Method Article
An in vivo Assay to Test Blood Vessel Permeability
W tym Artykule
Podsumowanie
We are presenting an in vivo assay to test blood vessel permeability. This assay is based on intravenous injection of a dye and subsequent visualization of its diffusion into interstitial spaces.
Streszczenie
This method is based on the intravenous injection of Evans Blue in mice as the test animal model. Evans blue is a dye that binds albumin. Under physiologic conditions the endothelium is impermeable to albumin, so Evans blue bound albumin remains restricted within blood vessels. In pathologic conditions that promote increased vascular permeability endothelial cells partially lose their close contacts and the endothelium becomes permeable to small proteins such as albumin. This condition allows for extravasation of Evans Blue in tissues. A healthy endothelium prevents extravasation of the dye in the neighboring vascularized tissues. Organs with increased permeability will show significantly increased blue coloration compared to organs with intact endothelium. The level of vascular permeability can be assessed by simple visualization or by quantitative measurement of the dye incorporated per milligram of tissue of control versus experimental animal/tissue. Two powerful aspects of this assay are its simplicity and quantitative characteristics. Evans Blue dye can be extracted from tissues by incubating a specific amount of tissue in formamide. Evans Blue absorbance maximum is at 620 nm and absorbance minimum is at 740 nm. By using a standard curve for Evans Blue, optical density measurements can be converted into milligram dye captured per milligram of tissue. Statistical analysis should be used to assess significant differences in vascular permeability.
Wprowadzenie
Formation and maintenance of selective permeable barriers are essential for proper organ development and performance 1,2. Endothelial cells line the blood vessel lumen and form a semi-permeable barrier that is essential in the selective transport between blood and the interstitial space of all organs. An adequate permeability barrier is maintained through tight cell-to-cell junctions that are strictly controlled by growth factors, cytokines and other stress related molecules 3. Disruption of the endothelial cell barrier can result in increased permeability and vascular leakage. These effects are seen in various disease states and the understanding of the underlining molecular signaling requires multidisciplinary methods 4,5. In this article, we describe an in vivo method to measure vessel permeability using a mouse model.
The assay we are describing, also known as Miles assay, is a well-established method to test vascular permeability in vivo. The assay is based on the fact that, under basal physiological conditions, albumin does not cross the endothelial barrier. Evans Blue, an azo dye with high affinity for albumin, is injected in the blood stream of an experimental animal, and under physiologic conditions, it is expected to be restricted within blood vessels. When a vascular permeability stimulus is added, either topically or systemically, blood vessels start to leak protein and thus, also the Evans blue that is bound to albumin. This results in a rapid bluish coloration of tissues that have permeable vessels.
Successful injection of the dye in the mouse lateral tail vein is critical for the good outcome of the experiment. Tail vein injection technique requires extensive practice and should be mastered prior to starting the experiment.
Vessel permeability is highly dependent on the age and weight of the animal, so when comparing different mouse strains it is imperative that the mice or other test subjects have close to identical birth dates and weight. Other factors that influence the permeability of the endothelial barrier are environmental conditions such as temperature, humidity, and very importantly, the handling stress of the mouse. Due to the multitude of factors that can influence the outcome of the experiment it is always advisable that the experiment is repeated at least three times and statistical analysis performed.
This assay can be used or to compare vessel permeability of mice that have been genetically modified, as well as mice with different genetic backgrounds. Permeability can be assessed in the presence or absence of a stimulus, depending on the function of the gene that is modulated. This assay can also be used or to test the effect of different compounds on vessel permeability.
Protokół
1. Intravenous Injection of Evans Blue in the Mouse Lateral Tail Vein
- Prepare a 0.5% sterile solution of Evans blue in PBS. If necessary, filter-sterilize the solution to remove any particulate matter that has not dissolved.
- Aspirate 200 μl Evans Blue solution into a syringe. Avoid all air bubbles that might have escaped into the syringe.
- Place mice that are 8-12 weeks old into a restraint device so that the animal is not freely mobile but its tail can be handled.
- Place the mouse restraint device on its side so the lateral tail vein is easily visible and are facing upward.
- Hold onto the tail with the non-dominant hand between the thumb and the forefinger.
- Insert the needle (small gauge, 27-30) at a 10-15 degree angle, bevel up, advance into the lateral tail vein towards the direction of the head. Keep the needle and syringe parallel to the tail.
- Do not apply back pressure to confirm proper placement as this might collapse the vein.
- Slowly inject 200 μl Evans Blue solution in the tail vein of the mouse.
- Observe the ease with which the plunger advances, as this is the proof of correct placement of the needle in the vein.
- Put the mouse back into its cage and observe it for 30 min.
2. Organ Collection and Extraction of Evans Blue from the Organs
- Sacrifice the mice through cervical dislocation. For Miles assay purposes cervical dislocation is recommended as it limits significant interference with vascular permeability. Sacrifice all the mice at the same time, as fast as possible. Work with cohorts of 6 mice or less, because shortly after death blood vessels become more permeable.
- Place the mice on their backs and pin their feet on a white board.
- Open the abdominal and thoracic cavity to expose thoracic and abdominal organs.
- Take representative pictures to show differences in Evans Blue extravasation. Include all mice in the same field in order to have identical lighting conditions for all mice.
- Collect organs of interest and put them in 1.5 ml tubes.
- Weigh an empty tube and bring the balance value to zero.
- Transfer the tissue sample and weight it. Repeat for all tissue samples. Tissues can be air dried to eliminate the water content variability between different organs.
- . Add 500 μl formamide to each tissue sample tube.
- Transfer all tubes to a 55 °C water bath or heat block. Incubate for 24-48 hr to extract Evans Blue from tissue.
3. Quantification of Evans Blue Extravasated in Interstitial Tissue
- Centrifuge the Formamide/Evans Blue mixture to pellet any remaining tissue fragments.
- Measure absorbance at 610 nm. Use 500 μl Formamide as blank.
- Calculate ng Evans Blue extravasated per mg tissue.
- Plot all data in a graph.
- Perform statistical analysis to determine significant differences.
Wyniki
We used an in vivo permeability assay to test vessel leakage in mice 8-12 weeks old. This test is useful in comparing the relative vascular permeability between animals of different genetic background or in a single strain of mice subjected to treatments that affect the vasculature. Our results show that a strain of genetically modified mice we created in our lab has a more permeable endothelium compared to wild-type mice. These changes are evident at macroscopic level in many organs (Figure 1).
We were able to quantify the difference in vessel permeability by spectophotometricaly measuring the Evans Blue that was captured per gram of tissue in different organs. As we observed in Figure 2 different organs show different response to permeability inducer signals and thus show different dye accumulation. This is also a function of the levels of vascularization in different tissues.
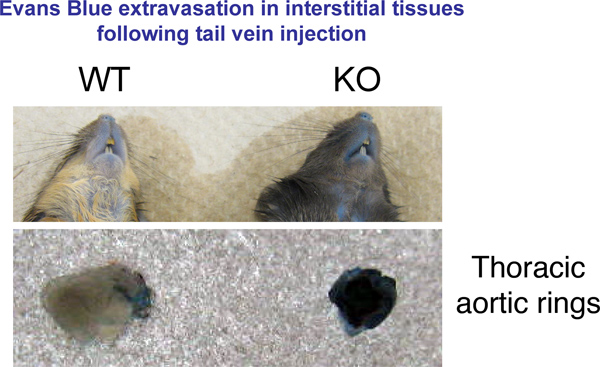
Figure 1. Evans Blue Extravasation in Tissues. 30 min after Evans blue injection mice were sacrificed through cervical dislocation. Representative pictures of organs of interest were taken.
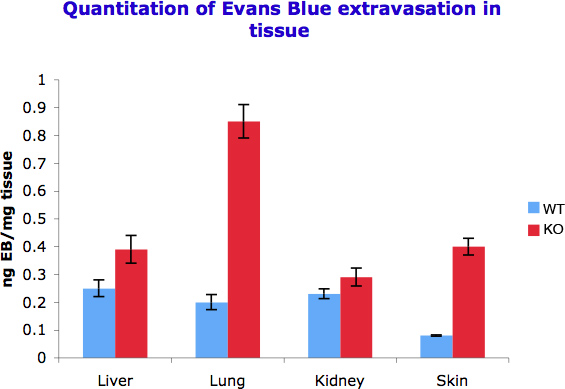
Figure 2. Quantitation of Evans Blue extravasation in different tissues. 50-100 mg tissue was incubated with 500 μl formamide to extract extravasated Evans Blue. Optical density was measured at 610 nm and the measurements converted into ng dye extravasated per mg tissue. The experiment was repeated three times.
Dyskusje
Vascular permeability is a critical marker for blood vessel status. Increased vascular permeability has been shown to be present in several systemic diseases, including diabetes, hypertension, and autoimmune diseases 6,7,8. Increased vascular permeability has been shown to be mediated by shear stress, growth factors like vascular endothelial growth factor and fibroblast growth factor, inflammatory mediators like serotonin, histamine and bradykinin 9. Extravasation of water and small molecules is thought to occur through small openings between endothelial cells. The strength of cell-to-cell junctions is strictly regulated by interaction between molecules 10.
In physiological conditions endothelium is permeable to water and ions, and impermeable to proteins. Thus, in the absence of inflammatory stimuli, albumin is restricted to the blood flow and does not move into the extracellular fluid. By injecting Evans Blue, a dye that binds albumin, we can monitor the extent of protein leakage from the blood stream into the interstitial tissue.
A modified version of this assay uses fluorescent-labeled microspheres. By using different size microspheres or different molecular weight fluorescent- labeled dextran (4-70 KDa) one can better assess the extent of endothelial damage. However, this option eliminates the ease of visualization and it requires fixing the tissues and fluorescent microscopy imaging, or measurement using a fluorescence plate reader.
In vitro studies of vessel permeability have been used successfully in the literature 11. An essential ingredient of any in vitro permeability study is an intact, confluent cell monolayer. These assays use a classic double chamber where endothelial cells are cultured in a monolayer on a permeable membrane situated in the upper chamber. A dye is applied to the upper chamber and endothelial cell permeability is assessed by measuring the amount of dye that reaches the lower chamber. The results mimic in most cases the in vivo assay results. However, they lack the appropriate physiological context and are thereby complicated by the complexity of results. The in vitro approach also eliminates the role of pericytes, cells that, in living tissues, are in close contact with endothelial cells and send signals for endothelial cell proliferation, vessel growth and branching.
The Miles test results should ideally be accompanied by molecular studies that further examine the hypothesis that is being tested. As mentioned, in a whole organism, vascular permeability is dependent on many variables, and thus the results of an in vivo permeability assay need to be interpreted in light of the complexity of the system analyzed.
Ujawnienia
The authors have no conflict or competing financial interests.
Podziękowania
This work was supported by a grant from the National Institutes of Health, R01CA142928.
Materiały
| Name | Company | Catalog Number | Comments |
| REAGENT | |||
| EVANS BLUE | SIGMA | E2129 | |
| FORMAMIDE | INVITROGEN | 15515-026 | |
| PBS | 0.2M Phosphate 1.5M NaCl pH 7.4 | ||
| EQUIPMENT | |||
| SPECTROPHOTOMETER | EPPENDORF | 952000006 | |
| MOUSE RESTRAINT DEVICE | HARVARD APPARATUS | 340012 | |
| SYRINGE | BD | 309659 | |
| NEEDLES | BD | 305106 | The gauge of the needle depends on the size of the animal. |
| BALANCE | DENVER INSTRUMENT | TP-64 | |
Odniesienia
- Beck, K. F., et al. Inducible NO synthase: role in cellular signaling. J. Exp. Biol. 202, 645-653 (1999).
- Bertglia, S., Giusti, A. Role of nitric oxide in capillary perfusion and oxygen delivery regulation during systemic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 288, H525-H531 (2005).
- Miles, A. A., Miles, E. M. Vascular reactions to histamine, histamine-liberator and leutaxine in the skin of guinea pigs. J. Physiol. (London). 118, 228-257 (1952).
- Weis, S. M. Vascular permeability in cardiovascular disease and cancer. Curr. Opin. Hematol. 15, 243-249 (2008).
- Kumar, P., Shen, Q., Pivetii, C. D., Lee, E. S., We, M. H., Yuan, S. Y. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 30, 11-19 (2009).
- Viazzi, F., et al. Vascular permeability, blood pressure, and organ damage in primary hypertension. Hypertens. Res. 31, 873-879 (2008).
- Scheppke, ., et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J. Clin. Invest. 118, 2337-2346 (2008).
- Blanchet, M. R., et al. Loss of CD34 Leads To Exacerbated Autoimmune Arthritis through Increased Vascular Permeability. J. Immunol. 184, 1292-1299 (2010).
- Dvorak, A. M. Mast cell-derived mediators of enhanced microvascular permeability, vascular permeability factor/vascular endothelial growth factor, histamine, and serotonin, cause leakage of macromolecules through a new endothelial cell permeability organelle, the vesiculo-vacuolar organelle. Chem. Immunol. Allergy. 85, 185-204 (2005).
- Le Guelte, A., Gavard, J. Role of endothelial cell-cell junctions in endothelial permeability. Methods Mol. Biol. 763, 265-279 (2011).
- Martins-Green, M., Petreaca, M., Yao, M. An assay system for in vitro detection of permeability in human "endothelium". Methods Enzymol. 443, 137-153 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone