s infrahepatic caval vein end-to-side to the recipient`s caval vein with a 8-0 Ethilon running suture.
- After rinsing with heparin/saline solution, introduce the stent in the portal vein into the right renal artery (Figure 3). Connect the portal vein and renal artery with 2 stitches (8-0 Ethilon). Fix the linking stent in the renal artery with a ligature (braided silk black 7-0).
- To allow reperfusion, first remove the arterial clamp, then both venous clamps (Figure 4).
- Prepare the infrarenal aorta, then place a proximal and distal microclamp and incise the aorta right between both clamps. Perform an end-to-side anastomosis between the aorta and the donor’s celiac trunk, using an aortic patch, with a 10-0 Ethilon running suture.
- Insert the bile duct into the duodenum: Apply a pursestring suture in the duodenal wall, make a small incision in the duodenal wall right in the middle of the pursestring. Introduce the stented bile duct into the duodenum, and tighten the pursestring suture. Finally, hold the duodenum and the bile duct tightly together with two stitches (Ethilon 8-0) (Figure 5).
- Then perform a subtotal resection of the native liver, first described by Emond et al. to mimic acute hepatic failure.7
- Fix the suprahepatic caval vein of the graft, together with a cuff of diaphragm, to the lateral abdominal wall of the recipient with 4-0 Vicryl suture. Rinse the abdomen with saline solution (Figure 6).
- For the muscular layer use a 4-0 Vicryl running suture and for the cutaneous a 3-0 Vicryl running suture.
4. Recovery and Postoperative Management
- Let the animals recover from anesthesia under an infrared warming lamp.
- Inject Buprenorphine at a dose of 0.03-0.05 mg/kg B.W. sc every 8-12 hr for 2 days.
- Examine the animals concerning the general condition and signs of illness or infections.
5. Calculation of the Initial Native Liver Weight
Calculate the initial native liver weight in the following manner: subtract the weight of the resected liver lobes from the complete liver weight (2.66% of body weight (this value with a standard deviation of +/- 0.49% was determined in previous experiments with n=80 animals of the same strain and age)).15
Wyniki
The mean duration of the transplantation was about 150 min. The cold ischemia time was 64 +/- 7 min, the warm ischemia time was less than 25 min.
The perioperative survival rate was 90% and the 6-week survival rate was 80%. The 20 % loss was due to peritonitis because of stent dislocation and biliary leakage (four animals) during the first postoperative week and due to pneumonia, intra-abdominal abscess and ileus (seven animals) during the following five weeks. The surviving animals were in excellent general condition.
The parameters of liver-synthesis (Quick’s value: 110 +/- 7.8 %, AT III: 104 +/- 6 %) were within the normal range six weeks after operation.
At this time point, the native liver had regenerated, showing an increase in weight from 2.3±0.8 g to 9.8±1 g (P: 0.0065). The graft’s weight decreased from 3.3±0.8 g to 2.3±0.8 g (P: 0.06). The total weight of both livers was within the physiological ratio of liver weight/body weight (Figure 7).
In the histological stain of the grafts with Hematoxylin-Eosin at 6 weeks after HALT, regular hepatocytes were observed with neither edema nor fatty degeneration (Figure 8). All grafts showed slight proliferation of bile ductuli and cholangitis due to sludge formation (Figure 8). Low-grade intra-parenchymatous hepatocellular necroses could be observed in only two grafts. After six weeks the histology of the native liver revealed no alterations.

Figure 1. Schematic presentation of the heterotopic auxiliary liver transplantation (HALT) with portal vein arterialization (PVA) in human. The portal vein (3) is arterialized via an iliac artery bifurcation interposition graft (5) which is also the vessel feeder of the hepatic artery (6).
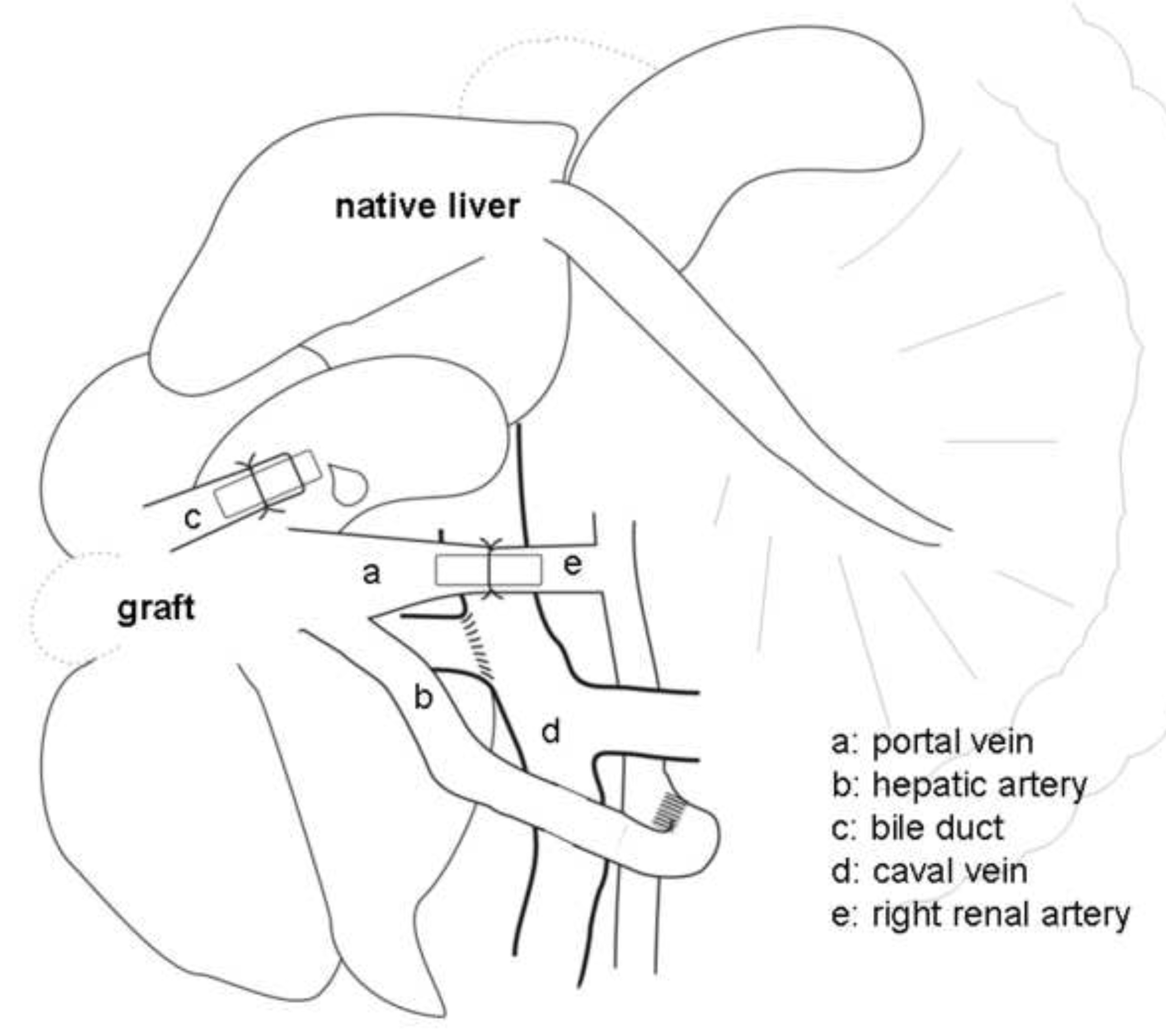
Figure 2. Schematic presentation of the heterotopic auxiliary liver transplantation (HALT) with portal vein arterialization (PVA) in the rat. The portal vein (a) is arterialized via the right renal artery (e) by a stent.

Figure 3. Portal vein arterialization during HALT with PVA in the rat. (A) The stent in the portal vein is introduced in the right renal artery. (B) The portal vein and the right renal artery are connected with two sutures. Please click here to view a larger version of this figure.

Figure 4. Situs after graft reperfusion via the arterialized portal vein in the rat. Please click here to view a larger version of this figure. Please click here to view a larger version of this figure.

Figure 5. Choledocho-duodenostomy during HALT in the rat. Please click here to view a larger version of this figure.
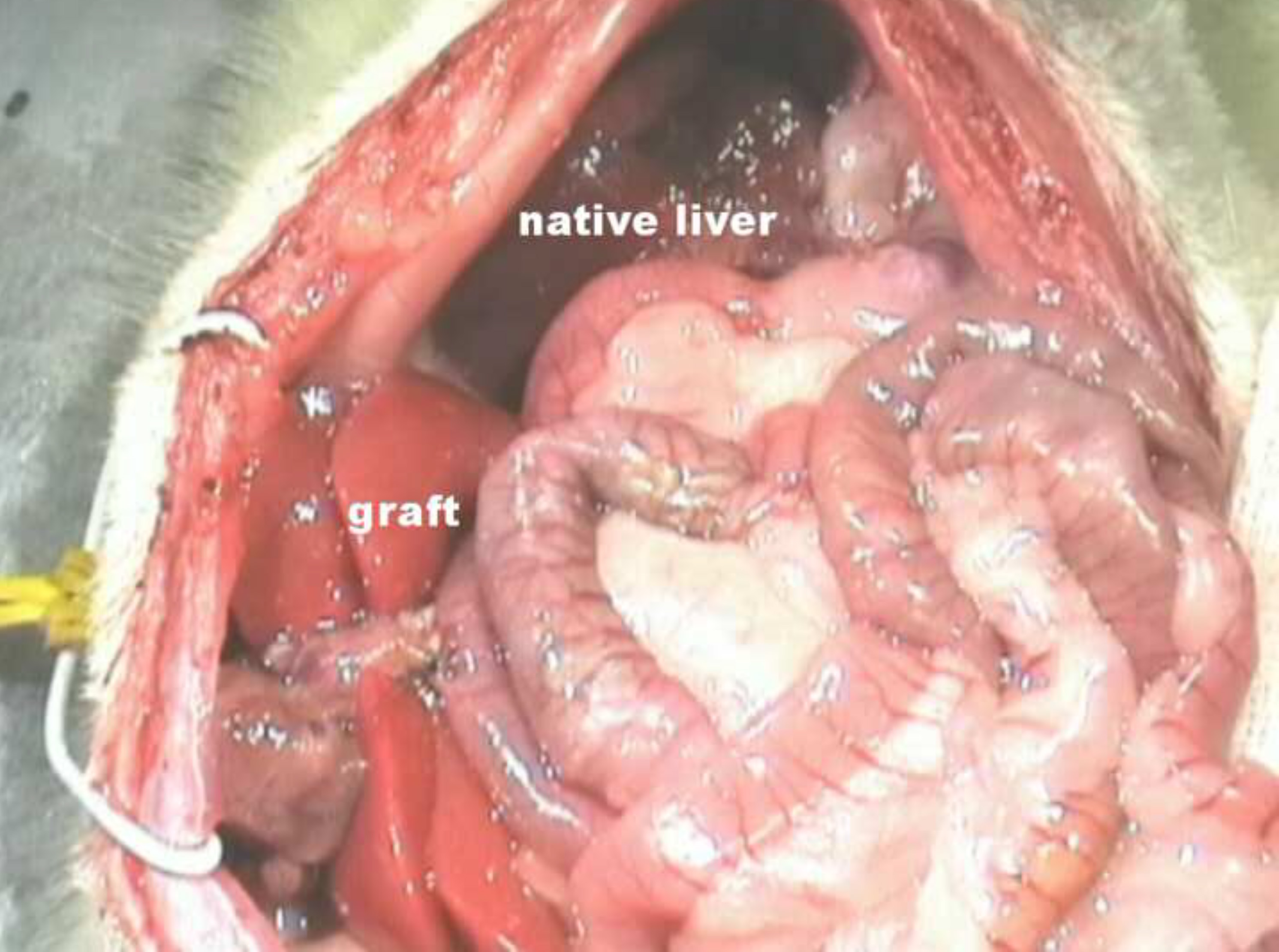
Figure 6. Situs after HALT with PVA in the rat. Please click here to view a larger version of this figure.

Figure 7. Graphic presentation of the liver weight (gr) of native liver, graft and total liver, at time point of operation and after six weeks.

Figure 8. Histological section of the graft after six weeks, stained with Hematoxylin-Eosin (HE) (magnification x100 (A) and x400 (B)). Please click here to view a larger version of this figure.
Dyskusje
The first heterotopic auxiliary rat liver transplantation was described by Lee et al.,16 in 1966 followed by Hess.17 The portal vein of the graft was anastomosed with the portal vein of the recipient. During this procedure, the portal venous blood supply to the native liver is compromised.
In 1991 Hong performed HALT with PVA via the iliac artery of the recipient.13 Without blood flow regulation massive portal and sinusoid congestions occurred.
Furthermore Aguirrezabalaga et al., performed HALT with PVA, nevertheless without paying the necessary attention to the blood flow regulation in the arterialized portal vein.18
The use of a stent having an inner diameter of 0.3 mm and a length of 8 mm yields an average portal blood flow after reperfusion of the arterialized portal vein that is within the upper physiological range.12
The biliary drainage is the Achilles heel of liver transplantation. The recipient animals are in danger to die due to biliary leakage and peritonitis. For this purpose we used a stent to perform the choledocho-duodenostomy. Additionally we applied a pursestring suture with a pair of additional sutures to avoid leakage and disconnection. Consequently, the biliary complications were reduced to 4 %.
In this current study, a subtotal resection of the native liver was performed to induce acute liver failure. This technique was first described by Emond, it is standardized and shows no side effects on other organ systems or on the graft.7
In 2002, Palmes published the APOLT technique in the rat with a survival rate of 70-80 %, the animals were sacrificed after two weeks.19 In this model the left lobe of the graft was transplanted after resection of the left lobe of the native liver. The graft’s portal vein was anastomosed end-to-end to the left segmental portal vein of the recipient, thus resulting in surgical difficulty to perform the operation, since the lumen of the vessels becomes very small.
Unlike orthotopic liver transplantation, during heterotopic liver transplantation there is no anhepatic phase. The anastomoses can be performed without any time-pressure. Moreover, in the heterotopic position, there is no interference by the moving diaphragm. Furthermore, the recipient’s portal vein is not clamped, thereby avoiding venous congestion of the small intestine, spleen, pancreas and stomach.
We obtained good long-term results concerning graft microcirculation, morphology and function.20 HALT with flow regulated PVA can reliably bridge an acute liver failure phase while the native liver regenerates. 15
HALT with flow regulated PVA is an innovative technique, relatively easy to establish. This model enables future studies on liver regeneration and inter-liver competition and portal vein arterialization, portal vein hyperperfusion and portal blood flow regulation.20, 21
Ujawnienia
All authors declared no competing interests.
Podziękowania
This study was funded by the “Deutsche Forschungsgemeinschaft” (DFG). K. Schleimer was supported by the Lise Meitner Fellowship of the Ministry of Science and Research of NRW. We would like to thank Mrs. Mary-Joan Blümich for her excellent editing assistance. Dr. Maria Kokozidou was financed from Fresenius 2011_A61.
Materiały
| Name | Company | Catalog Number | Comments |
| Braided Silk black 7-0 | Ethicon Products | 892100 | |
| Vicryl 3-0 | Ethicon Products | J285G | |
| Vicryl 4-0 | Ethicon Products | J284G | |
| Ethilon 8-0 | Ethicon Products | 1714G | |
| Ethilon 10-0 | Ethicon Products | 7770G | |
| Vasofix safety 14G | B Braun | 2758853 | |
| Vasofix safety 20G | B Braun | 2758818 | |
| 2 French (0.012”/0.3 mm ID x 0.025”/0.6 mm OD) x 24”/60 cm polyurethane catheter | Access technologies a division of Norfolk Medical, Inc., Illinois, USA | CNC-2P | |
| HTK solution, Custodiol | Dr. Franz Köhler Chemie GmbH, Alsbach-Hähnlein, Germany | ||
| Isofluorane 5 % | Any genericon | ||
| Heparin | Any genericon | ||
| 0.9% saline | Any genericon | ||
| Buprenorphine | Any genericon |
Odniesienia
- Boudjema, K., et al. Auxiliary liver transplantation for fulminant and subfulminant hepatic failure. Transplantation. 59, 218-223 (1995).
- Boudjema, K., Jaeck, D., Siméoni, U., Bientz, J., Chenard, M. P., Brunot, P. Temporary auxiliary liver transplantation for subacute liver failure in a child. The Lancet. 342, 778-779 (1993).
- Chenard-Neu, M. P., et al. Auxiliary liver transplantation: Regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure - a multicenter European study. Hepatology. 23, 1119-1127 (1996).
- Gubernatis, G., Pichlmayr, R., Kemnitz, J., Gratz, K. Auxiliary partial orthotopic liver–transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 15, 660-666 (1991).
- Erhard, J., Lange, R., Giebler, R., Rauen, U., de Groot, H., Eigler, F. W. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. Transplantation. 60, 877-879 (1995).
- Erhard, J., et al. Auxiliary liver transplantation with arterialization of the portal vein for acute hepatic failure. Transpl Int. 11, 266-271 (1998).
- Emond, J., Capron-Laudereau, M., Meriggi, F., Bernuau, J., Reynes, M., Houssin, D. Extent of Hepatectomy in the rat. Eur Surg Res. 21, 251-259 (1989).
- Mas, A., Rode`s, J. Fulminant hepatic failure. Lancet. 349, 1081-1085 (1997).
- Terpstra, O. T. Auxiliary liver grafting: a new concept in liver transplantation. The Lancet. 342, 758 (1993).
- Terpstra, O. T., Reuvers, C. B., Schalm, S. W. Auxiliary heterotopic liver transplantation. Transplantation. 45, 1003-1007 (1988).
- Schleimer, K., Lange, R., Rauen, U., Erhard, J. Auxiliary liver transplantation in acute liver failure in the rat - an illustrated description of a new surgical approach. Langenbecks Arch Surg. 384 (2), 204-208 (1999).
- Schleimer, K., et al. Physiologic microcirculation of the heterotopically transplanted rat liver with portal vein arterialization depending on optimal stent diameter. Med Sci Monit. 28 (BR140-145), (2006).
- Hong, H. Q., et al. Study of portal arterialization with auxiliary liver in rats. Hiroshima J. Med. Sci. 40, 29-33 (1991).
- Higgins, G. M., Anderson, R. M. Experimental pathology of the liver. I--Restoration of the liver of the white rat following partial surgical removal. Arch. Path. 12, 186-202 (1931).
- Schleimer, K., et al. Auxiliary liver transplantation with flow-regulated portal vein arterialization offers a successful therapeutic option in acute hepatic failure--investigations in heterotopic auxiliary rat liver transplantation. Transpl Int. 19 (7), 581-588 (2006).
- Lee, S., Edgington, T. S. Liver transplantation in the rat. Surg. Forum. 17, 220-222 (1966).
- Hess, F., Willemen, A., Jerusalem, C. Auxiliary liver transplantation in the rat, influence of the condition of the recipient's liver on the fate of the graft. Eur. Surg. Res. 9, 270-279 (1977).
- Aguirrezabalaga, J., Arnal, F., Marini, M., Centeno, A., Fernandez-Selles, C., Rey, I., Gomez, M. Auxiliary liver transplantation with portal arterialization in the rat: description of a new model. Microsurgery. 22, 21-26 (2002).
- Palmes, D., Dietl, K. H., Drews, G., Holzen, J. P., Spiegel, H. U. Auxiliary partial orthotopic liver transplantation: treatment of acute liver failure in a new rat model. Langenbecks Arch Surg. (386), 534-541 (2002).
- Schleimer, K., et al. Improved microcirculation of a liver graft by controlled portal vein arterialization. J Surg Res. 116 (2), 202-210 (2004).
- Schleimer, K., et al. Portal hyperperfusion causes disturbance of microcirculation and increased rate of hepatocellular apoptosis: investigations in heterotopic rat liver transplantation with portal vein arterialization. Transplant Proc. 38 (3), 725-729 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone