Method Article
Comparing Transperitoneal Retroperitoneal Access and Percutaneous Puncture for Lumbar Disc Degeneration Modeling in Rabbits
* Ces auteurs ont contribué à parts égales
Dans cet article
Résumé
This protocol compared percutaneous and trans-retroperitoneal punctures in a rabbit intervertebral disc degeneration (IVDD) model. Both methods induced IVDD; however, the trans-retroperitoneal approach resulted in more extensive changes and lower mortality.
Résumé
This study compares the efficacy of two methods for inducing intervertebral disc degeneration (IVDD) in rabbits: percutaneous and trans-retroperitoneal puncture of the annulus fibrosus. Fifteen healthy male New Zealand White rabbits were randomly assigned to three groups: sham, percutaneous puncture, and trans-retroperitoneal puncture. A comprehensive assessment, including mortality rates, morphological and histological evaluations, radiological imaging, and biomarker analysis, was conducted to ensure an accurate and detailed comparison between the two methods. The results demonstrate that both puncture techniques successfully induced IVDD in the rabbit model. However, the trans-retroperitoneal approach resulted in more pronounced degenerative changes in the intervertebral discs while maintaining a significantly lower mortality rate compared to the percutaneous method. These findings highlight the advantages of the trans-retroperitoneal approach in IVDD modeling. This study provides valuable insights into the establishment of IVDD models and lays a foundation for future investigations into effective treatment strategies for low back pain, ultimately improving patient outcomes.
Introduction
Over the past few decades, low back pain (LBP) has emerged as the most significant musculoskeletal disorder affecting quality of life1. LBP has become an increasingly important public health concern, imposing a substantial economic burden on society due to lost labor and additional medical expenses2,3. In the United States alone, the direct and indirect costs associated with LBP exceed $100 billion annually, including medical expenditures, income losses, and labor losses4. LBP is often caused by intervertebral disc degeneration (IVDD)5,6,7,8. Given the high prevalence and economic impact of LBP, accurately modeling IVDD is crucial for exploring treatment strategies.
To understand the pathophysiology of IVDD and evaluate treatment strategies, various preclinical in vivo animal models have been developed and utilized9. Multiple methods have been employed in these models to induce disc degeneration, including surgical or chemical disc injury, non-invasive mechanical stress, genetic modification, and natural occurrence10. Among these methods, surgical injury accounts for up to 64.9% of IVDD induction, with needle puncture being the primary surgical technique11. The needle puncture model is characterized by its ease of establishment and minimal damage to experimental animals. Common needle puncture approaches include open retroperitoneal access to the lumbar disc space and percutaneous posterolateral puncture. The depth of insertion can be determined using radiographic monitoring or needle length. Notably, the percutaneous approach may reduce iatrogenic tissue damage compared to open surgical methods, while retroperitoneal access provides the benefit of direct visualization-features that have not been quantitatively compared in prior literature. While studies have investigated the effects of using needles of different diameters12 and puncturing different discs10 on IVDD induction, comparative studies focusing on different needle puncture approaches remain limited. The selected rabbit model offers particular utility for researchers requiring cost-effective longitudinal studies with frequent imaging assessments, given its anatomical similarity to human discs and its advantages over rodent models in terms of size and structure13.
In this study, rabbit models of lumbar IVDD were established using two methods: open retroperitoneal access to puncture the lumbar disc space and percutaneous posterolateral puncture. A comprehensive set of outcome measures, including morphological, histological, and radiological changes, was analyzed.
Protocole
The animal experimental procedures strictly adhered to the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health and were approved by the Experimental Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine (Ethics Approval Number: 2021-23). Fifteen healthy, 4-month-old, clean-grade New Zealand White rabbits (2.25 kg ± 0.25 kg) were used, including seven males and eight females. The animals were housed in an environment with a room temperature of 23 °C ± 3 °C and a humidity of approximately 60% ± 10% for one week of adaptation, with free access to water and food. Prior to the experiment, the 15 rabbits were randomly assigned to one of three groups: the sham group (Group A), the percutaneous annulus fibrosus puncture group (Group B), and the trans-retroperitoneal space annulus fibrosus puncture group (Group C), with five rabbits in each group. The details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Establishment of rabbit IVDD model via percutaneous annulus fibrosus puncture
NOTE: The rabbit IVDD model was established using the percutaneous annulus fibrosus puncture method. The procedure followed the puncture modeling method described by Luo TD et al.14 and was performed under X-ray guidance (Figure 1).
- Prepare the rabbit.
- Fast the rabbits for 24 h before surgery, ensuring access to water.
- Administer anesthesia via intravenous injection of 3% pentobarbital sodium (1.3 mL/kg) into the ear vein (following institutionally approved protocols).
- Confirm successful anesthesia by checking for immobility, relaxed muscles, lack of corneal reflex, and absence of pain response.
- Position and mark the rabbit.
- Fix the rabbit in a prone position on a fixation board.
- Shave and prepare the surgical area, then palpate bone landmarks.
- Palpate the bony landmarks on the rabbit's lumbar back. Locate the lowest rib on the rabbit, which typically corresponds to the vertebra just above the L1 spinous process.
- Identify the spinous process immediately below this vertebra to determine the L1 spinous process.
- Locate the highest points of the iliac crests, approximately level with the L6 vertebra.
- Trace down from the L1 spinous process to sequentially identify each spinous process down to L7.
- Use a marking pen to mark the L1 spinous process clearly on the rabbit's back.
- Move to the next spinous process and mark it as L2.
- Continue marking each subsequent spinous process as L3, L4, L5, L6, and L7. Ensure that each mark is distinct and in sequential order for clear identification.
- Locate and mark the puncture site.
- Palpate the transverse processes and locate the midpoint between the distal ends of L5 and L6.
- Mark this point and prepare to insert the puncture needle approximately 1 cm above it.
- Insert the puncture needle.
- Hold the puncture needle horizontally and insert it towards the ground, breaking the skin.
- Advance the needle to reach the L4 vertebral body and verify the correct positioning under X-ray guidance.
- Tilt the needle slightly cephalic at an angle of approximately 20° toward the L4-5 intervertebral disc. Puncture the disc and confirm the accuracy of the puncture under X-ray examination.
- Perform disc punctures.
- Precisely puncture the annulus fibrosus, using X-ray guidance if necessary.
- Repeat the puncturing process for the L2-3 and L3-4 intervertebral discs, puncturing each once.
- Maintain a puncturing depth of approximately 5 mm with a dwell time of 5 s for each disc.
- Post-procedure care
- Disinfect and bandage the puncture site.
- Inject penicillin intramuscularly into the gluteus maximus at a dosage of 40,000 U per rabbit daily for 3 days.
NOTE: Adjust the approach if the needle encounters hard tissue. Use X-ray guidance for precise puncturing. Monitor the rabbit's recovery and provide appropriate care.
2. Establishment of rabbit IVDD model via trans-retroperitoneal space annulus fibrosus puncture
NOTE: The rabbit IVDD model was established using the trans-retroperitoneal space annulus fibrosus puncture method12 (Figure 2).
- Fast the rabbits for 24 h before surgery, allowing access to water.
- Anesthetize the rabbit by intravenously injecting 3% pentobarbital sodium (1.3 mL/kg) into the ear vein (following institutionally approved protocols).
- Ensure that the rabbit is immobile, with relaxed muscles, no corneal reflex, and no pain response to pressure to confirm successful anesthesia.
- Fix the rabbit in a prone position on a fixation board.
- Shave and prepare the surgical area.
- Palpate bone landmarks, marking the L1-L7 lumbar spinous processes on the rabbit's lumbar back with a marking pen.
- Re-palpate the transverse processes of the rabbit to determine the surgical incision location.
- Place a sterile drape and disinfect the local skin to ensure aseptic conditions.
- Use a posterior retroperitoneal approach to dissect the fascia and muscles layer by layer, exposing the lateral aspect of the lumbar intervertebral disc.
- Puncture the annulus fibrosus with a puncture needle to a depth of approximately 5 mm and a dwell time of 5 s.
- Sequentially puncture the L3-4, L4-5, and L5-6 intervertebral discs, ensuring that each disc is punctured only once.
- Suture the tissues layer by layer using a 0.25 mm diameter suture thread.
- Disinfect and bandage the puncture site after modeling.
- Inject penicillin intramuscularly into the gluteus maximus of the rabbit daily at a dosage of 40,000 U per rabbit for three consecutive days.
NOTE: Use penicillin with a specification of 800,000 units/vial and approval number Veterinary Drug 140051251.
3. Selection of IVDD models and outcome evaluation
- Mortality and general condition assessment of rabbits
- Observe rabbits weekly to determine survival and record general condition, including mental status, activity patterns, food and water intake, as well as fecal and urinary output.
- Record observations accurately and note any changes in condition.
- Weight monitoring of rabbits
- Record the body weight of rabbits before and after model establishment, as well as prior to tissue collection.
- Ensure accurate weight recording and note any significant changes.
- Radiological assessment
- Obtain sagittal 1.5T T2-weighted magnetic resonance imaging of the entire lumbar vertebral explant sequence of each white rabbit before and 4 weeks after model establishment.
- Observe the extent of intervertebral disc degeneration.
- Perform a quantitative assessment of intervertebral disc degeneration using the modified Pfirrmann grading system proposed by Griffith et al.15. Have three independent blinded radiologists evaluate T2-weighted MRI sequences according to established criteria: disc height, nucleus pulposus signal intensity, and annulus fibrosus integrity.
- Determine final grades through consensus when discrepancies exceed one grade level. Conduct all assessments using standardized DICOM viewing software with calibrated display settings.
- Histopathological evaluation and scoring
- Euthanize the rabbits 4 weeks after modeling using an intravenous overdose of pentobarbital sodium (following institutionally approved protocols), then rapidly harvest the L2-L3, L3-L4, and L4-L5 intervertebral discs on ice15.
- Fix the L2-L3 discs in 4% paraformaldehyde and store the remaining samples at -80 °C.
- Immerse the fixed discs in a decalcifying solution, such as 10% EDTA, ensuring complete submersion. Change the decalcifying solution every 2-3 days to maintain effectiveness.
- Monitor the decalcification process regularly until full decalcification is achieved, which may take several days to a week, depending on disc size and thickness.
- Rinse the decalcified discs thoroughly with running water to remove any traces of the decalcifying solution.
- Dehydrate the discs by immersing them in a series of graded ethanol solutions, starting with 70% ethanol and gradually increasing to 100% ethanol. Conduct each dehydration step for 1-2 h per grade.
- Infiltrate the dehydrated discs with paraffin wax (melting point 56-58 °C) for a minimum of 2 h, ensuring complete infiltration.
- Embed the infiltrated discs in a wax block, positioning them for sectioning. Allow the wax block to cool and solidify completely.
- Section the embedded discs into thin, uniform slices (5-10 µm) using a microtome. Mount the sections onto glass slides for further analysis, such as histological staining or immunohistochemistry12,13.
- Perform hematoxylin and eosin (HE) staining, capture images under an optical microscope, and assign HE staining scores using the IVD histopathological grading scale12.
- TUNEL assay
- Dewax and rehydrate intervertebral disc tissue sections, then perform antigen retrieval and membrane permeabilization.
- Add a mixture of reagent 1 (TdT) and reagent 2 (dUTP) at a ratio of 1:9 and incubate in a humidified chamber.
- Wash sections with PBS buffer, apply DAPI stain, and incubate in the dark at room temperature for 10 min.
- Capture images using a fully automated panoramic scanner and processing software.
- Cytokine detection
- Euthanize the experimental rabbits (step 3.4.1) and collect blood samples from the abdominal aorta.
- Centrifuge rabbit blood samples at 2000 × g for 10 min at 25 °C to separate serum from blood cells. Carefully collect the supernatant (serum), ensuring no cellular debris is included.
- Follow the instructions provided in the ELISA kit to detect the expression of TGF-β in serum samples.
- Prepare reagents and standards as directed in the ELISA kit.
- Add serum samples to the appropriate wells in the ELISA plate.
- Incubate the plate at the recommended temperature and duration according to the kit instructions.
- Wash the plate as instructed to remove unbound reagents.
- Add the detection antibody and other necessary reagents, following the kit's protocol.
- Incubate the plate again for the specified time and temperature.
- Wash the plate thoroughly to remove excess reagents.
- Add the substrate solution to the wells and incubate for the recommended period to allow for color development.
- Measure the optical density (OD) using a spectrophotometer at the wavelength specified in the kit instructions.
- Calculate sample concentrations by substituting OD values into the provided equation or using the standard curve generated from the known concentrations of the standards.
4. Statistical analysis
- Perform statistical analysis using commercially available software.
- Express continuous variables as mean ± standard deviation.
- Use one-way ANOVA to test differences between groups.
- Apply LSD tests for pairwise comparisons.
- Use repeated measures ANOVA for analyzing repeated measures data.
- Perform Spearman correlation analysis to assess the correlation between variables.
- Set the significance level at α = 0.05 and consider P values less than 0.05 as statistically significant.
Résultats
The surgical procedures were performed without complications. One rabbit from Group B (percutaneous puncture group) died after the procedure. All other animals resumed normal feeding and activity patterns postoperatively and survived throughout the experimental period. No prolonged bleeding or infection was observed at the surgical sites.
Mortality and general condition assessment
The mortality rate was 0% for both Group A and Group C, while it was 20% for Group B (Table 1). Rabbits in Group A exhibited normal general conditions. Before modeling, rabbits in Group B displayed similar conditions to those in the sham group. However, on the second day after modeling, rabbits in Group B showed reduced vitality, decreased activity, a preference for huddling in the corners of their cages, significantly reduced food intake, relatively unchanged water intake, duller fur, increased fecal output, and loose stools. Over time, one rabbit from Group B died, while the remaining rabbits gradually regained their food intake to pre-modeling levels. However, their vitality, activity, fur condition, and stool consistency remained inferior to pre-modeling levels. In contrast, rabbits in Group C exhibited better vitality, activity, food intake, fur glossiness, and fecal consistency compared to those in Group B, beginning in the second week after modeling.
Body weight assessment
The body weight of rabbits was similar across all groups before modeling. However, before sampling, rabbits in Group B weighed more than those in Groups A and C (Figure 3).
Radiological assessment of intervertebral discs
Loss of intervertebral disc (IVD) height was observed in Groups B and C starting one week postoperatively (Figure 4). Pfirrmann grading demonstrated significant intergroup differences (p < 0.001, Kruskal-Wallis test with Dunn's post hoc analysis). The sham group (Group A) maintained intact disc morphology, with uniform grade 1 scores across all specimens (median [range]: 1 [1-1]). The percutaneous puncture group (Group B) exhibited moderate-to-severe degeneration (median [range]: 6 [5-6]), while the retroperitoneal approach group (Group C) showed a comparable degree of degeneration (median [range]: 5 [5-6]). Pairwise comparisons revealed significant differences between: (1) Group A vs. Group B (p = 0.0039); (2) Group A vs. Group C (p = 0.0039); (3) Group B vs. Group C (p = 0.206). Inter-rater reliability remained excellent (Krippendorff's α = 0.87) (Table 2).
Histopathological assessment and scoring
Qualitative histological analysis revealed differences in ultrastructure, vascular infiltration, and granulation tissue deposition among Groups A, B, and C (Figure 5). Pathological scoring indicated significantly higher scores in Groups B and C compared to Group A, with Group C exhibiting a higher score than Group B (Table 3).
TUNEL assay results
No significant green punctate apoptotic cells or nucleus pulposus cell apoptosis were observed in Group A. In contrast, Groups B and C exhibited numerous green punctate apoptotic cells and severe nucleus pulposus cell apoptosis compared to Group A. However, Group B displayed relatively fewer green punctate apoptotic cells and less nucleus pulposus cell apoptosis than Group C (Figure 6).
TGF-β detection results
Quantitative analysis of TGF-β levels revealed significant intergroup variations (one-way ANOVA, F(2,12) = 87.3, p < 0.0001). The sham group (Group A) demonstrated baseline TGF-β concentrations (mean ± SD: 1944.6 pg/mL ± 182.3 pg/mL), while the percutaneous puncture group (Group B) showed a moderate elevation (2635.4 pg/mL ± 136.7 pg/mL). Notably, the retroperitoneal approach group (Group C) exhibited substantial TGF-β upregulation (4143.7 pg/mL ± 353.7 pg/mL), exceeding Group B levels by 57.2% (Tukey's post hoc: p < 0.0001). Individual biological variations remained within 15% of group means (coefficient of variation range: 5.2%-8.5%) (Figure 7, Table 4).

Figure 1: Percutaneous annulus fibrosus puncture modeling under X-ray guidance. X-ray imaging was used to ensure precise puncturing of the annulus fibrosus. The figure shows the puncture needle localized under X-ray before disc penetration. Please click here to view a larger version of this figure.
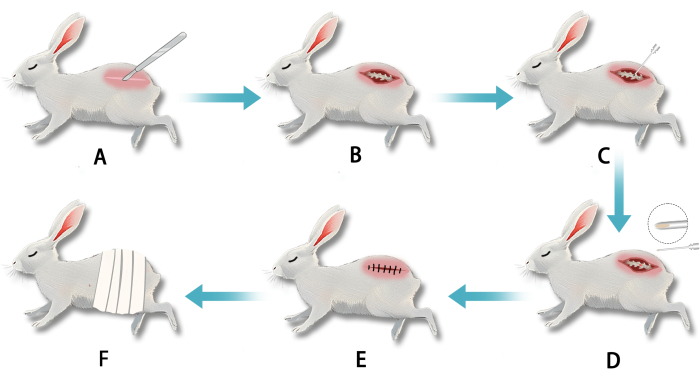
Figure 2: Establishment of a white rabbit model of intervertebral disc degeneration (IVDD) using a transperitoneal retroperitoneal gap puncture of the annulus fibrosus. (A) After anesthesia, an incision was made in the lumbar vertebral segment. (B) Blunt dissection exposed the lumbar annulus fibrosus. (C) The annulus fibrosus was punctured with a needle to disrupt the nucleus pulposus. (D) White, jelly-like nucleus pulposus tissue was visible at the needle tip upon withdrawal. (E) The incision was sutured after puncture completion. (F) The wound was dressed postoperatively. Please click here to view a larger version of this figure.

Figure 3: Mean body weights of white rabbits in each group before modeling and sampling. Please click here to view a larger version of this figure.

Figure 4: MRI images of rabbit intervertebral discs before and after modeling. Significant height loss was observed in Groups B and C post-modeling, with greater loss in Group C compared to Group B. Please click here to view a larger version of this figure.

Figure 5: Histological images of rabbit intervertebral discs. (A) Group A exhibited a regular nucleus pulposus shape, abundant cell distribution, vacuoles in the gelatinous matrix (black arrows), and organized fibrocartilaginous plates (yellow arrows). (B) Group B displayed an irregular nucleus pulposus shape, reduced cell count, clustered matrix distribution, large cavities (blue arrows), and disrupted fibrocartilaginous plates separated from the nucleus pulposus (green arrows). (C) Group C exhibited an irregular nucleus pulposus shape, significant cell loss (purple arrows), clustered matrix distribution, irregular shapes, and disrupted fibrocartilaginous plates (red arrows). Scale bar: 200 µm (applies to all panels). Please click here to view a larger version of this figure.
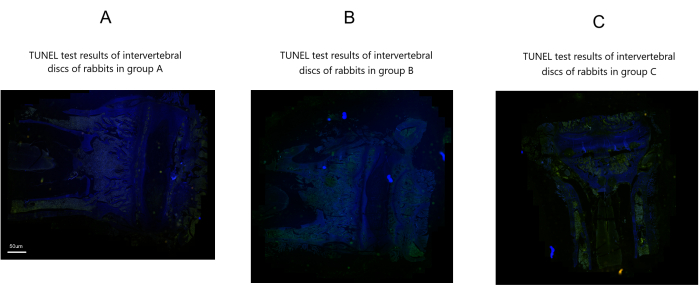
Figure 6: TUNEL assay results of rabbit intervertebral discs post-modeling in three groups. Scale bar: 50 µm (applies to all panels). Please click here to view a larger version of this figure.
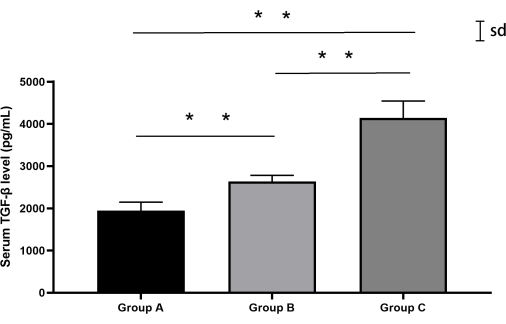
Figure 7: Serum TGF-β levels in rabbits from each group. Both Groups B and C exhibited higher TGF-β levels compared to Group A, with Group C showing the highest levels. Error bars represent mean ± standard deviation (SD). Please click here to view a larger version of this figure.
| Group | A | B | C |
| Mortality | 0 | 20% | 0 |
Table 1: Mortality rate post-modeling in rabbit groups.
| n | Pfirrmann Grade Distribution | Median [Range] | Intergroup Comparison (p-value) |
| 5 | 1 (100%) | 1 [1-1] | A vs B: 0.0039 |
| 5 | 5 (40%), 6 (60%) | 6 [5-6] | A vs C: 0.0039 |
| 5 | 5 (60%), 6 (40%) | 5 [5-6] | B vs C: 0.206 |
Table 2: Comparative analysis of intervertebral disc degeneration using the modified Pfirrmann grading system.
| Group | A | B | C |
| Pathology score | 4 | 10 | 11 |
Table 3: Average pathological scores of rabbits in each group.
| Group | n | Mean ± SD (pg/mL) | Median [Range] (pg/mL) | Pairwise Comparisons (Tukey's HSD) |
| A | 5 | 1944.6 ± 182.3 | 2054.5 [1709.1-2126.6] | A vs B: p = 0.0012 |
| B | 5 | 2635.4 ± 136.7 | 2544.6 [2526.6-2889.6] | A vs C: p < 0.0001 |
| C | 5 | 4143.7 ± 353.7 | 4090.3 [3694.9-4595.5] | B vs C: p < 0.0001 |
| All values normalized to total protein content (μg/mg tissue) ANOVA assumptions verified (Levene's test p = 0.18, Shapiro-Wilk p > 0.15) Effect sizes: Cohen's f = 2.16 (large effect)* |
Table 4: TGF-β concentration profiles across experimental groups.
Discussion
The findings of this study indicate that both percutaneous and trans-retroperitoneal puncture approaches are effective in inducing intervertebral disc degeneration (IVDD) in rabbit models. Notably, based on a comprehensive evaluation of general condition, mortality, histopathological assessment, TUNEL assay, and serum TGF-β levels, the trans-retroperitoneal puncture model resulted in more extensive degenerative changes in the intervertebral discs while maintaining a lower mortality rate.
Surgical injury is the most commonly used method for establishing IVDD models. Since Lipson and Muir (1981) successfully developed an IVDD model in rabbits using surgical scalpel annulotomy16, various injury techniques have emerged, including needle puncture12,14,17, annulus fibrosus excision18, total discectomy, partial and total nucleotomy19, nucleus pulposus aspiration20,21,22, and drill injury23,24,26. These surgical methods impose different physiological burdens on animals, leading to varying degrees of IVDD induction. Compared to scalpel injury models, needle puncture models better mimic the slow and progressive nature of disc degeneration14.
While Masuda et al.12 introduced the trans-retroperitoneal puncture technique, this open surgical approach requires longer operative time, greater surgical skill, and more extensive surgical resources. Additionally, the incision created during open surgery may increase the risk of infection, postoperative pain, and mortality in experimental animals. In contrast, the percutaneous posterolateral puncture technique proposed by Luo et al.14 is more straightforward and feasible. The results of this study corroborate that the trans-retroperitoneal puncture model induces more extensive degenerative changes in intervertebral discs while maintaining a lower mortality rate.
In needle puncture models, the extent and nature of degenerative changes in the intervertebral disc are influenced by multiple mechanical and biological factors that vary over time27,28. These factors are well-known pathological contributors to disc degeneration. Needle puncture disrupts the integrity of the annulus fibrosus, reduces the elasticity of the nucleus pulposus, promotes nucleus pulposus cell apoptosis, and alters the mechanical stress distribution within the spine29. Additionally, the injured site elicits inflammatory and early repair responses, including capillary proliferation in the outer annulus fibrosus and the deposition of granulation and fibrotic tissues. These pathological reactions have been described in numerous animal models of disc degeneration30,31and are also observed in human lumbar disc herniation.
In this study, these pathological responses were evident in both models. Compared to percutaneous puncture, trans-retroperitoneal puncture offers a clearer puncture view, facilitating more precise disruption of the annulus fibrosus and leading to greater inflammation at the injury site. This may explain why the trans-retroperitoneal puncture model induces more extensive degenerative changes in the intervertebral discs. Conversely, the limited visibility in percutaneous puncture increases the likelihood of mispuncturing the spinal cord or other structures, potentially accounting for the higher mortality rate observed in this study.
Needle puncture models for disc degeneration are commonly established in small animals such as rats and mice32, as well as rabbits12,14,16. However, this technique is also applicable to larger animals, including dogs33, sheep34, cattle35, and rhesus monkeys36, for IVDD model development. Although concerns exist regarding the differences between quadrupedal animal models and the bipedal human spine, biomechanical studies suggest that quadruped spines primarily experience axial compression, similar to human spines37,38. The intense bending and torsional forces acting on quadruped spines are counteracted by paraspinal muscles and ligaments that generate considerable tensile forces along the long axis38. Given ethical and practical constraints, larger quadrupeds are costly and difficult to acquire, making rabbits an ideal model for IVDD research. Furthermore, the anatomical homology between rabbit and human intervertebral discs-particularly the presence of facet joints, paraspinal muscles, and ligaments-further supports the suitability of rabbits for this research13,39.
However, significant differences exist between these animal models and human intervertebral discs, representing a limitation of this study. These differences include variations in disc size, anatomical characteristics, the presence of notochordal cells, and the translatability of experimental results. Consequently, this study faces certain challenges. Additionally, in clinical practice, patients often present with pre-existing degenerative changes in the intervertebral disc, which can ultimately lead to disc herniation and neural compression. In contrast, the animal models in this study induced degeneration by injuring healthy discs. Degenerated discs may have reduced self-repair and regenerative capabilities after injury or may exhibit more severe degeneration compared to the healthy discs examined in this experiment. Future research on animal models of intervertebral disc degeneration should take these factors into full consideration.
Both percutaneous and trans-retroperitoneal puncture approaches are effective in establishing intervertebral disc degeneration models in rabbits. However, the trans-retroperitoneal puncture technique induces more extensive degenerative changes while also being associated with a lower mortality rate.
Déclarations de divulgation
None.
Remerciements
This project was supported by the National Natural Science Foundation of China (No. 82004497), China Postdoctoral Science Foundation (No. 2021M693788), National Natural Science Foundation of China (No. 82105043), and Natural Science Foundation of Sichuan Province (No. 2023NSFSC1814).
matériels
| Name | Company | Catalog Number | Comments |
| 0.3 T Veterinary Maenetic Resonance lmaging(MRI) | NINGBO CHUANSHANJIA | CSJ-MR | |
| Alcohol medical | LIRCON | 20230107 | |
| Benzylpenicillin potassium | Jiangxi Keda Animal Pharmaceutical | 140051251 | |
| Haemostatic forceps | SHINVA | 20211239 | |
| Injection syringe | CONPUVON | 20153151307 | |
| Knife blades | Hons Medincal | 20210615 | |
| Medical absorbent cotton ball | Cofoe | 20210006 | |
| Medical suture needle | Shanghai Xiaoyi Medical Devices | 20192020430 | |
| Medullo-puncture needle | Yangzhou Jiangzhou Medical Devices | 20190902 | Used to puncture lumbar disc |
| Physiological saline | NeilMed | C1210504D2 | |
| Povidone iodine solution | Sichuan IJIS Medical Technology | 20221209 | |
| Quasi-microbalance | Explorer | ||
| Rabbit dissection operating table | Zhenhua Biomedical | ZH-BXT-3Z | |
| Shaver | AUX | ||
| Statistical analysis softeare | IBM | SPSS | |
| Sterile gauze | Cofoe | 20202140675 | |
| Surgical gloves | DR.LERSH | 20172140028 | |
| Surgical knife | Hons Medinca | 20210019 | |
| Surgical tweezers | SHINVA | 20210233 | |
| USB-C data transmission line | KINI | ||
| White light photography microscope | Nikon | Eclipse Ci-L |
Références
- Vos, T., et al. Years lived with disability (YLDS) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 380 (9859), 2163-2196 (2012).
- Daly, C., Ghosh, P., Jenkin, G., Oehme, D., Goldschlager, T. A review of animal models of intervertebral disc degeneration: Pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016, 5952165 (2016).
- Risbud, M. V., Shapiro, I. M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 10 (1), 44-56 (2014).
- Katz, J. N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 88 (Suppl 2), 21-24 (2006).
- Cheung, K. M. The relationship between disc degeneration, low back pain, and human pain genetics. Spine J. 10 (11), 958-960 (2010).
- Knezevic, N. N., Candido, K. D., Vlaeyen, J. W. S., Van Zundert, J., Cohen, S. P. Low back pain. Lancet. 398 (10294), 78-92 (2021).
- Livshits, G., et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK twin spine study. Ann Rheum Dis. 70 (10), 1740-1745 (2011).
- Takatalo, J., et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young finnish adults. Spine (Phila Pa 1976). 36 (25), 2180-2189 (2011).
- Singh, K., Masuda, K., An, H. S. Animal models for human disc degeneration. Spine J. 5 (6 Suppl), 267s-279s (2005).
- Liang, T., et al. Constructing intervertebral disc degeneration animal model: A review of current models. Front Surg. 9, 1089244 (2022).
- Poletto, D. L., Crowley, J. D., Tanglay, O., Walsh, W. R., Pelletier, M. H. Preclinical in vivo animal models of intervertebral disc degeneration. Part 1: A systematic review. JOR Spine. 6 (1), e1234 (2023).
- Masuda, K., et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 30 (1), 5-14 (2005).
- Kroeber, M. W., et al. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine (Phila Pa 1976). 27 (23), 2684-2690 (2002).
- Luo, T. D., et al. A percutaneous, minimally invasive annulus fibrosus needle puncture model of intervertebral disc degeneration in rabbits. J Orthop Surg (Hong Kong). 26 (3), 2309499018792715 (2018).
- Griffith, J. F., et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 32 (24), E708-E712 (2007).
- Lipson, S. J., Muir, H. Experimental intervertebral disc degeneration: Morphologic and proteoglycan changes over time. Arthritis Rheum. 24 (1), 12-21 (1981).
- Wang, Y., Wu, Y., Deng, M., Kong, Q. Establishment of a rabbit intervertebral disc degeneration model by percutaneous posterolateral puncturing of lumbar discs under local anesthesia. World Neurosurg. 154, e830-e837 (2021).
- Gruber, H. E., et al. A new small animal model for the study of spine fusion in the sand rat: Pilot studies. Lab Anim. 43 (3), 272-277 (2009).
- Alini, M., et al. Are animal models useful for studying human disc disorders/degeneration. Eur Spine J. 17 (1), 2-19 (2008).
- Gandhi, S. D., et al. Intradiscal delivery of anabolic growth factors and a metalloproteinase inhibitor in a rabbit acute lumbar disc injury model. Int J Spine Surg. 14 (4), 585-593 (2020).
- Omlor, G. W., et al. A new porcine in vivo animal model of disc degeneration: Response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine (Phila Pa 1976). 34 (25), 2730-2739 (2009).
- Serigano, K., et al. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 28 (10), 1267-1275 (2010).
- Daly, C. D., et al. A comparison of two ovine lumbar intervertebral disc injury models for the evaluation and development of novel regenerative therapies. Global Spine J. 8 (8), 847-859 (2018).
- Kim, J. S., et al. The rat intervertebral disk degeneration pain model: Relationships between biological and structural alterations and pain. Arthritis Res Ther. 13 (5), R165 (2011).
- Lim, K. Z., et al. Ovine lumbar intervertebral disc degeneration model utilizing a lateral retroperitoneal drill bit injury. J Vis Exp. (123), e55753 (2017).
- Zhang, Y., et al. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Phila Pa 1976). 36 (19), 1519-1527 (2011).
- Freemont, A. J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 48 (1), 5-10 (2009).
- Vergroesen, P. P., et al. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 23 (7), 1057-1070 (2015).
- Natarajan, R. N., Andersson, G. B., Patwardhan, A. G., Verma, S. Effect of annular incision type on the change in biomechanical properties in a herniated lumbar intervertebral disc. J Biomech Eng. 124 (2), 229-236 (2002).
- Hoogendoorn, R. J., Wuisman, P. I., Smit, T. H., Everts, V. E., Helder, M. N. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine (Phila Pa 1976). 32 (17), 1816-1825 (2007).
- Melrose, J., Roberts, S., Smith, S., Menage, J., Ghosh, P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine annular lesion model of experimental disc degeneration. Spine (Phila Pa 1976). 27 (12), 1278-1285 (2002).
- Elmounedi, N., et al. Impact of needle size on the onset and the progression of disc degeneration in rats. Pain Physician. 25 (6), 509-517 (2022).
- Tellegen, A. R., et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J Control Release. 286, 439-450 (2018).
- Vadalà, G., et al. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine. 38 (6), E319-E324 (2013).
- Yang, J. J., Li, F., Hung, K. C., Hsu, S. H., Wang, J. L. Intervertebral disc needle puncture injury can be repaired using a gelatin-poly (γ-glutamic acid) hydrogel: An in vitro bovine biomechanical validation. Eur Spine J. 27 (10), 2631-2638 (2018).
- Xi, Y., et al. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine (Phila Pa 1976). 38 (10), E579-E586 (2013).
- Elliott, D. M., Sarver, J. J. Young investigator award winner: Validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine (Phila Pa 1976). 29 (7), 713-722 (2004).
- Smit, T. H. The use of a quadruped as an in vivo model for the study of the spine - biomechanical considerations. Eur Spine J. 11 (2), 137-144 (2002).
- Romaniyanto, F. N. U., et al. Effectivity of puncture method for intervertebral disc degeneration animal models: Review article. Annals Med Surg. 85 (7), 3501-3505 (2023).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon