Method Article
Endoscope-assisted Anterior Cervical Discectomy and Fusion for the Treatment of Cervical Spondylotic Myelopathy
Dans cet article
Résumé
We describe a minimally invasive surgery using Endoscope-assisted Anterior Cervical Discectomy and Fusion for the Cervical Spondylotic Myelopathy.
Résumé
Cervical spondylotic myelopathy (CSM) is a common cause of cervical spinal cord disease. Spinal endoscopy offers surgical advantages such as a magnified view and a water-mediated clear surgical field. This study describes an endoscope-assisted anterior cervical discectomy and fusion (ACDF) procedure. The addition of spinal endoscopy to traditional ACDF surgery magnifies the surgical field and allows for more precise operations, thereby improving surgical safety. Postoperatively, patients experienced significant improvements in neurological function, with no complications such as dysphagia, hematoma, or spinal cord injury. Postoperative imaging revealed that spinal cord compression was completely relieved, with sufficient decompression of the spinal cord and optimal placement of the fusion cage. The clear visual field provided by spinal endoscopy improves the identification of cervical anatomical structures during surgery, effectively reducing the risk of injury to the spinal cord and nerves. Endoscope-assisted ACDF has demonstrated excellent clinical and radiological outcomes in the treatment of CSM.
Introduction
Cervical spondylotic myelopathy (CSM) is one of the more severe forms of cervical spondylosis. CSM is a group of syndromes caused by degenerative changes in the cervical spine, leading to degeneration of the surrounding structures such as intervertebral discs and ligaments. These structures subsequently compress the spinal cord, resulting in limb dysfunction or even paralysis. Early diagnosis and timely intervention are critical for improving patient prognosis. Surgical intervention is often required when conservative treatments fail or spinal cord dysfunction worsens1,2.
Several surgical options are available for the treatment of cervical spondylotic myelopathy (CSM), including traditional anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), cervical disc replacement (CDR), and hybrid surgery (HS), which combines ACDF and CDR3. Traditional anterior cervical discectomy and fusion (ACDF) is a common treatment approach for CSM that effectively alleviates symptoms by directly decompressing the spinal cord and nerve roots. However, this traditional surgery has limitations, including a narrow surgical field and challenges with intraoperative hemostasis. These issues are particularly pronounced in patients with ossification of the posterior longitudinal ligament (OPLL), which prevents the compressive material from being completely removed during surgery and substantially elevates the risk of spinal cord injury4,5,6. In 1983, Bollati reported 57 anterior cervical surgeries performed with the assistance of a microscope; these patients demonstrated substantially reduced postoperative complications and increased safety and efficacy7. Compared with traditional ACDF, microsurgical techniques offer certain advantages; however, challenges such as insufficient precision, poor hand-eye coordination, and blind spots in the surgical field are still present, limiting the clinical application of these techniques8.
Spinal endoscopic surgical techniques, characterized by greater visual clarity, excellent tissue identification, and operational flexibility, have been widely applied in lumbar spine surgeries and have achieved favorable clinical outcomes9,10,11. Therefore, we integrated spinal endoscopic techniques with traditional ACDF to thoroughly remove ossification or free nucleus pulposus tissue located anterior to the spinal cord under the clear surgical field provided by the spinal endoscope. This approach eliminates blind spots associated with traditional surgeries and substantially reduces the risk of intraoperative spinal cord injury. This article aims to introduce the key technical aspects of endoscope-assisted ACDF. As traditional ACDF surgery has been extensively described in previous studies12, it will not be reiterated in this paper.
Protocole
This study was approved by the Ethics Committee of Hebei General Hospital. Informed consent was obtained from all individual participants.
1. Preoperative preparation
- Before surgery, place the patient in a supine position on the hospital bed, with soft pillows under both shoulders and a cylindrical pillow under the occiput to maintain the cervical spine in a hyperextended position.
NOTE: This step serves two purposes: (1) to allow the patient to adapt to the surgical position and (2) to assess, while the patient was awake, whether hyperextension aggravates any neurological symptoms. - Operation planning on the image: Acquire preoperative computed tomography (CT) with sagittal and coronal reconstructions to detect ossification of the posterior longitudinal ligament (OPLL). Perform magnetic resonance imaging (MRI) to evaluate preoperative spinal cord compression.
NOTE: The distance of ossification on sagittal CT images can aid in determining the upper and lower ranges of the window.
2. Skin marking and anesthesia
- Place the patient in a supine position on the operating table, and make skin markings at the surgical segment.
- Disinfect the surgical site by applying iodine applied twice and followed by a single application of 75% alcohol, using a circular motion from the incision site outward. Plan the incision as a transverse incision on the right anterior neck.
- The surgical disinfection range primarily covers the anterior neck and upper chest. Ensure that, vertically, it extends from the inferior border of the mandible to the superior border of the sternum and clavicle, and horizontally, it reaches the lateral borders of the sternocleidomastoid muscles on both sides.
- Administer preoxygenation using a 5 L/min pure oxygen face mask for at least 3 min to maintain a pulse oxygen saturation of 100% before initiating general anesthesia induction. Have a single anesthesiologist intravenously administer sufentanil citrate injection (0.3-0.5 µg/kg), cisatracurium besylate for injection (0.15-0.20 mg/kg), and etomidate injection (0.2-0.4 mg/kg), while continuous monitoring the electrocardiogram (ECG), blood pressure, and oxygen saturation to ensure intraoperative safety.
3. Exposure of the affected segment
- Make a transverse incision approximately 3 cm in length on the right anterior neck (Figure 1).
- Using the Smith-Robinson approach, expose the anterior aspect of the cervical vertebral body and intervertebral disc12. Use intraoperative C-arm fluoroscopy to confirm the target disc space.
- Incise the annulus fibrosus of the affected segment, and gradually remove the intervertebral disc and cartilage endplates until the posterior margin of the vertebral body is reached.
- Install a Caspar retractor, and insert the spinal endoscope system into the intervertebral space (Figure 2).
4. Endoscopic procedures for the cervical spine
- Under spinal endoscopic visualization, address bony spurs at the posterior margin of the cervical vertebral body, and enlarge the intervertebral space. Use a burr to remove the upper and lower bony spurs as well as the attachment points of the annulus fibrosus.
- The range of bony removal on both sides extends to the uncovertebral joints; determine the extent of cranial and caudal excision on the basis of the location and size of the ossification observed on preoperative imaging, ensuring sufficient excision at both ends.
- Excise the deep annulus fibrosus. Use nucleus pulposus forceps, Kerrison punches, and other tools alternately to decompress the spinal canal, progressively extending the decompression toward the spinal cord (Figure 3).
NOTE: During the burring process, lateral grinding and upward traction grinding techniques were prioritized to avoid excessive removal of superficial bone, thereby protecting the bony endplate. The use of a spinal endoscope with a 30° angle, tilting capability, and multidirectional burr further expanded the decompression range and ensured greater surgical precision.
- Incise the soft the posterior longitudinal ligament (PLL) to expose the spinal cord. After the annulus fibrosus and the superficial layer of the PLL are incised, use a Kerrison punch and a hook knife to cut through the soft PLL and separate the space between the PLL and the spinal cord (Figure 4).
NOTE: Once the gap between the spinal cord and the PLL are separated, subsequent decompression becomes significantly easier. PLL removal and spinal cord exposure are critical steps in this procedure. - For patients with OPLL, begin the procedure with the removal of the soft PLL and exposure of the spinal cord. Use a nerve root dissector to assess the space between the ossification and the spinal cord to confirm whether there is adhesion between the ossification and the dura mater. Use a 45° nucleus pulposus forceps or Kerrison punch to gradually remove the ossified ligament until the dura mater is fully exposed. Perform endoscopic visualization to check if the pulsation of the dura mater is satisfactory (Figure 5).
NOTE: During this process, a burr, nerve root dissector, nucleus pulposus forceps, or Kerrison punch were used alternately to increase operational flexibility and safety. - Use bipolar electrocoagulation probes for hemostasis, and apply fluid gelatin to assist in controlling bleeding. Once the surgical field is confirmed to be free of active bleeding, slowly withdraw the spinal endoscope (Figure 6).
NOTE: Avoid rapid removal of the spinal endoscope to prevent dural herniation caused by negative pressure. - Fill a properly sized titanium mesh cage with autologous bone and insert it into the intervertebral space. Select a titanium plate long enough to cover the surgical segment, and implant six screws into the vertebrae to secure the titanium plate, following the standard procedure of traditional ACDF12 to ensure postoperative stability and successful fusion.
5. Postoperative care
- Perform continuous ECG monitoring and administer intermittent oxygen inhalation and nebulization; encourage the patient to cough and expectorate.
- Assess the patient's pain intensity by inquiry and administer nonsteroidal analgesics promptly as needed.
- Closely monitor changes in limb muscle strength and sensory function.
- Remove the drainage tube when the drainage volume is less than 30 mL within 24 h postoperatively.
Résultats
This study included 20 patients who underwent endoscope-assisted ACDF surgery from January 2024 to November 2024. The average age was 62.2 years, and the study sample included 9 females and 11 males. The average operative time was 125.5 min, and the mean volume of blood loss was 59.0 mL (Table 1 and Table 2). All patients achieved successful relief of spinal cord symptoms, with lower postoperative visual analog scale (VAS) scores compared with the preoperative scores and significantly improved postoperative JOA scores. The average JOA score improvement rate was 60.7%. No complications, such as dysphagia, hematoma, or spinal cord injury, occurred (Table 1 and Table 2). Postoperative imaging confirmed complete removal of the compressive material, sufficient spinal cord decompression, and proper placement of the fusion cage (Figure 7 and Figure 8).
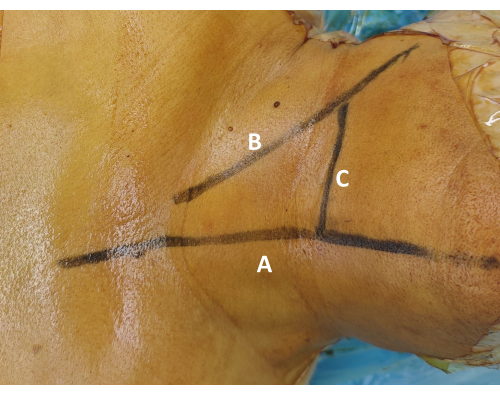
Figure 1: Surface marking of the surgical segment. (A) Draw the midline marking along the tracheal midline to ensure surgical symmetry. (B) Draw the transverse localization line along the superior border of the clavicle. Palpate the sternocleidomastoid muscle and cricoid cartilage to guide this marking. (C) Confirm the target level marking using fluoroscopic guidance, and mark the corresponding skin projection of the intended intervertebral disc. Please click here to view a larger version of this figure.
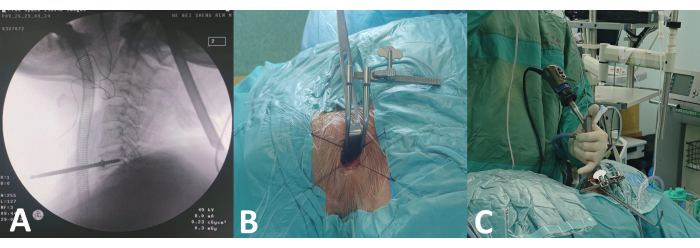
Figure 2: Preoperative procedures for spinal endoscopy. (A) Intraoperative C-arm fluoroscopy positioning. (B) Installation of Caspar retractor. (C) Spinal endoscopy. Please click here to view a larger version of this figure.
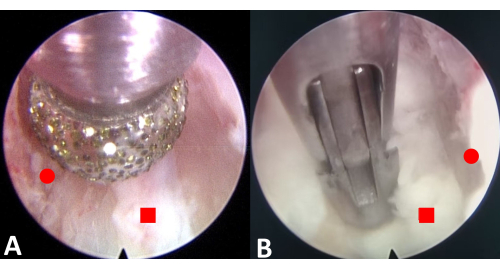
Figure 3: Endoscopic excision of the osteophytes and annulus fibrosus. (A) Burr the upper and lower osteophytes. (B) Resect the annulus fibrosus. The red circle represents the osteophyte, and the red square represents the annulus fibrosus. Please click here to view a larger version of this figure.
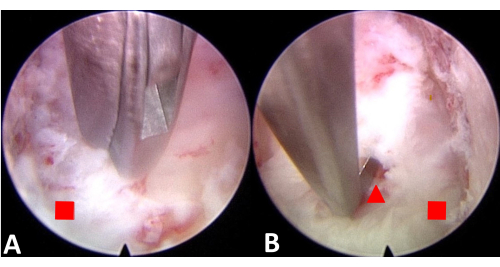
Figure 4: Endoscopic incision of the PLL and separation of the PLL from the spinal cord to create space. (A) Incise the PLL. (B) Separate the PLL from the spinal cord. The red square represents the posterior longitudinal ligament, and the red triangle represents the spinal cord. Please click here to view a larger version of this figure.
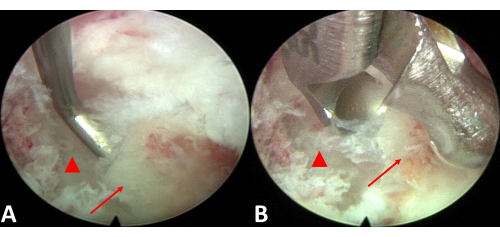
Figure 5: Endoscopic excision of the ossification. (A) Evaluate the space between the ossified tissue and the spinal cord. (B) Excise the ossified ligament. The red triangle represents the spinal cord, and the red arrow points to ossification of the posterior longitudinal ligament. Please click here to view a larger version of this figure.
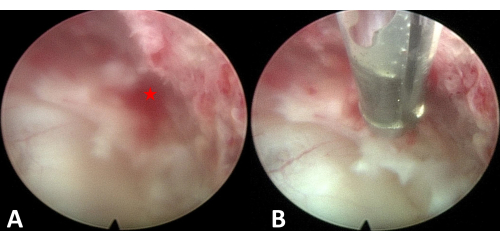
Figure 6: Hemostasis. (A) Bleeding point. (B) Hemostasis under endoscopic visualization. The asterisk represents the bleeding point. Please click here to view a larger version of this figure.

Figure 7: Pre and postoperative imaging. A patient with cervical spondylotic myelopathy before surgery, after surgery, and 3 months post surgery. Please click here to view a larger version of this figure.
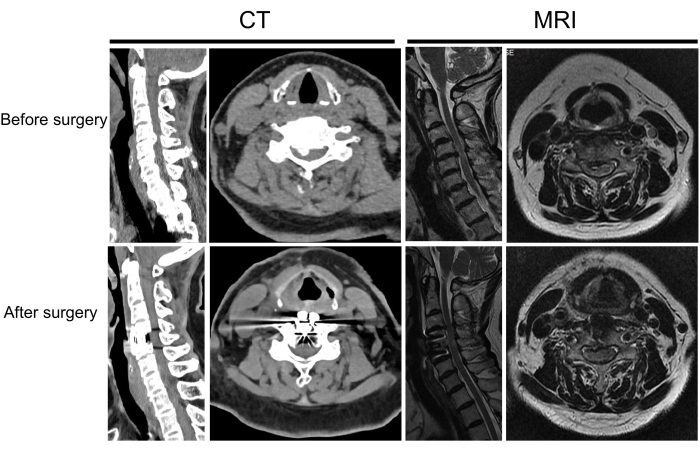
Figure 8: Pre and postoperative imaging of another patient. A patient with cervical spondylotic myelopathy before and after surgery. Please click here to view a larger version of this figure.
Table 1: Patient information. Please click here to download this Table.
Table 2: Surgical characteristics and postoperative outcomes. Please click here to download this Table.
Discussion
Anterior cervical discectomy and fusion (ACDF) is a common surgical method for treating cervical spondylotic myelopathy (CSM) and has satisfactory clinical outcomes13,14. However, traditional surgery faces challenges such as a limited surgical field and difficulties with hemostasis. These problems are particularly pronounced in cases of ossification of the posterior longitudinal ligament (OPLL) or distant disc herniation; in these cases, the compressive material cannot be completely removed intraoperatively, and the risk of spinal cord injury remains high15.
With advancements in minimally invasive spinal surgery techniques, the application of microscopic endoscopy in ACDF has gradually increased, expanding the surgical field and improving operative precision. However, the microscope-assisted procedure still has limitations, such as poor hand-eye coordination and blind spots in the field of view8. Full-endoscopic anterior cervical discectomy (FACD) is a minimally invasive procedure. Compared to conventional surgery, FACD significantly reduces soft tissue damage, operative time, and complications. The recovery process is rapid, with no need for additional surgery-related interventions16.
However, endoscope-assisted ACDF offers several advantages over full-endoscopic anterior cervical decompression, particularly in terms of postoperative stability, broader indications, and ease of surgical execution. By incorporating interbody fusion with a titanium cage and plate fixation, it enhances segmental stability and minimizes the risk of postoperative disc height loss or recurrence, which is a concern with non-fusion full-endoscopic techniques. Additionally, endoscope-assisted ACDF is better suited for multi-level disease, severe disc degeneration, and cases involving ossification of the posterior longitudinal ligament (OPLL), where full-endoscopic decompression may be insufficient. This technique also provides a wider surgical field, enabling more comprehensive neural decompression and reducing the risk of residual compression. Furthermore, it follows the standard ACDF procedure, making it easier for surgeons to adopt compared to the technically demanding full-endoscopic approach.
While full-endoscopic anterior decompression is advantageous for select cases with single-level soft disc herniation, endoscope-assisted ACDF remains a more versatile and reproducible option for a broader range of cervical spine pathologies. Endoscope-assisted ACDF has achieved excellent clinical and radiological outcomes, demonstrating reliable efficacy and high surgical safety. Wu et al.17. found that endoscope-assisted ACDF provides significant advantages over traditional ACDF, including a clearer surgical field, less intraoperative blood loss, and a lower risk of nerve injury. However, they also reported a longer operative time due to the complexity of endoscopic techniques. Our findings are consistent with their results, as our study also demonstrated a slightly prolonged surgical time with endoscope-assisted ACDF. Nevertheless, the extended duration does not significantly impact clinical outcomes, and the benefits of improved visualization and reduced trauma outweigh this limitation, making endoscope-assisted ACDF a viable alternative to traditional ACDF.As a new magnification tool for ACDF, spinal endoscopy provides clearer visualization and greater operational flexibility, effectively addressing these issues.
The advantages of endoscopic-assisted operations are as follows. First, under spinal endoscopy, the PLL can be distinguished in greater detail, enabling sharp dissection using Kerrison punches. The endoscope provides a clearer view of the spinal cord, allowing the surgeon to accurately assess the adequacy of decompression and effectively minimize the risk of spinal cord injury. Additionally, the use of a burr allows for a close-proximity operation, ensuring more precise and thorough removal of bony spurs. Compared with the naked eye and microscope, the endoscope offers superior visualization during the exposure and decompression of the bilateral uncovertebral joints.
Second, the spinal endoscope offers increased freedom of operation with its integrated 30° viewing angle and adjustable burr head, enabling a broader decompression range. This feature enhances flexibility and precision when working in complex anatomical regions. Third, water-mediated endoscopy allows for more accurate identification of bleeding points. For bleeding at the posterior margin of the vertebral body, the curved radiofrequency probe can reach and address the bleeding site precisely, significantly improving hemostatic effectiveness and surgical precision. Fourth, the endoscopic system minimizes interference from the surgeon's hands, reducing the number of blind spots in the surgical field. The coaxial design of the endoscope aligns with the operating habits of endoscopic surgeons, preventing instrument collisions and ensuring smoother and more efficient procedures.
However, the application of spinal endoscopic techniques also presents certain challenges, such as prolonged operative time and the requirement for surgeons with advanced surgical skills and detailed knowledge of microanatomy. Additionally, although endoscopic surgery offers a broader visual field, the field may still be limited in extremely complex cases. With the continuous advancement of technology and improvements in surgical instruments, we believe that the use of spinal endoscopy in cervical spine surgery will gradually mature and that the operative time will further decrease.
Déclarations de divulgation
The authors have no conflicts of interest to declare.
Remerciements
None
matériels
| Name | Company | Catalog Number | Comments |
| 75% alcohol | Hebei Ruihe Medical Equipment Co., Ltd | CC-01A | PEEK |
| Anterior Cervical Nail Plate Fixation System | Hebei Ruihe Medical Equipment Co., Ltd | PN-03 | Plate:TA3G, Nail:TC4 |
| Cervical Fusion Cage | |||
| cisatracurium besylate | SPINENDOS GmbH | SP081430.030 | Inner diameter:4.3 mm; Outer diameter:7.0 mm; Field angle: 80 °; Visual angle: 30 °; Working length: 181 mm |
| Endoscope system | SPINENDOS GmbH | SP082628.351 | Φ2.5 mm × 310 mm |
| Endoscopic forceps | SPINENDOS GmbH | SP082700.040L | Φ4.0 mm × 360 mm |
| Endoscopic hook | XIYI | MQZ | Φ3.2 mm × 328 mm |
| Endoscopic rongeur | ELLIQUENCE | DTF-40 | 40 cm |
| etomidate | SPINENDOS GmbH | SP082781.835 | Φ2.5 mm × 330 mm |
| High-speed burr | Neusoft Corporation | ||
| Interventional radiology | Ferrosan Medical Devices A/S | MS0010 | |
| iodine | Sichuan Guona Technology Co.,LTD | NNBP/40D/ | |
| Neusoft PACS/RIS | Elliquence, LLC | DTF-40 | |
| n-HA/PA66 | SPINENDOS GmbH | SP082615.265 | Φ7.2 mm × 178 mm |
| sufentanil citrate injection | |||
| SURGIFLO Haemostatic Matrix | |||
| Trigger-Flex Bipolar System | |||
| Working sheath |
Références
- Bakhsheshian, J., Mehta, V. A., Liu, J. C. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 7 (6), 572-586 (2017).
- McCormick, J. R., Sama, A. J., Schiller, N. C., Butler, A. J., Donnally, C. J. III. Cervical spondylotic myelopathy: A guide to diagnosis and management. J Am Board Fam Med. 33 (2), 303-313 (2020).
- Visocchi, M. et al. Hybrid implants in anterior cervical decompressive surgery for degenerative disease. J Craniovertebr Junction Spine. 12 (1), 54-60 (2021).
- Lee, C. J., Boody, B. S., Demeter, J., Smucker, J. D., Sasso, R. C. Long-term radiographic and functional outcomes of patients with absence of radiographic union at 2 years after single-level anterior cervical discectomy and fusion. Global Spine J. 10 (6), 741-747 (2020).
- Luo, H. T. et al. Meta-analysis of the treatment of cervical spondylosis by microscopy-assisted and traditional anterior cervical decompression under direct vision. Chin J Tissue Eng Res. 24 (9), 1369-1377 (2020).
- Marawar, S. et al. National trends in anterior cervical fusion procedures. Spine (Phila Pa 1976). 35 (15), 1454-1459 (2010).
- Bollati, A., Galli, G., Gandolfini, M., Marini, G., Gatta, G. Microsurgical anterior cervical disk removal without interbody fusion. Surg Neurol. 19 (4), 329-333 (1983).
- Damodaran, O., Lee, J., Lee, G. Microscope in modern spinal surgery: Advantages, ergonomics and limitations. ANZ J Surg. 83 (4), 211-214 (2013).
- Liu, L., Dong, J., Wang, D., Zhang, C., Zhou, Y. Clinical outcomes and quality of life in elderly patients treated with a newly designed double tube endoscopy for degenerative lumbar spinal stenosis. Orthop Surg. 14 (7), 1359-1368 (2022).
- Han, S. et al. Clinical application of large channel endoscopic systems with full endoscopic visualization technique in lumbar central spinal stenosis: A retrospective cohort study. Pain Ther. 11 (4), 1309-1326 (2022).
- Liang, J., Li, H., Tao, Y., Yuan, W., Wang, H. Efficacy and complications of unilateral biportal endoscopic spinal surgery for lumbar spinal stenosis: A meta-analysis and systematic review. World Neurosurg. 159, e91-e102 (2022).
- Tian X, Rudd S, Yang D, Ding, W., Yang, S. Anterior cervical hybrid decompression and fusion surgery to treat multilevel cervical spondylotic myelopathy. J Vis Exp. (196), 65034 (2023).
- Sun, X. et al. The frequency and treatment of dural tears and cerebrospinal fluid leakage in 266 patients with thoracic myelopathy caused by ossification of the ligamentum flavum. Spine (Phila Pa 1976). 37 (12), E702-707 (2012).
- Wang, T. et al. Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion in multilevel cervical spondylotic myelopathy: A meta-analysis. Medicine (Baltimore). 95 (49), e5437 (2016).
- Nakajima, H. et al. Long-term outcome of anterior cervical decompression with fusion for cervical ossification of posterior longitudinal ligament including postsurgical remnant ossified spinal lesion. Spine (Phila Pa 1976). 44 (24), E1452-E1460 (2019).
- Ruetten, S., Komp, M., Merk, H., Godolias, G. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop. 33 (6), 1677-1682 (2009).
- Wu, Z. P., Wei, Z. Y., Song, X. L. Comparison of efficacy between endoscope-assisted anterior cervical discectomy and fusion (ACDF) and open ACDF in the treatment of single-segment cervical spondylotic myelopathy. J Orthop Surg Res. 19 (1), 35 (2024).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon