Method Article
Development of a Benchtop Model for Evaluating the Compatibility of Wound Dressing Materials with Negative Pressure Wound Therapy Systems
In This Article
Summary
This study presents a benchtop model designed to evaluate the compatibility of wound dressing materials with negative pressure wound therapy systems by assessing pressure and fluid collection over 72 h under continuous and intermittent pressure settings.
Abstract
Negative pressure wound therapy (NPWT) systems facilitate wound healing by applying sub-atmospheric pressure to the wound bed, which promotes granulation tissue formation and reduces inflammation. Wound dressings can be used with these systems to enhance healing; however, the effects of dressings on NPWT device performance are challenging to assess. The purpose of this study was to develop a benchtop flesh analog model for testing the compatibility of wound dressing materials with NPWT devices. In this study, a chitosan-based advanced wound care device was evaluated for its effects on NPWT performance under maximum and minimum therapy pressures. The goal was to use the model to compare pressure readings and fluid collection for samples with and without the chitosan wound care device. The benchtop model was constructed using a plastic box connected to multiple pressure gauges. A circular defect was created on a piece of pork belly, used as the flesh analog, and inserted into the box. The defect was filled with standard NPWT foam or foam combined with the wound dressing. Simulated body fluid containing bovine serum was added to the box, which was then tested at either maximum (-200 mmHg) or minimum (-25 mmHg) pressures for 72 h. Pressure and fluid collection were recorded every 12 h. The NPWT system successfully maintained pressure over the 72 h test period, both with and without the test dressings. The addition of the wound dressings did not impact fluid collection. The test box proved effective as a benchtop model, as it could be sealed and maintained vacuum conditions over the 72 h testing period. This model successfully demonstrated its utility in evaluating the compatibility of wound dressing materials with NPWT systems.
Introduction
Different therapeutic approaches exist to aid in the management and healing process of wounds. Such therapeutic approaches include advanced wound dressings, growth factors, hyperbaric oxygen therapy, skin substitutes, and negative pressure wound therapy (NPWT)1. NPWT refers to wound dressing systems that continuously or intermittently apply sub-atmospheric pressure to the system, which provides negative pressure to the surface of the wound. NPWT has become a popular treatment modality for the management of acute or chronic wounds2. The NPWT system consists of an open cell foam, adhesive wound dressing, a fluid collection system, and a suction pump3. The suction pump, or vacuum, is used to maintain a steady pressure on the wound, which helps increase blood flow and reduce the risk of infection4. NPWT promotes granulation tissue formation by removing fluid from the wound and reducing swelling1. Clinically, the amount of suction pressure used for wounds ranges from -20 mmHg to -200 mmHg, but the most relevant pressure tested is -125 mmHg5.
Ex vivo experiments of NPWT are a challenge due to a lack of adequate benchtop models for testing. Current methods for testing NPWT systems include finite element analysis (FEA) computer simulations, which have been used to test how NPWT affects incision sites6. Other models include benchtop agar-based wound models, which can be used to test fluid uptake7. In vivo, porcine models also have been used to examine wound healing8. These models have advantages such as being easy to simulate on a computer for predicting how a wound should heal in theory, as well as testing fluid being pulled through a model material. In vivo testing is definitive for determining whether the system works in live subjects8. These models all have disadvantages as well. A computer simulation may not accurately represent how a wound would heal in real life. An agar-based model may show good fluid collection being pulled through the wound but may not represent how fluid would be pulled through tissue and muscle7. In vivo models are expensive and require significant resources to complete a study. Also, it can be difficult to keep animals semi-immobile, so there may be challenges with them pulling at the system, which may have confounding results.
A benchtop model is needed for NPWT so that new materials can be tested for use with the system using actual tissue. The new model should be able to reflect how fluid collection is affected by tissue and muscle. The new model should also be able to provide pressure readings inside the wound bed to determine whether the wound was receiving as much pressure as the vacuum pump was supplying. New materials/devices may also be tested, such as additional wound dressings, different types of foam, and different adhesive dressings on top of the wound.
Certain wounds require additional wound dressings to aid in the healing process by reducing the risk of infection. Another reason additional wound dressing materials may be required is to prevent tissue ingrowth between the surface of the wound bed and the open-cell foam. This additional dressing reduces the risk of the wound bed adhering to the open-cell foam, which helps reduce damage and pain when stopping the NPWT system9. These additional dressings can be placed around the open-cell foam to act as a barrier membrane between the wound bed and the foam. Certain materials have been used as an interface between the wound bed and foam, such as paraffin or Vaseline-embedded gauze. Paraffin has shown positive potential as a wound dressing by not affecting the transfer of pressure from the system to the ound9. However, Vaseline-embedded gauze was reported to inhibit fluid collection and thus was not considered to be an appropriate additional material9.
Chitosan-based wound dressings may be a good additional dressing to add during NPWT due to their antimicrobial effects and biocompatibility10,11. Chitosan is an N-deacetylated derivative of chitin, which is a natural polysaccharide found in fungi and arthropods12,13. Chitosan has exhibited inherent antibacterial properties in a broad spectrum of gram-negative and gram-positive bacteria14. Therefore, chitosan membranes have become popular in the treatment of wounds because they can be easily produced, have a long shelf life, and show innate antimicrobial effects10. These membranes also show good biocompatibility biodegradation, and are non-toxic10.
In this study, Foundation DRS, a chitosan and glycosaminoglycan advanced wound care device, was examined to determine its biocompatibility with NPWT. Foundation DRS is a biodegradable dermal regeneration scaffold manufactured for ideal handling characteristics and porosity to promote cellular invasion and neo-angiogenesis in wounds. This device is advantageous for healing in a range of different injuries and uses. It was created for intended use in a wide range of wounds, such as pressure ulcers, diabetic foot ulcers, first-degree burns, trauma wounds, dehisced wounds, and surgical wounds10,11. Foundation DRS is a good option for use in NPWT due to its manufacturing process, which prevents the device from turning into a hydrogel when it is wet. This device maintains an open pore structure when wetted, which should allow fluid to flow during the application of NPWT12,13.
The objective of this study was to develop a benchtop flesh analog model that could be used to test the compatibility of wound dressing materials with NPWT devices. Clinically, pressures range from -80 mmHg to -125 mmHg for most NPWT applications4. To simulate worst-case clinical use conditions, a higher and lower pressure setting were used (-25 mmHg and -200 mmHg). Another objective of this study was to determine if the addition of the chitosan wound care device interfered with the pressure readings and fluid collection of the NPWT. Disruptions in fluid collection or losses of pressure during NPWT could lead to poor wound healing and clinical outcomes. The fluid collection should be similar to the test groups with and without the chitosan wound care device. Pressure readings should also be similar across the test groups over 72 h. In clinical settings, the wound dressing is changed every 48-72 h, so each sample was tested for 72 h in this study3. During testing, the pressure readings should be observed to ensure there is not a drop in pressure.
Protocol
The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Creation of the test box
- Obtain a 3.2-cup plastic container.
- Create a 2-inch diameter hole in the center of the container lid. Also, make two 3/8 holes in two corners of the container lid approximately 1/2 inch from the edge seal. Use a hole saw to create the holes.
NOTE: A schematic showing the overall testing setup using a commercial NPWT machine connected to a lab-built benchtop flesh analog box is shown in Figure 1. This schematic outlines how the box is used for experiments. The box created for this experiment is shown in Figure 2. - On the first of the 3/8 holes, connect a pressure gauge directly to the hole.
NOTE: This gauge was used to monitor for pressure drops outside of the test tissue, which would indicate leaks in the tissue. - On the second 3/8 hole, feed a small flexible IV tube with an outer diameter of less than 3/8 through the hole to a length of 7 inches on the inner side of the lid. Then, fit the pressure tube to a low-pressure gauge outside the container.
NOTE: The pressure tube was placed in the wound bed during testing.
2. Flesh analog preparation
- Use commercially available salted pork belly, known hereto as tissue, to simulate the muscle and fat tissue for NPWT testing.
- Create a circular wound defect in the surface of the tissue using a #21 blade scalpel that is approximately 1.5 inches wide by 0.75 inches deep. Then, fenestrate the tissue through the fat on each side with a #21 blade scalpel.
- After the wound defect is created, wipe down the tissue to remove excess fat from the skin, and then soak overnight in deionized water to remove excess salt.
3. Loading of the test chamber
- Fill the bottom of the test chamber with open-cell foam that is 1.5 inches thick. Then, place the tissue on top of the foam.
NOTE: Manually center the tissue sample so the wound defect created is directly under the hole in the top of the lid. - For the experimental groups, add the chitosan wound care device inside the wound defect so that the bottom and sides of the defect are covered. Then, fill the rest of the defect with the open cell foam.
- Insert the pressure tube connected to the pressure gauge on the testing chamber into the open cell foam that is used to fill the defect. Ensure that this tube is placed approximately halfway down from the surface of the wound defect.
- Cover the tissue with the adhesive wound dressing. Then, create a small cut on the adhesive dressing, directly on top of the middle of the open cell foam, filling the wound defect.
- Thread the vacuum nozzle through the lid of the testing chamber and place it on top of the adhesive dressing, where the small cut was made. After placing the vacuum nozzle, close the lid of the testing chamber to press the adhesive wound dressing and vacuum nozzle down, which helps create a seal.
- Connect the 500 mL fluid collection cannister to the vacuum pump and then connect the vacuum nozzle to the fluid collection cannister.
4. Creation of the simulated body fluid
- Create a simulated body fluid according to Marques et al.15.
- Make the simulated body fluid by combining 8.035 g of NaCl, 0.355 g of NaHCO3, 0.225 g of KCl, 0.231 g of K2HPO43H2O, 0.311 g of Cl2Mg6H2O, 0.292 g of CaCl, 0.072 g of NaSO42-, 6.118 g of (HOCH2)3CNH2, and 39 mL of 1 M HCl in 960 mL of deionized water to bring the total solution to 1 L.
NOTE: The composition of the simulated body fluid is shown in Table 1. - Then, combine the simulated body fluid with bovine serum in a 3:1 ratio. Supplement the final solution with 5% of 10x antibiotics/antimycotics for microbial control. Stir the solution after adding the bovine serum and antibiotics/antimycotics, and then store it in a refrigerator.
NOTE: The final solution will be referred to as the complete solution. This solution should not be kept sterile and should be made fresh before each sample is tested.
5. Test conditions
- Adjust the settings on the vacuum pump for the samples depending on the test condition.
NOTE: The test groups are: Group 1 Control (n = 3): Foam alone with continuous suction at -200 mmHg; Group 2 Control (n = 3): Foam alone with intermittent suction from 0 to -200 mmHg; Group 3 (n = 3): Chitosan Wound Care Device under Foam with continuous suction at -200 mmHg; Group 4 (n = 3): Chitosan Wound Care Device under Foam with intermittent suction from 0 to -200 mmHg; Group 5 Control (n = 3): Foam alone with continuous suction at -25 mmHg; Group 6 Control (n = 3): Foam alone with intermittent suction from 0 to -25 mmHg; Group 7 (n = 3): Chitosan Wound Care Device under Foam with continuous suction at -25 mmHg; Group 8 (n = 3): Chitosan Wound Care Device under Foam with intermittent suction from 0 to -25 mmHg. - For maximum pressure test groups, set the pressure at -200 mmHg. For minimum pressure testing groups, set the pressure at -25 mmHg. Then, place the vacuum pump settings on intermittent or continuous pressure. Run all samples for 72 h.
NOTE: The continuous setting applied pressure continuously for 72 h. The intermittent setting applied pressure at a 5/2 ratio (5 min of pressure, followed by 2 min with no pressure) for 72 h. The maximum and minimum values were chosen based on the pressure range that clinical NPWT systems can use. A 72 h cycle was chosen based on the length of time NPWT is typically used clinically before performing a bandage change3. - During testing, record the pressure on the pressure gauge and the amount of fluid in the fluid collection canister every 12 h for 72 h.
- If the amount of body fluid analog drops below 75% of the top of the testing chamber, as observed visually, remove the secondary pressure gauge and add a complete solution to the chamber.
NOTE: The preparation of samples and testing setup can be seen in Figure 3. - After 72 h, turn the vacuum pump off and disconnect the fluid collection canister from the vacuum nozzle. Remove the fluid collection canister from the vacuum pump.
- Remove the tissue from the testing chamber and pull the adhesive wound dressing off. Then, take out the open cell foam and observe whether the chitosan wound care device was still intact. It is considered intact if it can be removed without breaking, ripping, or tearing; however, minor tears or thinning are acceptable if the membrane can be removed completely.
6. Statistical analysis
- Use the pressure values that were recorded every 12 h during the testing period from the three test specimens per test condition for statistical analysis. For statistical analysis, the final fluid collection value from the three test specimens was used per test condition.
NOTE: For all statistical analyses, the level of significance was set at α = 0.05. - Calculate the mean and standard deviations(n = 3/group) at each time point. Before performing the statistical analysis, perform normality testing for each group using the Shapiro-Wilk test (e.g., continuous suction at -200 mmHg, continuous suction at -25 mmHg, intermittent suction at -200 mmHg, and intermittent suction at -25 mmHg) to determine if ANOVA or Kruskal-Wallis test is appropriate.
- Analyze data for experimental and control groups subjected to the same pressure test conditions (e.g., continuous suction at -200 mmHg; continuous suction at -25 mmHg; intermittent suction at -200 mmHg or intermittent suction at -25 mmHg) using a two-way ANOVA or Kruskal Wallis test using membrane type and time as main factors.
- If statistical differences were identified, perform post-hoc analyses. Use Tukey's HSD post-hoc test after the ANOVA or the Dunn post-hoc test after the Kruskal-Wallis test to determine which groups are different.
- Using the final fluid collection values for each sample in the control and experimental groups, perform a two-tailed t-test assuming unequal variances.
NOTE: Pressure was analyzed at each time point to ensure there was no significant drop in pressure across the duration of the testing period. While fluid collection was examined at each time, it was only analyzed at the final time point. This is because each tissue had different fat and muscle profiles, resulting in different fluid collection rates, making overall fluid collection more useful for analysis than fluid collection by time points.
Results
The goal of the study was to develop a benchtop model for NPWT that uses a tissue analog and to use the model to investigate the compatibility of wound dressing materials with a negative pressure wound therapy machine. The model was used to study if the NPWT machine was able to maintain pressure over time with the addition of a wound care device. The model was also used to determine if the pressure generated and fluid collected by the NPWT machine in the presence of a wound care device were different as compared to the absence of the device.
The mean ± standard deviation pressures were calculated at each time point over the 72 h test for each control and experimental group. For each group, pressure readings were compared to determine if there were any pressure drops or pressure increases over time. For all four test conditions at maximum and minimum pressure and for both control and experimental groups, there was no statistical change in pressure over the 72 h testing period (p > 0.7). Since no pressure drop was seen within any group over the 72 h testing period, the addition of the chitosan membrane did not affect the performance of the vacuum pump during testing.
At maximum pressure (Figure 4), there was no difference seen between pressure readings from the control and experimental groups for the continuous test conditions, but there was a difference for the intermittent test condition. For the continuous test condition, the experimental group showed similar pressure readings (-169.6 mmHg ± 1.56 mmHg) as compared to control (-172.9 mmHg ± 2.18 mmHg) (p = 0.27).
At minimum pressure (Figure 5), there was a difference seen between pressure readings from the control and experimental groups for the continuous test condition, but there was no difference for the intermittent test condition. For the continuous test condition, the experimental group showed lower pressure readings (-21.8 mmHg ± 0.7 mmHg) as compared to the control (-27.1 mmHg ± 1.75 mmHg) (p = 6 x 10-7). For the intermittent test condition, the experimental group showed similar pressure readings (-20.6 mmHg ± 1.45 mmHg) as compared to control (-23.4 mmHg + 1.83 mmHg) (p = 0.29). Fluid collection was observed to be similar across all groups (Figure 6 and Figure 7).
There was variation in pressure readings among test specimens. The variation was largely attributed to the amount of fat each tissue had and how fenestrated the tissue was before testing and not to the testing chamber since it was routinely checked for leaks, and if detected, the leak was fixed prior to sample testing.
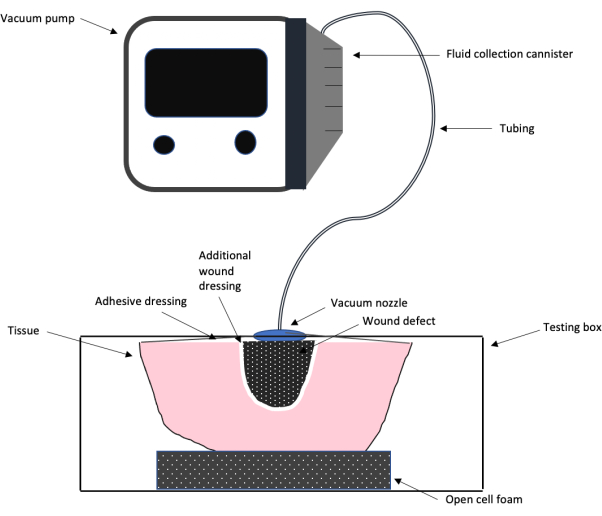
Figure 1: Schematic of the NPWT test setup. A schematic showing the overall testing design setup of the negative pressure wound therapy system used in this work, including the vacuum pump, tubing, adhesive wound dressing and foam, additional wound dressing, and wound defect. Please click here to view a larger version of this figure.

Figure 2: NPWT benchtop model design. A representative image of the plastic box design created for the NPWT benchtop model. Please click here to view a larger version of this figure.
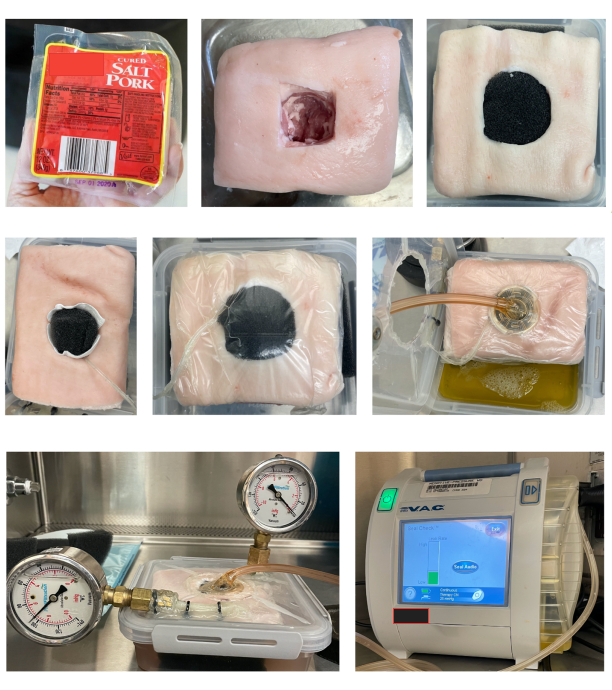
Figure 3: Steps in preparing specimens and assembling components for testing. The steps taken for testing the sample such as sample preparation, loading of the test chamber, and the overall setup. The testing chamber setup in this experiment shows two pressure gauges: one connected on its side to read the pressure (left) and one used as a failsafe and to remove to add more fluid to the chamber (right). The testing chamber also shows the placement of the tissue filled with foam, wound dressing, and vacuum nozzle placed on top. Please click here to view a larger version of this figure.

Figure 4: Maximum pressure comparisons. The mean ± standard deviation pressure readings for control and experimental groups (Groups 1-4, n = 3/group) at maximum pressure (-200 mmHg) for 72 h. The dotted blue line and dashed grey line show the continuous pressure groups. The small-dashed orange line and dashed and dotted yellow lines show the intermittent pressure groups. The pressure readings were averaged from the three samples in each group from 0-72 h in 12-h increments. Please click here to view a larger version of this figure.
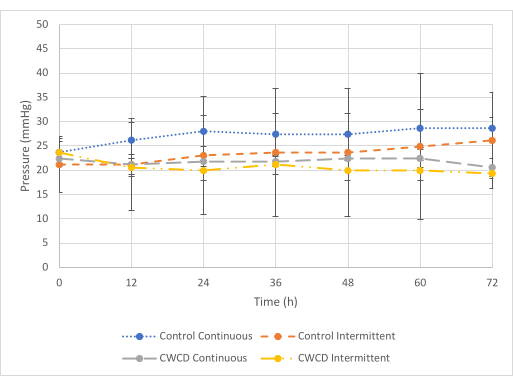
Figure 5: Minimum pressure comparisons. The mean ± standard deviation pressure readings for control and experimental groups (Groups 5-8, n = 3/group) at minimum pressure (-25 mmHg) for 72 h. The dotted blue line and dashed grey line show the continuous pressure groups. The small-dashed orange line and dashed and dotted yellow lines show the intermittent pressure groups. The pressure readings were averaged from the three samples in each group from 0-72 h in 12-h increments. Please click here to view a larger version of this figure.

Figure 6: Representative images of the final fluid collection for Groups 1 through 4. (A) A representative image of the fluid collection after 72 h for Group 1 control sample under continuous suction at maximum pressure. (B) A representative image of the fluid collection after 72 h for the Group 2 control sample under intermittent suction at maximum pressure. (C) A representative image of the fluid collection after 72 h for the Group 3 test sample under continuous suction at maximum pressure. (D) A representative image of the fluid collection after 72 h for the Group 4 test sample under intermittent suction at maximum pressure. Please click here to view a larger version of this figure.

Figure 7: Representative images of the final fluid collection for Groups 5 through 8. (A) A representative image of the fluid collection after 72 h for the Group 5 control sample under continuous suction at minimum pressure. (B) A representative image of the fluid collection after 72 h for the Group 6 control sample under intermittent suction at minimum pressure. (C) A representative image of the fluid collection after 72 h for group 7 test sample under continuous suction at minimum pressure. (D) A representative image of the fluid collection after 72 h for the Group 8 test sample under intermittent suction at minimum pressure. Please click here to view a larger version of this figure.
| Reagent | Amount |
| Sodium chloride | 8.035 g |
| Sodium bicarbonate | 0.355 g |
| Potassium chloride | 0.225 g |
| Potassium phosphate dibasic trihydrate | 0.231 g |
| Magnesium chloride hexahydrate | 0.311 g |
| 1 M hydrochloric acid | 39 mL |
| Calcium chloride | 0.292 g |
| Sodium sulfate | 0.072 g |
| Tris(hydroxymethyl) aminomethane | 6.118 g |
Table 1: The reagents required to prepare the simulated body fluid in 1 L of DI water.
Discussion
There are a few benchtop models for NPWT, but they have significant limitations. Loveluck et al. developed an FEA computer model to determine how NPWT affected sutured incision sites but did not account for additional wound dressing materials6. Rycerz et al. developed agar-based models to evaluate instillation solution distribution to wounds during NPWT7. While the agar provided a medium for assessing the distribution of water-soluble materials/dyes in the different models, it is a simple homogeneous material that does not replicate the complex heterogeneous structure of wounds involving muscle and fat tissues subjected to NPWT. Also, these tests were performed for relatively short time periods of up to 3.5 h, while in clinical conditions, it is common for the vacuum to be on for 48-72 h before changing the wound dressing3. Alternatively, in vivo, porcine models can be used but are expensive and require special oversight and approval processes prior to use7. No current benchtop models exist that use a realistic tissue composition and structures to assess the fluid collection and pressures inside the wound bed relative to the vacuum pump under different experimental conditions.
To address this limitation, a benchtop model was designed and built to be able to read pressure over the 72 h period from inside of the wound defect to see how much pressure was being applied to the wound relative to the vacuum pump reading. This was achieved by using the pressure gauge connected to the pressure tube that ran through the middle of the foam. To replenish fluid in the container during testing and not affect the vacuum, a second pressure gauge was added, which could be removed for pipetting in additional fluid. This second pressure gauge also acted as a failsafe because it should read zero, and if it did not, then the testing chamber was being pressurized instead of the tissue sample, which would render the test invalid. Another issue that the system addressed was ensuring there was enough of a seal on the sample; this was achieved by placing a piece of foam under the sample to raise it that way when the lid of the testing chamber was closed, the tissue was pushed down enough to close the chamber without there being extra space.
The pork belly was used to address the need for a wound tissue analog. Pork belly, which comes from the underside of the pig's stomach, was used because it has layers of fat and muscle, which mimic the complex human muscle and fat tissues and better simulate wound tissue characteristics. In addition, pork belly is easily obtained inexpensively, and only minimal manipulation is needed to remove curing salts prior to use. The different compositions of tissue samples used might have caused some of the pressure differences seen across groups. Different fat and tissue compositions, or how the foam was placed in the tissue, may have affected the pressure the machine was able to pull. Clinically, patients will also exhibit variations of muscle, fat, and tissue, so the differences seen due to the pork belly composition might be representative of the variation in how the device works in patients.
This benchtop model design also allows for new applications for NPWT to be tested in vitro, from different pressures to different wound dressing materials. It also allows pressure to be recorded from inside the wound bed where the foam is located versus only observing the pressure readings from the vacuum pump. This shows whether the pressure applied from the pump is being applied inside the wound. The model created in this study is helpful because it allows fluid collection and pressure to be recorded without using computer simulations or in vivo testing. Similarly, this benchtop model is advantageous because it allows a look at how the NPWT machine works on muscle and fat tissues that more closely mimic the clinical condition instead of agar-based materials. This model is more affordable than in vivo testing since it does not require the use of living animals, and it is created from low-cost items.
This model was used to compare the pressures generated and fluid collected by an NPWT machine with and without a commercial chitosan wound care device. The NPWT machine was able to maintain pressures over the 72 h period in the presence or absence of the chitosan advanced wound care device. When the NPWT machine was used with or without the wound care device, there were no or only small differences in pressure readings between the control (no wound care device) and test (with wound care device) groups over a 72 h period under the maximum and minimum pressure test conditions. However, there were no differences in fluid collection between the control and test membranes across the treatment groups.
Certain steps in the protocol were critical in ensuring pressure and fluid collection readings were accurate for each sample. The most important steps in the testing process were found in Step 1. To ensure precise pressure readings were recorded, the testing box created had to show no leaks; otherwise, the pressure gauges would not work properly. Other critical steps in this process include steps 2.2 and 3.1. Step 2.2 was crucial because the wound defect had to be deep enough to penetrate through the final layer of fat in the sample, and the tissue needed to be fenestrated for fluid collection. Step 3.1 was critical because the defect needed to be filled with open-pore foam. The foam must fill the entire wound defect and fit snugly; otherwise, it will not create a proper seal with the vacuum, which causes pressure reading issues. Step 3.4 was critical because the adhesive wound dressing needed to cover the entire sample. If the adhesive wound dressing did not cover the entire sample, there could be pressure leaks inside the testing chamber.
A limitation of this work is that there is no comparable model to compare. Replication and use of the model by others will help confirm the utility of the model. Another limitation of this model is the potential for air leaks by connecting the pressure tube to the pressure gauge. A better way to minimize risks of air leaks by using a different connection approach could be helpful in future studies.
This benchtop model was needed for NPWT so that new material could be tested for compatibility by examining fluid collection and pressure generation to ensure that the additional wound dressing did not alter the performance of the NPWT system. The model created has many potential applications in NPWT. It may be used to test different wound dressing materials and local drug delivery options in wound dressing materials. Several types of wounds may be created and tested using this model, such as incisional, tunneling, or burn wounds. For the different wound types, pressure and fluid collection could be examined to determine the optimal NPWT settings. This model also opens other avenues for deciding how to test NPWT in skin samples, whereas before, models were mostly restricted to other materials or simulated tests. In conclusion, the designed benchtop model was successfully able to evaluate the pressures inside of a wound bed for wound dressings to determine if they were compatible with the NPWT system.
Overall, the tested chitosan wound care device did not appear to inhibit pressure or fluid collection in the NPWT system for the conditions tested. In this work, a benchtop model was developed to overcome the limitations of current benchtop models by allowing for testing over extended periods of time, using a tissue analog, and the ability to accommodate the use of wound dressing materials.
Disclosures
This work was supported by a grant from Bionova Medical, Inc. (Germantown, TN).
Acknowledgements
This research was made possible with the help of the University of Memphis Department of Biomedical Engineering and Bionova Medical.
Materials
| Name | Company | Catalog Number | Comments |
| 100x antibiotics/mycotics | Gibco | 15240062 | This is the 100X antibiotics/antimycotics used in the simulated body fluid |
| 3 M KCI ACTIV.A.C Therapy System | KCI Mdical Products | VFTR006619 | This is the vacuum pump used in the study. |
| 3 M KCI InfoV.A.C Canister w/Gel 500 mL | eSutures.com | M8275063 | These are the fluid collection canisters used in the study |
| 3 M KCI V.A.C GranuFoam Medium Dressing Kit, SensaT.R.A.C | eSutures.com | M8275052 | These are the wound dressing packs with the vacuum nozzle including the open cell foam. |
| Bovine Serum | Gibco | 16170086 | This was used to mix with the simulated body fluid and the antibiotics/antimycotics |
| Calcium Chloride | Fisher Scientific | C614-500 | This was used to create the simulated body fluid |
| Excel/Powerpoint | Microsoft Office | N/A | This was used to run the statistics and create the schematic for Figure 1 |
| Foundation DRS Solo | BioNova Medical | N/A | This is the advanced chitosan wound care device used in the study. |
| Hydrochloric Acid | Fisher Scientific | SA54-1 | This was used to create the simulated body fluid |
| Magensium Chloride | Fisher Scientific | M33-500 | This was used to create the simulated body fluid |
| Phosphate buffered saline | Thermo Scientific | J62036.K3 | This was used to dilute the 100x antibiotic/antimycotic to 10x |
| Potassium Chloride | SIGMA | P-3911 | This was used to create the simulated body fluid |
| Potassium Phosphate Dibasic | Fisher BioReagents | BP363-500 | This was used to create the simulated body fluid |
| PRM Vacuum Gauge 0 to -10 in Hg | PRM Filtration | PGCNBTY630652J10HG | Two pressure gauges are needed for the testing chamber. |
| Salted Pork Belly | Hormel Food Corporations | UPC: 0003760037988 | Salted pork belly can be bought from Kroger. It cannot be sliced. It is best to pick samples that have less fat, and more muscle. |
| Sodium Bicarbonate | SIGMA | S5761-500G | This was used to create the simulated body fluid |
| Sodium Chloride | Fisher Scientific | S640-500 | This was used to create the simulated body fluid |
| Sodium Sulfate | Fisher Scientific | BP166-100 | This was used to create the simulated body fluid |
| Tris(hydroxymethyl) aminomethane | Fisher Scientific | BP152-500 | This was used to create the simulated body fluid |
| Tupperware Brands Corp, Kissimmee , FL | Tupperware | N/A | This is the box used as the testing chamber. |
References
- Liu, S., et al. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: Systematic review and meta-analysis. Ther Clin Risk Manag. 13, 133-142 (2017).
- Capobianco, C. M., Zgonis, T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg. 26 (4), 619-629 (2009).
- Venturi, M. L., Attinger, C. E., Mesbahi, A. N., Hess, C. L., Graw, K. S. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: A review. Am J Clin Dermatol. 6 (3), 185-194 (2005).
- Ren, Y., Chang, P., Sheridan, R. L. Negative wound pressure therapy is safe and useful in pediatric burn patients. Int J Burns Trauma. 7 (2), 15-23 (2017).
- Argenta, L. C., Morykwas, M. J. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 38 (6), 563-576 (1997).
- Loveluck, J., Copeland, T., Hill, J., Hunt, A., Martin, R. Biomechanical modeling of the forces applied to closed incisions during single-use negative pressure wound therapy. Eplasty. 16, e20 (2016).
- Rycerz, A. M., Allen, D., Lessing, C. M. Science supporting negative pressure wound therapy with instillation. Int Wound J. 10 (S1), 25-31 (2013).
- Hodge, J. G., et al. Novel insights into negative pressure wound healing from an in situ porcine perspective. Wound Repair Regen. 30 (1), 64-81 (2022).
- Birke-Sorensen, H., et al. Evidence-based recommendations for negative pressure wound therapy: Treatment variables (pressure levels, wound filler and contact layer) - Steps towards an international consensus. J Plast Reconstr Aesthet Surg. 64 (Suppl. 1), S1-S16 (2011).
- Burkatovskaya, M., et al. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 27 (22), 4157-4164 (2006).
- Noel, S. P., Courtney, H., Bumgardner, J. D., Haggard, W. O. Chitosan films: A potential local drug delivery system for antibiotics. Clin Orthop Relat Res. 466 (6), 1377-1382 (2008).
- Chen, S., Hao, Y., Cui, W., Chang, J., Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J Mater Sci. 48 (19), 6560-6568 (2013).
- Guo, S., et al. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J Biomater Sci Polym Ed. 31 (2), 106-118 (2020).
- Qasim, S. B., Najeeb, S., Delaine-Smith, R. M., Rawlinson, A., Rehman, I. U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent Mater. 33 (1), 71-83 (2017).
- Marques, M. R. C., Loebenberg, R., Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolut Technol. 18 (3), 15-28 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved