Method Article
Lipid Exchange Assay in Living Cells
In This Article
Summary
We describe a method for using cyclodextrin to mediate exchange between lipids of the plasma membrane with exogenous lipids. This technique can be paired with experiments studying transmembrane proteins, which behave differently in lipid raft-like environments than they do in non-raft-like environments.
Abstract
Lipid rafts are dynamic, ordered domains in the plasma membrane often formed during membrane protein clustering and signaling. The lipid identity of the outer leaflet drives the membrane's propensity to form lipid rafts. The transient nature of lipid rafts makes it difficult to study in living cells. Therefore, methods that add or remove raft-forming lipids at the outer leaflet of living cells facilitate studying the characteristics of rafts, such as their effects on membrane proteins. Lipid exchange experiments developed in our lab utilize lipid-loaded cyclodextrins to remove and add exogenous phospholipids to change the lipid constitution of the plasma membrane. Substituting the membrane with a raft or non-raft-forming lipid can aid in studying the effects on transmembrane protein activity. Here, we describe a method for lipid exchange on the outer leaflet of the plasma membrane using lipid-loaded cyclodextrin. We demonstrate the preparation of the exchange media and the subsequent treatment of attached mammalian cells. We also showcase how to measure the efficiency of exchange using HP-TLC. This protocol yields a nearly complete replacement of the outer leaflet with exogenous lipids without altering cellular viability, permitting further experimentation on modified intact plasma membranes.
Introduction
The plasma membrane is composed of a lipid bilayer enriched with various membrane proteins, including transmembrane receptors and ion channels. Lipid domains within the membrane have been elucidated through detergent-soluble and insoluble regions identified in detergent-resistant membrane (DRM) fractionation experiments1. The insoluble fractions were characterized by being enriched in cholesterol, tightly packed sphingomyelins and saturated phospholipids, exhibiting higher melting points, in contrast to the soluble fractions that predominantly consist of lower melting temperature and loosely packed unsaturated phospholipids. The tightly packed regions are referred to as liquid-ordered (Lo) lipid domains, or lipid rafts, while the more loosely organized liquid-disordered (Ld) lipid domains are the non-raft regions of the plasma membrane2,3. Lipid raft regions are known to facilitate signaling processes, with evidence indicating that the active insulin receptor associates with these rafts4,5. However, due to the dynamic nature of the cell membrane and the generally small size of domains, directly visualizing the presence of rafts in live cells presents significant challenges. In this context, we present a method to investigate the impact of lipid rafts on the insulin receptor through lipid exchange techniques.
Cyclodextrins (CDs) are formed by linked glucose monomers that create a ring-like structure with a central cavity. The size of this cavity is determined by the number of glucose units: six units form alpha-cyclodextrin (α-CD), while seven units create beta-cyclodextrins (β-CDs). CDs are highly water-soluble molecules capable of encapsulating lipids within their cavity, thus facilitating their transport to the cell membrane6. Beta-cyclodextrins have been extensively used to add and remove lipids from membranes7; however, its larger cavity lacks specificity for cholesterol or phospholipids8. In contrast, alpha-cyclodextrins, with their smaller cavity, exhibit greater selectivity in binding lipid molecules over sterols. Specifically, methyl-α-cyclodextrin (methyl-α-CDs) does not interact with sterols and has been effectively used to exchange phospholipids and sphingomyelins without altering the cholesterol composition of the cell membrane8,9.
In this manuscript, we provide a detailed protocol for using methyl-α-CDs (MαCD) to exchange lipids in the outer leaflet of the cell membrane with exogenous lipids that have properties either promoting or disrupting lipid raft formation. This exchange is used to investigate the impact of lipid rafts on insulin receptor activity. The demonstration will focus on the introduction of a phospholipid and sphingomyelin affecting the formation of liquid-ordered (Lo) domains in the plasma membrane of Chinese Hamster Ovary (CHO) cell lines stably overexpressing the insulin receptor (IR)10. The extent of lipid exchange in the CHO IR cells will be assessed through high-performance thin-layer chromatography (HP-TLC), while changes in insulin receptor activity will be quantified by western blot analysis following insulin stimulation post-lipid exchange.
Protocol
1. Preparation of Methyl-α-CD solution
- Add 20 mL of phosphate-buffered saline (PBS) to ~10 g of MαCD powder in a glass bottle. Incubate in a warm water bath (45 °C) to dissolve, stirring occasionally until well dissolved.
NOTE: The solution may still be cloudy due to insoluble CD species. - Pass solution through a 0.22 µm syringe filter. The solution will become clear.
- Use a refractometer to determine the exact concentration of MαCD.
- Place 10 µL of sample on the sample area of a refractometer, illuminate it using a white incandescent bulb, and record the solution's refractive index.
- Calculate the MαCD concentration using the equation
RI = (1.49 x 10 x C) + 1.33
where RI = Refractive index, C = concentration of MαCD (mM). The equation was obtained through gravimetric analysis, or measuring the refractive index of a known weight of MαCD dissolved in a known volume.
- Close the glass bottle with a lid and wrap the lid in a transparent film to prevent evaporation. Store at 4 °C.
2. Preparation of multilamellar vesicles (MLVs)
- Maintain stock solutions of desired exogenous lipids dissolved in chloroform at concentrations of 20 mM to 50 mM and store at -20 °C or below to minimize solvent evaporation.
NOTE: All lipids in Table 1 can be fully dissolved in chloroform stocks. However, it is possible that alternative lipids may require 1:1 chloroform: methanol to fully dissolve. - Aliquot lipid stock into borosilicate tubes using a positive-displacement pipette with a glass tip. Alternatively, a glass microsyringe can be used.
- Dry lipid aliquot on a heating block at a low setting of about 50 °C under a stream of N2 gas until all apparent chloroform evaporates.
- Remove the remaining solvent from the dried lipid by placing the tube in a vacuum chamber and exposing it to a high vacuum (under 200 mTorr) for 1 h.
- Add serum-free Ham's F-12 media to dry lipid film to reach a final concentration of 20 mM. Cover with a lid or Teflon tape and heat in a 70 °C water bath for 5 min.
- Vortex to suspend lipids and form MLVs. The media should now appear cloudy.
NOTE: Some saturated lipids may not be fully suspended by vortexing alone and may need to be suspended by pipetting up and down until the film is no longer visible.- Transfer the entire volume to a microcentrifuge tube. MLVs can be stored for up to 3 days at 4 °C.
3. Preparation of lipid exchange media
- Add MαCD stock to a final concentration of 40 mM, along with prepared MLVs to the final lipid concentration found in Table 1.
NOTE: If using MLVs stored at 4 °C, they will be settled at the bottom of the tube. Warm up to room temperature and ensure they are resuspended by flicking or agitating the tube.- Load lipids onto MαCD by incubating for 30 min in a 37 °C or 55 °C water bath according to Table 1. The temperature chosen should be above the gel-to-liquid phase melting point of the chosen lipid. The media should go from cloudy to clear.
- Let lipid exchange media cool to room temperature for 30-60 min.
4. Lipid exchange treatment of cells
- Grow CHO IR cells at 37 °C and 5 % CO2 in Dulbecco's modified Eagle's medium (DMEM, 4.5 g/L glucose) supplemented with 10% fetal bovine serum (FBS), 300 µg/mL L-glutamine, 100 µg/mL non-essential amino acids, 50 µg/mL G418, 2 µM methotrexate, and 1x antibiotic-antimycotic.
- Seed 1.5 x 106 cells in 60 mm plates and grow until they are 80%-90% confluent.
- Wash cells 3x by adding 1 mL of PBS and then aspirating. Starve cells overnight in 2 mL of serum-free Ham's F12 media.
- Wash cells 3x with 1 mL of PBS. Add 1 mL of prepared exchange media or serum-free media as a control to the cells and incubate for 1 h at room temperature (25 °C -27 °C). Swirl cells every 15 min to ensure even exposure to exchange media.
- Wash cells 3x with 1 mL of PBS. Each plate can be subsequently processed for lipid extraction in step 5, or for IR autophosphorylation in step 7.
5. Lipid extraction on cell culture plate
- Completely remove PBS and set the plate at a 45° angle for 10 min or until fully dry, removing any buffer that may collect along the bottom.
- Add 1 mL of 3:2 (v:v) hexane: isopropanol solution to the dried cells and incubate for 10 min on a shaker at room temperature.
- Transfer the solution to a borosilicate tube. Cover with Teflon tape and store at -20 °C.
- Dissolve the remaining cell debris by adding 500 µL of 1N NaOH and shaking for 10 min at room temperature.
- Use this solution in a protein quantification assay such as the Bradford Assay to determine the protein concentration of each sample. These concentration values can be used to normalize loading volumes of the lipid extracts on HP-TLC plates for equal lipid loading across all samples.
6. Checking exchange efficiency with HP-TLC
- Make 100 mL of 65:25:5 (v:v:v) chloroform:methanol:30% (v/v) ammonium hydroxide and pour it into a glass TLC tank. Cover tightly and allow vapor to equilibrate for at least 1 h.
- Dry lipid extract sample on a heating block at low setting under a stream of N2 gas until all apparent organic solvent evaporates.
- Dissolve the lipid film in 50 µL of 1:1 (v:v) chloroform: methanol.
- Use a 10 µL Hamilton syringe to load 1-10 µL of sample in 1 cm bands on a silica HP-TLC plate. Load a maximum of 10 bands on a 20 cm plate. Use normalized values to determine volumes needed to load equal amounts of lipids across all samples.
NOTE: Before loading each sample, the plate should be activated by placing it on a hot plate at a medium-high setting. - Place the plate upright in the TLC tank and allow the solvent front to travel 8 cm to ensure the separation of phospholipid species.
- Let the plate dry for 10 min. Spray with an aqueous solution of 3% (w/v) cupric acetate and 8% (v/v) phosphoric acid.
- Let the plate dry for 30 min at room temperature or with a heat gun. The plate should turn from translucent blue to opaque white.
- Char plate in a 180 °C-200 °C oven for 5-10 min or until the black lipid bands become detectable.
7. Checking receptor activation with autophosphorylation assay and western blot
- Incubate cells with 500 µL of 100 nM Insulin in serum-free media at room temperature for 5 min.
- Wash cells with ice-cold PBS and put cells on ice to halt stimulation. Promptly add 1 mL of ice-cold PBS to the cells and harvest with a cell scraper. Pellet cells at 3000 x g for 5 min at 4 °C.
- Add 100-200 µL of complete RIPA lysis buffer (50 mM Tris pH 8, 200 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate, 1 mM activated sodium orthovanadate, 10 µg/mL aprotinin, 10 µg/mL leupeptin) to cell pellet on ice. Pipette up and down 30x-40x to lyse.
- Incubate lysate on ice for 10 min. Clear lysate of cell debris by spinning at 16,000 x g for 10 min at 4 °C and collecting the supernatant. Reserve about 20 µL of lysate to determine protein concentration with Bradford assay.
- Combine lysate with 5x Laemmli buffer (350 mM Tris HCl pH 6.8, 30% v/v glycerol, 10% w/v SDS, 25% v/v β-mercaptoethanol, 0.002 g of bromophenol blue) and boil at 95 °C for 5 min. The volume of buffer added should be sufficient to achieve a final concentration of 1x Laemmli buffer.
- Run lysates through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
- Load equal mass of protein for all samples along with molecular weight marker onto an SDS-PAGE gel. Run at 100 V-150 V in running buffer (2.5 mM Tris pH 8, 19.2 mM glycine, 0.01% (w/v) SDS) until well resolved in the 100-250 kDa range.
- Transfer onto a polyvinylidene fluoride (PVDF) membrane in transfer buffer (2.5 mM Tris pH 8, 19.2 mM glycine) for 1 h at 100V and 4 °C.
- Block PVDF membrane for 30 min at room temperature in 5% BSA solution in TBST (50 mM Tris pH 8, 150 mM NaCl, 0.1% (v/v) Tween 20).
- Incubate with pYpY IR antibody or IR-β antibody at a 1:1000 concentration in 5% BSA solution in TBST for 1 h at room temperature or overnight at 4 °C.
NOTE: The pYpY IR antibody specifically recognizes phosphorylated tyrosines 1162 and 1163 and serves to indicate autophosphorylation of the receptor. The IR-β antibody recognizes the beta subunit of the receptor indiscriminately regardless of phosphorylation state and serves to indicate global levels of the receptor. - Wash 3x with TBST at room temperature. Incubate with α Rabbit-HRP antibody at a 1:3000 concentration in 1% BSA solution in TBST for 30 min at room temperature.
- Wash 3x with TBST at room temperature. Incubate with enhanced chemiluminescent (ECL) substrate for 1 min and image the film.
Results
To demonstrate the observable change in cellular lipid composition after the exchange, we performed HP-TLC on CHO IR cells following brain SM (bSM) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) exchange (Figure 1). In cases where sphingomyelins like bSM are being used for exchange, an increase in the SM band intensity is apparent, along with a decrease in PC band intensity relative to the untreated control. Conversely, when exchanging phosphatidylcholines like DOPC, the PC band becomes more intense while the SM band becomes less intense. Exchange efficiency can be determined by measuring the intensities of the exchanged lipid class with ImageJ software and taking the ratio of the untreated and treated samples. The loading standard, made from several dilutions of a commercially available lipid, demonstrates an option for estimating the amount of a class of lipids before and after exchange. By measuring the intensity of the SM standard bands and generating a standard curve, the SM intensities of all samples can be fitted onto the curve for lipid quantification.
Next, we show how receptor autophosphorylation can be assayed after lipid exchange by western blot (Figure 2). In the case of the insulin receptor, insulin-dependent autophosphorylation levels can be compared between untreated cells and cells exchanged with raft-forming lipids (bSM) or non-raft-forming lipids (DOPC). DOPC exchange negatively impacts IR phosphorylation, as do other lipids, such as 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC)4. Sphingomyelins maintain or moderately increase IR phosphorylation, as does 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)4. By blotting total receptor levels with IR-β antibody, phosphorylation levels from the pYpY IR antibody can be normalized to total receptor levels. ImageJ intensity values for pYpY IR divided by IR-β yields normalized values (Table 2). This accounts for any differences in expression level or otherwise unequal loading protein amounts, as can be seen in the first lane.
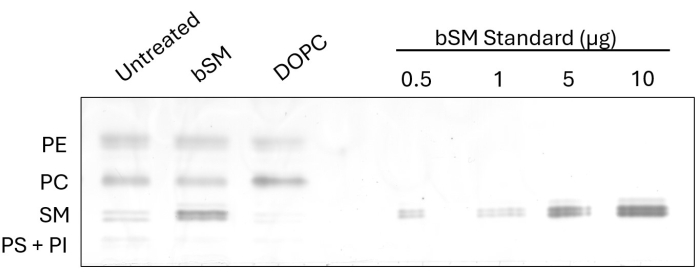
Figure 1: HP-TLC of bSM and DOPC exchange in CHO IR cells. CHO IR cells were starved and incubated with lipid exchange media (bSM or DOPC) or serum-free media as a control. Equal volumes of lipid extracts were loaded onto an HP-TLC plate along with increasing amounts of bSM. Please click here to view a larger version of this figure.

Figure 2: Western blot image of IR autophosphorylation after bSM and DOPC exchange. Control plates were incubated with (positive control) or without (negative control) 100 nM insulin. Lipid exchange plates (bSM or DOPC) were incubated with 100 nM insulin after exchange. Equal amounts of total protein were run on SDS-PAGE and blotted for pYpY IR and IR-β. The IR-β blot serves as a loading control. Please click here to view a larger version of this figure.
| Lipid | Exchange Media Preparation Temperature (°C) | Exchange Concentration (mM) | Saturation Character |
| DOPC | 37 | 4 | Unsaturated |
| POPC | 37 | 4 | Unsaturated |
| DLPC | 37 | 4 | Saturated |
| DMPC | 37 | 2 | Saturated |
| DPPC | 55 | 1 | Saturated |
| DSPC | 55 | 0.5 | Saturated |
| bSM | 37 | 1 | Saturated# |
| eSM | 37 | 1 | Saturated |
| #bSM is a mixture of sphingomyelin species. Most of these species are of saturated character with the exception of 24:1 sphingomyelin. It is unknown how well 24:1 sphingomyelin is able to be loaded onto MαCD relative to the saturated species. | |||
Table 1: Lipids used in exchange media.
| pYpY | IR-β | pYpY/IR-β | pYpY/IR-β (normalized) | |
| Negative control | 142.6 | 1229 | 0.12 | 0.09 |
| Positive control | 5844 | 4446 | 1.31 | 1.00 |
| bSM | 5886 | 5173 | 1.14 | 0.87 |
| DOPC | 2031 | 2740 | 0.74 | 0.56 |
Table 2: Intensity values of IR autophosphorylation western blot.
Discussion
Since the conceptualization of the existence of lipid rafts in the cell membrane there have been numerous attempts to visualize them in cells and study lipid and receptor association. Experiments involving microscopy11 in cells used fluorescently tagged biomarkers, usually, proteins and lipids known to associate with rafts, to visually study the localization of ordered lipid domains in the cell12. However, the cell membrane is full of folds13,14, caveolae invaginations1,13, and protein clustering2, which hinder the visualization of raft biomarker dynamics. An alternate approach has been used to study rafts in model membranes by preparing vesicles by using the appropriate lipids15. While easy to synthesize and work with, these vesicles are not an accurate representation of the cell membrane in terms of biophysical properties and chemical composition of the cellular bilayer. A natural membrane system is giant plasma membrane vesicles (GPMVs). GPMVs are plasma membrane vesicles with a chemically accurate composition of the cellular membranes. These are produced by incubating cells with reagents that form vesicles by budding off the cell membrane of live cells16. However, conventional solutions for forming GPMV are known to chemically modify and/or crosslink lipids and proteins during the vesiculation process and constitutively activate receptors in the vesicles. The protocol developed in our lab influences the ability of the membrane to form lipid rafts by exchanging exogenous lipids with varying propensities to support or destroy the ordered regions of the cell membrane in intact cells and without chemically modifying proteins4,8.
Lipid exchange experiments can be immediately followed by assays that measure the properties of the plasma membrane. HP-TLC provides confirmation that exchange has taken place and has been maintained after an experiment, adding confidence to the results of any biochemical experiments carried out after exchange assays. By visualizing the sphingomyelin and phospholipid makeup of the cell before and after exchange, one can also determine the efficiency of exchange under a new set of conditions, such as new cell lines or cyclodextrins. Steps leading up to the exchange segment of the protocol play a significant role in ensuring the precision of replicates and efficient lipid exchange in cells. Calibration, pipetting, and drying down the lipids should be viewed as critical steps due to the small volumes of organic solvent being used and the delicate concentration requirements for proper MαCD loading. The proper storage and calibration of lipid stock solutions are critical preliminary steps in the protocol. Stock solutions are typically prepared in chloroform and can evaporate over time, even when stored at -20 ˚C, leading to gradual changes in concentration. Drying the specified volume of lipids to achieve the desired concentration is essential for ensuring the correct amount of lipid is loaded onto the cyclodextrin for the exchange process. To prevent an error in pipetting, it is advised to calibrate the concentration of stock solutions on a monthly basis.
After calibrating the stock solutions, the volume of lipid required is pipetted into clean borosilicate tubes, for example, with a positive displacement Drummond pipette, which uses glass bores. High lipid stock concentrations could necessitate pipetting exceedingly small volumes, leading to inevitable error and variability in the final exchange conditions. To prevent this, it is advisable to pipette a volume corresponding to an intermediate concentration between that of the stock and the final exchange solution. This approach ensures optimal pipetting accuracy to form the multilamellar vesicles (MLVs) in the working exchange solution. Lipids are typically dried on a heating block under a steady stream of nitrogen gas maintained at about 10 psi for 10-20 min. Excessive gas pressure or placing the gas nozzle close to the bottom of the tube may cause the drying liquid to splatter against the walls, resulting in a loss of lipid volume and potentially altering the concentration of the MLVs. To prevent cross-contamination, all components of the drying equipment are washed with 100% ethanol both before and after the drying process. To ensure complete drying, the tube is placed under a high vacuum for 1 h and then sealed with transparent film to maintain the purity of the dried lipid.
Limitations of the experiment can be attributed to the role of flippases and scramblases in maintaining membrane asymmetry. The enzymes responsible for the movement of lipids between the inner and outer leaflets of the bilayer preserve the asymmetric nature of the cell membrane17. During the course of the experiment, the rate at which flippases and scramblases redistribute the exogenously added lipids between the leaflets is unknown. This could establish the nature of the cell membrane and is an external factor that has not been unaccounted for.
Additionally, this flip-flop action could, under some conditions, expose lipids prematurely present in the inner membrane of cells, like phosphatidylserine (PS), a lipid that is inherently responsible for signaling cellular apoptosis18. Our experiments employ sphingomylein (SM) and phosatidylcholine (PC) lipid exchanges. These lipids are present in the outer leaflet of the plasma membrane and take long durations to flip towards the cytosolic leaflet catering the assay primarily to the outer leaflet9,19,20. Another issue is that not every exogenous lipid added to cells may fully support cell membrane integrity and structure9,19. Using such lipids will inadvertently damage the cell membrane post the exchange limiting the lipids used in the exchange protocol. Performing cell viability, assays can assist in gauging the extent of damage endured by cells post-incubation with the exchange media4. Flow cytometric analysis of trypsinized and propidium iodide treated cells is a method to analyze the viability of the lipid exchanged cells. Membrane damage could also result in receptor internalization. Large amounts of insulin receptor internalization will alter receptor autophosphorylation data. Flow cytometry performed on phycoerythrin-tagged antibodies bound to the ectodomains of the insulin receptor in cells treated with exchange media can account for any changes in surface receptor expression4,20.
In this manuscript, we reported concentrations of a variety of lipids loaded onto specific concentrations of MαCD for efficient lipid exchange in CHO IR cells. To perform exchange experiments in other cell lines, the first step is determining effective lipid concentrations in the exchange media tolerated by the cells without compromising membrane integrity or affecting cell viability8,9,21. A wide range of CD to exogenous lipid ratios should be tested alongside untreated cells. In this manner, an optimum ratio of CD: lipid will be identified where there is no visible rounding up of cells or detachment from the plate (in the case of adherent cells), and the morphology of treated cells is similar to untreated ones. Biochemically, cells treated with CD: lipid media should be checked for perturbations in the membrane post lipid exchanges using trypan blue treatment and propidium iodide leakage or Annexin V binding to test surface PS exposure21. Optimal temperature conditions and length of exchange periods vary for different lipid vesicles and should be determined for new cell lines. Our lipid exchange experiment has been performed exclusively in live cell membranes. To our knowledge, there is no data evidence for successful lipid exchange in cellular lysate. This lipid exchange technique can be extended to alter the membrane of organelles within the cell if they can be isolated from the cellular lysate.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
Funding was provided by NIH grant GM 122493. CHO IR cells were a kind gift from Dr Jonathan Whittaker (Case Western Reserve University).
Materials
| Name | Company | Catalog Number | Comments |
| 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) | Avanti Polar Lipids | 850335 | |
| 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) | Avanti Polar Lipids | 850345 | |
| 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) | Avanti Polar Lipids | 850375 | |
| 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) | Avanti Polar Lipids | 850355 | |
| 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) | Avanti Polar Lipids | 850365 | |
| 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC) | Avanti Polar Lipids | 850457 | |
| Anti-insulin receptor β antibody | Cell Signaling Technology | CST3025 | |
| Anti-pYpY1162/1163 Insulin receptor antibody | R&D Systems Inc. | AF2507 | |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signaling Technology | 7074 | |
| Borosilicate glass test tubes (12 x 75 mm) | Thermo Fisher Scientific | 14-961-26 | |
| Brain sphingomyelin (bSM) | Avanti Polar Lipids | 860062 | |
| Egg sphingomyelin (eSM) | Avanti Polar Lipids | 860061 | |
| Fetal bovine serum (FBS) | Corning | 35-016-CV | |
| G418 disulfate salt | Sigma Aldrich | A1720 | |
| Gibco Antibiotic-antimycotic solution (100x) | Thermo Fisher Scientific | 15240062 | |
| Gibco Dulbecco’s modified eagle medium (DMEM, 4.5 g/L glucose, L-glutamine, sodium pyruvate) | Thermo Fisher Scientific | 11965092 | |
| Gibco ham’s F12 media | Thermo Fisher Scientific | 11765054 | |
| Gibco L-glutamine | Thermo Fisher Scientific | 25030032 | |
| Gibco MEM Non-Essential Amino Acids Solution (100X) | Thermo Fisher Scientific | 11140050 | |
| Gibco phosphate buffered saline (PBS) without calcium and magnesium (0.144 g/L KH2PO4, 9 g/L NaCl, 0.795 g/L Na2- HPO4 (anhydrous)) | Thermo Fisher Scientific | 10010023 | |
| Gibco Trypsin-EDTA (0.05%), phenol red | Thermo Fisher Scientific | 25300054 | |
| High performance thin layer chromatography (HP-TLC) | Merck | HP-TLC Silica Gel 60 plates | |
| Immobilon-P PVDF Membrane | Millipore | IPVH00010 | |
| Methotrexate | Sigma Aldrich | 454126 | |
| Methyl-α-cyclodextrin (MαCD) | AraChem | CDexA076/BR | |
| Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | 32106 | |
| Sodium orthovanadate, Activated | Sigma Aldrich | 5.08605 |
References
- Brown, D. A., London, E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 14, 111-136 (1998).
- Brown, D. A. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 21, 430-439 (2006).
- Schroeder, R., London, E., Brown, D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 91 (25), 12130-12134 (1994).
- Suresh, P., Miller, W. T., London, E. Phospholipid exchange shows insulin receptor activity is supported by both the propensity to form wide bilayers and ordered raft domains. J Biol Chem. 297 (3), 101010 (2021).
- Delle Bovi, R. J., Kim, J., Suresh, P., London, E., Miller, W. T. Sterol structure dependence of insulin receptor and insulin-like growth factor 1 receptor activation. Biochim Biophys Acta Biomembr. 1861 (4), 819-826 (2019).
- Zidovetzki, R., Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 1768 (6), 1311-1324 (2007).
- Ohtani, Y., Irie, T., Uekama, K., Fukunaga, K., Pitha, J. Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur J Biochem. 186 (1-2), 17-22 (1989).
- Suresh, P., London, E. Using cyclodextrin-induced lipid substitution to study membrane lipid and ordered membrane domain (raft) function in cells. Biochim Biophys Acta Biomembr. 1864 (1), 183774 (2022).
- Li, G., et al. Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids. Proc Natl Acad Sci U S A. 113 (49), 14025-14030 (2016).
- Yoshimasa, Y., Paul, J. I., Whittaker, J., Steiner, D. F. Effects of amino acid replacements within the tetrabasic cleavage site on the processing of the human insulin receptor precursor expressed in Chinese hamster ovary cells. J Biol Chem. 265 (28), 17230-17237 (1990).
- Gaus, K., et al. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci U S A. 100 (26), 15554-15559 (2003).
- Klymchenko, S., Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem Biol. 21 (1), 97-113 (2014).
- Pike, L. J. Growth factor receptors, lipid rafts and caveolae: An evolving story. Biochim Biophys ActaMol Cell Res. 1746 (3), 260-273 (2005).
- Brown, D. A., London, E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes. Biochem Biophys Res Commun. 240 (1), 1-7 (1997).
- Lin, Q., London, E. Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS One. 9 (1), e87903 (2014).
- Sezgin, E., et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 7 (6), 1042-1051 (2012).
- Deveaux, P. F., Hermann, A., Ohlwein, N., Kozlov, M. M. How lipid flippases can modulate membrane structure. Biochimica et Biophysica Acta Biomembr. 1778 (7), 1591-1600 (2008).
- Balasubramaniam, K., Mirnikjoo, B., Schroit, A. J. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 282 (25), 18357-18364 (2007).
- Li, G., et al. Replacing plasma membrane outer leaflet lipids with exogenous lipid without damaging membrane integrity. PLoS One. 14 (10), e0223572 (2019).
- Contreras, F. X., Sanchez-Magraner, L., Alonso, A., Goni, F. M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Letters. 584 (9), 1779-1786 (2010).
- Suresh, P., London, E. MαCD-based plasma membrane outer leaflet lipid exchange in mammalian cells to study insulin receptor activity. Biophys Approach Study Memb Struct A Exp. 700, 485-507 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved