Method Article
Direct Cryosectioning of Drosophila Heads for Enhanced Brain Fluorescence Staining and Immunostaining
In This Article
Summary
This study presents a simplified protocol for tissue processing involving decapitation, fixation, cryosectioning, fluorescence staining, immunostaining, and imaging, which can be extended to confocal and multiphoton imaging. The method maintains efficacy comparable to complex dissections, bypassing the need for advanced motor skills. Quantitative image analysis provides extensive investigative potential.
Abstract
Immunostaining Drosophila melanogaster brains is essential for exploring the mechanisms behind complex behaviors, neural circuits, and protein expression patterns. Traditional methods often involve challenges such as performing complex dissection, maintaining tissue integrity, and visualizing specific expression patterns during high-resolution imaging. We present an optimized protocol that combines cryosectioning with fluorescence staining and immunostaining. This method improves tissue preservation and signal clarity and reduces the need for laborious dissection for Drosophila brain imaging. The method entails rapid dissection, optimal fixation, cryoprotection, and cryosectioning, followed by fluorescent staining and immunostaining. The protocol significantly reduces tissue damage, enhances antibody penetration, and yields sharp, well-defined images. We demonstrate the effectiveness of this approach by visualizing specific neural populations and synaptic proteins with high fidelity. This versatile method allows for the analysis of various protein markers in the adult brain across multiple z-planes and can be adapted for other tissues and model organisms. The protocol provides a reliable and efficient tool for researchers conducting high-quality immunohistochemistry in Drosophila neurobiology studies. This method's detailed visualization facilitates comprehensive analysis of neuroanatomy, pathology, and protein localization, making it particularly valuable for neuroscience research.
Introduction
Complex behaviors ranging from social interactions1, sensory perception and processing2, learning3, to movement4 are driven by the brain. Neurological disorders are also increasingly common and predicted to increase with time5,6. It is critical to study how the brain works in both health and disease. The central dogma of molecular biology suggests that one of the most important functions of biological units is proteins7, and both how much and where they are expressed are critical to understanding how the brain works.
Drosophila melanogaster, commonly known as the fruit fly, is a highly valuable model for studying brain function under aging and pathophysiological conditions8. The availability of advanced genetic tools in Drosophila enables researchers to explore the function of nearly any protein9, with comprehensive genetic libraries for almost every gene readily accessible10. Coupled with its short lifespan and high reproductive rate, these features make Drosophila an exceptional model for brain research11. This has led to significant achievements, including developing a complete brain map of the fly12, and even contributed to a Nobel Prize for elucidating the neuronal mechanisms of circadian rhythms and molecular clocks13,14,15. As a result, Drosophila remains a powerful and versatile system, driving forward our understanding of brain function and providing unprecedented insights into neurological processes.
Immunohistochemistry and immunofluorescence are foundational tools to study protein expression in situ. In contrast to techniques like Western Blot, which only allows for semiquantitative analysis and is typically conducted in bulk tissue16, or complicated and expensive techniques like mass spectrometry to measure protein level17, immunohistochemistry is relatively straightforward and allows for both the quantification of protein expression and for measuring the localization of a protein within a tissue or cell. Importantly, fluorescent immunohistochemistry can also be multiplexed to measure multiple proteins to identify specific cell types and tissues or answer multiple questions in the same tissue. Additionally, tissue fixation can allow comparisons across different experimental conditions, genotypes, ages, and circadian time points. However, fluorescent immunohistochemistry can be challenging, and many factors can influence image quality. This optimized cryosectioning and immunostaining protocol for Drosophila brains aims to enhance high-resolution imaging by improving tissue preservation, antibody penetration, and visualization of neural populations and protein markers. Developed to address challenges in traditional methods, such as complex dissection, tissue damage, and limited imaging resolution associated with whole-brain mounts18. This protocol combines cryosectioning with fluorescence staining to ensure structural integrity and sharp imaging across multiple z-planes. Compared to whole-mount preparations, this method minimizes distortion, facilitates deeper antibody diffusion, and provides clear neuroanatomical and protein localization analyses18. Its versatility allows adaptation for other tissues and model organisms, offering a reliable and efficient tool for neuroscience research19,20. It can be adapted to look at almost any protein and applied to study any condition, disease, or model.
Protocol
1. Preparation of equipment
- Ensure that the cryostat is powered on and set to -20 °C. Power on the slide warmer or a small incubator, ensuring it is set to 37 °C.
NOTE: At this stage, labeled slides can be placed on the warmer or incubator and left indefinitely until sectioning.
2. Preparation of solutions
- Prepare 50 mL of 1x Phosphate buffered saline (PBS), pH 7.4, from 10x PBS stock. Prepare a solution of 4% Paraformaldehyde in PBS with a final volume of 10 mL.
- Prepare a cleaning solution of 70% ethanol in water in a spray bottle. Prepare a blocking solution (3% Bovine serum albumin (BSA) in Tris-buffered saline from 20x stock). Prepare a primary antibody solution (dilution is experiment-dependent). Here, a 1:250 dilution of ApoE Mouse Antibody in 3% BSA in TBS is used.
- Prepare a secondary antibody solution (dilution is experiment-dependent). Here, a 1:500 dilution of Alexa Flour 750 A21037 Goat Anti-Mouse Secondary in 3% BSA in TBS is used.
- Prepare fluorescent stain solution (dilution is experiment-dependent). Here, a 5 µg/mL solution of Nile Red in PBS is prepared.
NOTE: All solutions, particularly fluorescents, should be refrigerated and stored in a dark refrigerated container. They should also be brought to room temperature just prior to use.
3. Collection of tissue
- After obtaining flies in aging vials, open the valve on the CO2 mat and dump the flies onto the mat quickly to avoid escape. Wait for the flies to become mostly unconscious, ceasing most movement. Position flies underneath the SZ61 microscope by moving the mat underneath the objective lens. Adjust magnification and focus such that the flies are clearly visible and comfortable to decapitate.
NOTE: The flies used for this example are ELAV/+ and ELAV>ApoE4 - Insert spring scissors between the thorax and head, squeeze firmly, and decapitate 5 to 10 fly heads per experimental group. Return unused flies to their aging vial.
NOTE: If concern regarding penetration of fixative exists, a small incision can be performed on the posterior of the head to allow for increased penetration of the fixative agent in step 4.1. - Using a brush, gently place heads into labeled 1.5 mL tubes on ice until ELAV/+ and ELAV>ApoE4 groups have been collected.
4. Fixation of whole tissue
- Remove the tubes from ice and pipette 100 µL of 4% Paraformaldehyde in PBS solution into each tube, ensuring that all heads are submerged in the solution. If heads are adhering to the walls of the tube and avoiding contact with the solution, use a brush to gently push them downward or tap lightly against a horizontal surface to ensure appropriate contact.
- Place on an orbital shaker using a medium setting for 15 min. After 15 min, discard the Paraformaldehyde solution and replace it with 1x PBS for 10 min, ensuring that all heads are submerged in the solution.
- Discarding the previous solution each time, wash the tissue with PBS 2x for 10 min each, for a total of 3 washes.
- After the final wash, transfer the heads to a 10% sucrose in PBS solution, ensuring the heads are submerged within the tube. Leave these overnight for optimal saturation.
NOTE: If same-day imaging is desired, the sucrose infusion can be reduced; however, cryoprotectant effects will also be reduced proportionally.
5. Mold preparation
- Fill a labeled mold approximately 50% with Optimal Cutting Temperature (OCT) compound, allowing it to spread to all 4 corners of the mold.
- Using a brush, carefully place the heads on the surface of the OCT within the mold. When placing multiple experimental groups into the same mold, ensure that these groups are separated within the mold to prevent confusion.
NOTE: It is important to prevent dilution of the OCT compound through contamination with the prior sucrose solution via the brush. In addition, placement deep into the OCT using the brush can create many bubbles around the heads. Avoid these mistakes to prevent sectioning issues later on. - Using the tip of the forceps, slowly push each head to the bottom of the mold with eyes facing the bottom. This orientation is selected for a cut through the coronal plane.
- Avoid puncturing or crushing the heads against the bottom of the mold during this process. Align all heads in the X, Y, and Z dimensions so that each section contains all subjects simultaneously. Ensure slow submersion to reduce air bubble formation.
- Once all heads are aligned properly, carefully place molds directly into -20 °C to freeze.
NOTE: If the alignment of heads is noted to be significantly off during sectioning, consider the use of dry ice or liquid nitrogen for initial snap freezing of the mold. - Once the mold has mostly frozen, fill the remainder with OCT and allow it to freeze before storing for the long term at - 80 °C.
6. Cryosectioning of molds
NOTE: It is generally advisable to prepare and cut a blank mold before cutting experimental group molds. This allows to ensure the proper functionality of the wheel, blade, and anti-roll glass immediately before sectioning tissue.
- Attach the chuck bit to the mold by applying a generous amount of OCT to the mold and placing the bit on top, pressing it flat. Allow this to freeze completely in the cryostat, usually within 5 min.
- Release the bit and OCT block from the mold and place it into the chuck, ensuring the mold remains properly oriented from top to bottom. Tighten the chuck key until the bit is secure.
- Align the block with the blade using the adjustment knobs and chuck depth controls.
- Set the section width to 20 µm. Cut each slice using slow but consistent motion, allowing the anti-roll glass to capture each slice. Capture sections using the warmed slides by touching the slide to the closer edge of the section and allowing the section to rise up onto the slide. Up to eight sections can be fit on a standard-size slide (25 mm x 75 mm x 1mm) when spaced appropriately.
NOTE: There is a matter of choice to be made when choosing which sections to collect. As a result of the embedding orientation, earlier sections will be from the anterior, and later sections will be from the posterior portions of the head. As such, if interest lies in one particular area more than another, section selection can be adjusted accordingly. Alternatively, all sections can be collected on multiple slides until no tissue remains. - Allow slides to dry at room temperature for at least 30 min but no more than 1 h.
7. Staining and IHC
NOTE: For this protocol, the method will detail IHC using an unconjugated primary antibody. Fluorochrome conjugated antibodies, or other fluorescence stains that can be performed in a single stage, should be used together with the secondary antibody if both are to be used together.
- Immediately following the drying period, remove OCT on the edges of the slide using a razor blade, leaving room for a hydrophobic border. Draw a hydrophobic border on each slide using the marker. Allow this to dry for 5 min.
- Wash all slides 3 times for 5 min each with PBS by pipetting gently on top of the slide, avoiding pipetting directly on top of tissue when possible.
NOTE: The 1000 µL pipette is most useful here. A standard-size slide with tissue should be completely covered with 750µL of any solution. Slide racks and buckets may also be used. - After the slides have been washed, pipette 3% BSA in TBS blocking solution onto the slides. Allow to incubate for 30 minutes.
- Discard the blocking solution and pipette the primary antibody solution onto the slides. In this case, a 1:250 dilution of SC-13521 ApoE in 3% BSA in TBS. Allow the antibody to incubate overnight at 4 °C or for 1 h at room temperature. Use wet tissue wipes or paper towels to prevent the solution from drying out overnight.
- Discard the primary antibody and using the pipette, wash 3 times for 5 minutes each with PBS.
- Add the secondary antibody solution. In this case, a 1:500 dilution of AF 750 Goat Anti-Mouse in addition to a 5 µg/mL concentration of Nile Red, all in 3% BSA in TBS. Allow this to incubate for 1 hour at room temperature.
NOTE: We simultaneously incubate a fluorescent stain and a secondary antibody in the same buffer, 3% BSA in TBS. This is preferred as long as there are no known conflicts between the two reagents. If conflicts exist, incubate separately and perform an additional 3 washes between incubations. - Wash slides 3 times with PBS for 5 min each. After the final wash, leave a small amount of PBS on the slide.
8. Mounting and preparation for imaging
- Using a 1000 µL pipette, add 3-5 drops of hardening mounting media containing DAPI (0.9µg/ml), evenly across the slide avoiding direct dropping on tissue.
- Place the coverslip on the slide, avoiding any air bubble formation.
NOTE: The use of forceps can aid in this by allowing the coverslip to slide close to the surface of the slide before finally releasing. - Handle freshly mounted slides with care and store them flat until completely dry. Seal slides using nail polish if long-term storage is anticipated.
- Capture images as soon as possible to avoid fluorescent decay.
9. Image acquisition
NOTE: For image capture, the use of Olympus Cell Sense Dimensions software will be detailed.
- Before imaging, inspect slides and wipe them using 70% ethanol in water solution. If slides are imaged immediately following mounting, take extra care when cleaning to avoid disturbing the coverslip. Allow the surfaces of the slide and coverslip to dry for 5 min before imaging.
- Select each desired channel for capture, in this case, the channels for DAPI, Nile Red, and AF 750.
- Select the desired magnification as described below. The choice of magnification is experiment-dependent; here, use 10x magnification.
NOTE: All magnifications are usable, including oil-based lenses. If desired, apply immersion oil to the slide surface. - For each channel, calibrate proper exposure times based on the brightest fluorescing subjects in an experiment. Avoid overexposure while generating as bright an image as possible.
NOTE: In general, the two main determinants of poor process capture are overexposure, resulting in the loss of definition, and too much background signal, which is difficult to separate from the true signal. In addition, exposures must be set independently for each unique magnification used. - Select the folder to save and name the merged images within the menu. Following capture, all images will be located in this folder and bear the name defined here.
- Capture images by first focusing on a subject in the channel that allows for the best focal point and then begin collection. Maintain the procedure of focusing on each subject in the same channel for consistency across the experiment.
- Capture all experimental groups used for later comparisons on the same day to avoid fluorescent decay between compared groups.
- Select the desired magnification as described below. The choice of magnification is experiment-dependent; here, use 10x magnification.
10. Quantification
NOTE: Quantification can be performed using a variety of softwares. Here, the use of Olympus CellSense Dimensions is referenced.
- Following the collection, review the images. Remove sections that possess imperfections or sectioning artifacts from the quantification folder. Select images that reflect the goal of quantification. Examples of imperfections are bubbles, torn sections, overlapping tissue, and dust or debris.
- To select appropriate images, ensure that all images featuring brain tissue are used for quantification to reflect overall expression throughout the brain. Alternatively, use images only reflecting the fore, mid, or hind brain to give insight into differences in expression in these areas.
- Within the count and measure menus, determine the parameters of interest and their boundaries for the quantification of images.
- For quantification of ApoE expression by mean intensity value comparison, select thresholding values such that all pixels for that channel are considered (0-infinity). In addition, outline regions of interest using available drawing tools.
- Run the count and measure either in a batch for all files or individually on each image.
- For batch processing, record the settings and execution of quantification and then select the folder to perform quantification. Processing in a batch is accessible through the macro manager tab and greatly accelerates workflow.
- Export the data table to a spreadsheet for plotting. To do this, use the count and measure results menu while opening or exporting to a table automatically when performing in a batch. These data tables will contain all selected parameters chosen in step 10.2.
- For statistical analysis and plotting, plot data using a spreadsheet directly, or through other software. Familiarity with the chosen software is key to generating an accurate representation of the experimental outcomes. Here, absolute intensity values of ApoE expression in the brain of the mutant were normalized to the control and then plotted using GraphPad Prism.
Results
The method described above allows for fluorescence imaging of adult fly brains reliably and without tedious dissection. Illustrated simply in Figure 1, the method is straightforward and can be performed rapidly if all specimens, equipment, and materials are readily available. Alternatively, using -80 °C storage during the OCT mold stage, specimens can be kept for use many weeks later. Researchers need not be trained long to learn the simple dissection and embedding techniques, making this method quite accessible.
Examples of fluorescence microscopy performed using this method can be seen in Figure 2A-D. The expression of both the antibody tag (ApoE) and the fluorescent stain (Nile Red) is well-defined. Additionally, the high integrity of brain tissue can also be seen. Images can be further clarified using common image processing software features such as deconvolution, which is also shown in panel Figure 2A'-D'. Deconvolution is useful for improving sharpness and contrast and reducing noise.
With regards to the quantification of images, all images can be quantified for standard parameters such as object count, mean object area, intensity, and area fraction. The limitations of quantification truly depend on the software of choice, but generally, the above-listed parameters allow for adequate investigation and are available in most applications. Figure 3 demonstrates a histogram generated from mean intensity values within the brain regions of multiple images. This serves to visualize the mean brain intensity of a given genotype. In this case ELAV/+ and ELAV>ApoE4.
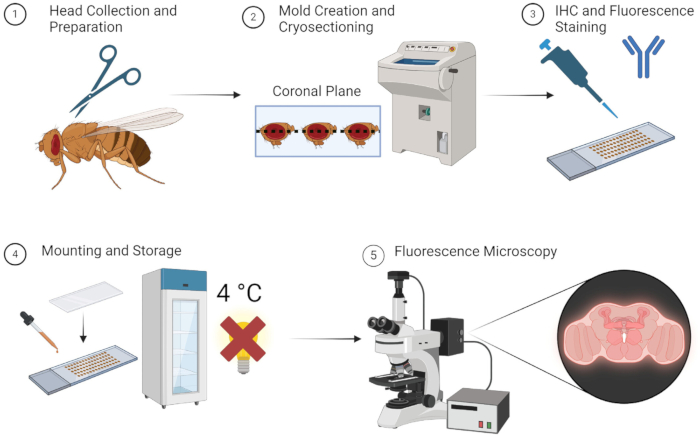
Figure 1: Workflow for Drosophila brain sectioning and imaging. 1. Drosophila heads are collected by decapitation using fine scissors. 2. After fixation, the collected heads are embedded with OCT in a mold and cryosectioned to obtain thin brain sections. 3. The brain sections are subjected to immunohistochemistry (IHC) staining with primary antibodies or fluorescent staining. 4. After staining, the sections are mounted with a cover slip and stored at 4 °C in the dark to maintain fluorescence integrity. 5. The brain sections are visualized using fluorescence microscopy to study the targeted proteins or cellular structures. Please click here to view a larger version of this figure.

Figure 2: Immunohistochemistry and fluorescence staining of cryosectioned heads. (A-D) Representative images of 7-week-old female fly heads stained for ApoE expression, lipids (Nile Red), and DAPI. These images demonstrate the expression of human ApoE4 (Elav>ApoE4), while control flies Elav/+ lack this ApoE expression. Lipid staining with Nile Red (Elav>ApoE4, and Elav/+). Brain tissue integrity using this method is evidenced here as well. (A'-D') These represent the A, B, C, and D after deconvolution. Please click here to view a larger version of this figure.

Figure 3: Relative quantification of ApoE. Showed are the results of quantification for ApoE protein expression in 7-week-old Drosophila brains. The absolute values of intensity were collected for both Elav/+ and Elav>ApoE4 mutant subjects and then normalized to Elav/+. Statistical significance between Elav>ApoE4 and Elav/+ is apparent. Please click here to view a larger version of this figure.
Discussion
Here, we present a protocol for precise fluorescent imaging of cryosectioned Drosophila heads. This is a straightforward approach that has several important positives. Namely, the methods are simple enough that anyone with basic laboratory safety training could complete, they are adaptable to measure the expression of any protein that high-quality antibodies exist for, and they allow for precise measurement of both how much a protein of interest is expressed and where that expression occurs throughout the head. If tissue and image quality are high enough, it could potentially allow for 3D mapping of expression throughout the entire Drosophila head.
In contrast to technically challenging approaches to isolate the brain for imaging combined with confocal microscopy, this protocol allows for imaging throughout the entire Drosophila head, not just the brain. This is especially important given that the fly visual system is an important model of neuronal function21, but some of these structures could be lost during isolation of the brain. It also provides in-section control tissues, for example, the muscles of the proboscis, to determine whether the expression of the protein of interest is specific to certain cell types or tissue types22. This allows for direct comparison between tissues, rather than using separate sections that could be impacted by slight differences in conditions. Another strength of this protocol and using Drosophila as a model system is that fly heads are very small19,20, so relatively high-content fluorescent imaging can be done. We have been able to put over 50 heads in a single block. With 8 sections able to be placed on an industry-standard size slide, 1 slide could contain 8 unique sections per fly for a total of 400 unique sections. Importantly, all sections are subjected to the same experimental conditions and time points to achieve great integrity when comparing between groups. Additionally, this protocol only requires the use of a standard fluorescent microscope rather than a confocal microscope, which may be cost-prohibitive for laboratories or institutions that lack high financial support and allow for access to this imaging more broadly.
One key limitation to this protocol, which is inherent to immunostaining, is the importance of using high-quality antibodies and appropriate controls when optimizing the antibodies. It is critical to verify that the antibodies are selective and sensitive to the protein of interest, as there is often off-target staining that can lead to challenges in determining if the fluorescent signal is real. To do so, test both the primary and secondary antibodies. Fortunately, it is straightforward to do this because of the easily available genetic tools in Drosophila. To verify the quality of the primary antibody, we would recommend completing the protocol with wild-type flies and experimental flies alongside positive control flies that overexpress the protein of interest and negative control flies that have the gene knocked out or knocked down. These control groups will establish the range of expression levels and expected fluorescence, serving as reference points to assess where the experimental flies fall within that spectrum. It is critical to test multiple dilutions of the primary antibody, as too little will lead to poor quality staining that does not lead to fluorescence above the background where protein is present, while too much antibody will lead to off-target staining where the protein is absent. This multiple dilution approach should include a group with no primary antibody, which will verify the quality of the secondary antibody, as there will only be fluorescence in this group if there is background fluorescence and provides an additional negative control group that shows the limits of fluorescent detection by the secondary antibody. If needed, use different incubation conditions, i.e., time or temperature, to maximize signal-to-noise in your final images. We reiterate that high-quality antibodies, along with high-quality tissue, are critical for high-quality immunofluorescent imaging.
Altogether, immunofluorescent imaging is a powerful tool to study biology more broadly and neuroscience more specifically. This, combined with the powerful genetic tools available in Drosophila, has the potential to uncover important knowledge of how proteins impact health and disease. The fly brain has been demonstrably important for discovering how the human brain functions and will continue to do so. Because of the adaptability of the protocol, we describe here, the role of almost any protein (or lipid) in the head can be studied in the context of any disease or condition, only limited by the quality of antibodies. Thus, immunofluorescent imaging will likely be critical for future research into how the brain works and will have a significant impact on human health and therapeutic development.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank members of the Melkani lab for their help with valuable feedback for developing the protocol. Fly stocks, Elav-Gal4 (BL#458) and UAS-ApoE4 (BL#76607) were obtained from Bloomington Drosophila Stock Center (Bloomington, IN, USA). This work was supported by National Institutes of Health (NIH) grants AG065992 and RF1NS133378 to G.C.M. This work is also supported by UAB Startup funds 3123226 and 3123227 to G.C.M.
Materials
| Name | Company | Catalog Number | Comments |
| 1000 uL Pipette | Eppendorf | 3123000063 | |
| 1000 uL Pipette Tips | Olympus Plastics | 23-165R | |
| 10X Phosphate Buffered Saline (PBS) | Fisher | J62036.K7 | ph=7.4 |
| 200 Proof Ethanol | Decon Laboratories | 64-17-5 | |
| 20X Tris Buffered Saline | Thermo Scientific | J60877.K2 | pH=7.4 |
| AF750 Goat Anti-Mouse Secondary Antibody | Alexa Fluor | A21037 | |
| Anti-Roll Glass | IMEB | AR-14047742497 | |
| ApoE Mouse Primary Antibody | Santa Cruz | SC13521 | |
| Bovine Serum Albumin | Fisher | 9048-46-8 | |
| Centrifuge Tubes 1.5 mL | Fisher | 05-408-129 | |
| Charged Slides | Globe Scientific | 1415-15 | |
| Cryosectioning Molds | Fisher | 2363553 | |
| Cryostat | Leica | CM 3050 S | |
| Cryostat Blades | C.L. Sturkey | DT554N50 | |
| Distilled Water | |||
| Dry Ice | ??? | ??? | |
| Fine Forceps | Fine Science Tools | 11254-20 | |
| Fly Pad | Tritech Research | MINJ-DROS-FP | |
| Hardening mounting Media with Dapi | Vectashield | H-1800 | |
| Kimwipes | Kimtech | 34120 | |
| Microscope | Olympus | SZ61 | |
| Nile Red | Sigma | N3013 | |
| Optimal Cutting Temperature Compound | Fisher | 4585 | |
| Orbital Shaker | OHAUS | SHLD0415DG | |
| Paraformaldehyde 20% | Electron Microscopy Sciences | 15713 | |
| Razor Blades | Gravey | #40475 | |
| Spring Scissors | Fine Science Tools | 15000-10 | |
| Sucrose | Fisher | S5-500 |
References
- Fabbri-Destro, M., Rizzolatti, G. Mirror neurons and mirror systems in monkeys and humans. Physiology. 23, 171-179 (2008).
- Faust, T. E., Gunner, G., Schafer, D. P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat Rev Neurosci. 22, 657-673 (2021).
- Roozendaal, B., McEwen, B. S., Chattarji, S. Stress, memory and the amygdala. Nat Rev Neurosci. 10, 423-433 (2009).
- Arber, S., Costa, R. M. Networking brainstem and basal ganglia circuits for movement. Nat Rev Neurosci. 23, 342-360 (2022).
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 7, e105-e125 (2022).
- Huang, Y., Li, Y., Pan, H., Han, L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J Glob Health. 13, 04160 (2023).
- Crick, F. Central dogma of molecular biology. Nature. 227, 561-563 (1970).
- Ugur, B., Chen, K., Bellen, H. J. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9, 235-244 (2016).
- Hales, K. G., Korey, C. A., Larracuente, A. M., Roberts, D. M. Genetics on the Fly: A primer on the Drosophila model system. Genetics. 201, 815-842 (2015).
- Larkin, A., et al. FlyBase: updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 49, D899-D907 (2021).
- Jeibmann, A., Paulus, W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci. 10, 407-440 (2009).
- Winding, M., et al. The connectome of an insect brain. Science. 379, eadd9330 (2023).
- Bargiello, T. A., Jackson, F. R., Young, M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 312, 752-754 (1984).
- Zehring, W. A., et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 39, 369-376 (1984).
- Huang, R. C. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed J. 41, 5-8 (2018).
- Mahmood, T., Yang, P. C. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 4, 429-434 (2012).
- Ong, S. E., Foster, L. J., Mann, M. Mass spectrometric-based approaches in quantitative proteomics. Methods. 29, 124-130 (2003).
- Behnke, J. A., Ye, C., Moberg, K. H., Zheng, J. Q. A protocol to detect neurodegeneration in Drosophila melanogaster whole-brain mounts using advanced microscopy. STAR Protoc. 2, 100689 (2021).
- Moraes, R. C. M., et al. Apolipoprotein E induces lipid accumulation through Dgat2 that is prevented with time-restricted feeding in Drosophila. Genes. 15 (11), 1376 (2024).
- Roth, J. R., et al. Rapamycin reduces neuronal mutant huntingtin aggregation and ameliorates locomotor performance in Drosophila. Front Aging Neurosci. 15, 1223911 (2023).
- Currier, T. A., Pang, M. M., Clandinin, T. R. Visual processing in the fly, from photoreceptors to behavior. Genetics. 224, (2023).
- McKellar, C. E., Siwanowicz, I., Dickson, B. J., Simpson, J. H. Controlling motor neurons of every muscle for fly proboscis reaching. Elife. 9, (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved