Method Article
Direct Vagus Nerve Injection Protocol For Rats
In This Article
Summary
We present a protocol for direct vagus nerve injection in rats, enabling drug delivery directly into the nerve without post-injection complications. This method applies to preclinical neurological studies involving autonomic nervous system manipulation. It can be used for direct nerve injection for other nerves in rats and other species, with necessary modifications.
Abstract
There is a relative abundance of strategies and methodologies to facilitate drug delivery to the central nervous system. However, drug delivery directly to the peripheral nervous system is less common, with fewer detailed methods publications available to aid researchers. Here, we describe a direct nerve injection method for peripheral nervous system drug delivery, using the vagus nerve as a model nerve. This method can be used in the treatment of autonomic nervous system disorders through targeting of the left vagus nerve, although this general injection method can be extrapolated to injection of other nerves with minor modification. This method explains all critical steps involved in the procedure involving microsurgery in anesthetized adult rats under a dissecting microscope. The use of a tracking dye is described to facilitate the monitoring of injection fidelity in real time. Illustrations of successful and failed injections are provided. If carried out properly, direct vagus nerve injections can be conducted in a safe manner that is well-tolerated by the rat without post-delivery complications. For example, once the surgeons were trained in this method, six out of six rats were successfully injected without any complications. This method of direct nerve injection for preclinical rat studies is capable of delivering agents (including but not limited to gene therapy) to peripheral nerves.
Introduction
The application of the correct method of drug administration is one of the critical factors in achieving successful therapeutic results. Despite the abundance of methods for therapeutic agent delivery to the central nervous system (CNS), only a few methods are reported for peripheral nervous system (PNS) delivery by direct nerve injection. Direct nerve injection, such as injection into the dorsal root ganglia (DRG) in rats, has been tried in preclinical studies for better understanding of pain mechanisms, drug toxicity, gene transfer1,2,3, and general method development1,4. Additional reports on direct nerve injection include spinal nerve injection4, sciatic nerve injection1, and vagus nerve injection in rats5 and mice6. Recently a method for suprachondrial injection has been proposed for the better distribution of therapeutics into the optic nerve head in rabbits7.
DRG is considered the ideal location for direct injection of transgene-loaded vectors such as adeno-associated virus (AAV) because of the sensory function of the cell bodies in the DRG2. Both surgical and non-surgical methods of DRG injections have been described1,8. However, controversial conclusions have been found about the consistency of results with the non-surgical method of DRG injection1. A surgical method involving a partial laminectomy has been suggested to be 100% successful for DRG injection in rats without any alteration in behavior outcomes3, as well as a method involving partial osteotomy in mice9. Several studies report the DRG injection methods of drug delivery, which have been used in preclinical gene therapy research in rats and mice1,2,10. Vector-based gene therapy studies involving localized injections may include the following benefits: decreased off-target expression, reduction in systemic toxicity, and smaller viral loads and injection volumes, decreased risk of immunogenic complications11,12.
Direct injection method into the sciatic nerve, the longest nerve of the body, has been tried by exposing the right sciatic nerve at the mid-thigh level of a rat. The method used a pulled glass pipette equipped with a microprocesser-controlled injection system to inject a total volume of 10 µL dye with a flow rate of 1.2 µL/min1. This experiment showed a lack of dye distribution to the level of DRG, and the distribution was mostly limited around the site of injection. Similarly, other methods of direct nerve injections, such as spinal nerve injections, have been tried with dye to evaluate the appropriate amount of injection volume and dye distribution pattern in rats. 2 µL is suggested to be optimum for spinal nerve injection, whereas 3 µL of dye by DRG injection showed the distribution in both dorsal and ventral root ganglia in rats1. The volume for DRG injection in mice has been reported to be optimum from 1.0 µL to 1.5 µL based on strain and body size2,9.
Direct vagus nerve injection method was used in rats5 and mice6 to evaluate the role of neural injury or cellular integrity in transferring human α-synuclein. These two studies, conducted by the same group of researchers, describe a brief method of directly injecting AAV vectors into the left vagus nerve at the cervical region. In rats, the method involved a glass capillary with a 60 µm tip diameter to inject 2 µL vector at a flow rate of 0.5 µL/min with a 5 µL Hamilton syringe. In mice, a total volume of 750 nL vector solution was injected at a flow rate of 160 nL/min using a 36-G blunt steel needle fitted onto a 10 µL NanoFil syringe6. These experiments showed that the transgene was delivered and expressed in axons in the pons and midbrain of the rats and mice. Similarly, the dorsal motor nucleus of the left vagus nerve showed positive immunoreaction with transgene. These pieces of evidence illustrate that the direct vagus nerve injection method might be a reliable method in gene therapy where the cellular transduction is extended into several locations of the brain, which project axons through the vagus nerve. However, these methods do not mention the use of any dye to track the injection fidelity.
Here, a method is described for direct injection into the left vagus nerve using nontoxic tracking dyes largely applicable to researchers in preclinical studies. Potential pitfalls that may cause difficulties in drug delivery and the ways to overcome them are discussed. These situations are illustrated with pictures to show what makes the delivery unsuccessful and the way to make it successful.
Protocol
The following protocol is conducted in accordance with institutional ethics guidelines and approval from the Institutional Animal Care and Use Committee (IACUC).
1. Housing preparation
NOTE: This protocol is for adult rats aged at least 2 months. Smaller animals (including mice and younger rats) are possible but are not recommended and will be considerably more difficult.
- Provide moist chow or any other soft food (veterinary recovery gel) for 48 h prior to vagus nerve surgery. As animals may face difficulty eating after the procedure, introduce these items to the animals before surgery to help them become familiar with the taste of these items and induce eating motivation.
- House rats individually in a cage at least 1 week prior to surgery, as well as 1 week post-surgery. That will enable them to acclimate to the stress of being alone and prevent scratching of wounds after surgery by a littermate.
2. Preparation of the surgical items and space
- Make a list of all necessary items ahead of time and double-check that all items are available for the surgery.
- Sterilize all items with appropriate methods of sterilization before the surgery.
- Set up the sterile surgical space under the dissecting microscope. Place a heating pad under the microscope to keep the rat warm during surgery.
- Place all surgical materials on a surgical table on a sterile drape. Keep enough space for surgery under the dissecting microscope. Make sure the microscope's focus is optimum and encompasses the surgical area to visualize the nerve.
- Place the syringe pump close to the surgical stage so that the length of the tubing is enough to reach the site of injection.
- Place the surgical tools towards the side of the surgeon's dominant hand.
3. Preparing a mixture of the candidate drug and the tracking dye
- Ensure the candidate drug is diluted to achieve the required concentration in the recommended diluent such that 5 µL of the drug mixed with dye gives the required amount of the candidate drug to be delivered. A maximum volume of injection by the direct vagus nerve method is recommended to be 5 µL (including tracking dye, see step 3.2).
- Mix a compatible dye with the candidate drug to enable the tracking of injection fidelity in real-time. For example, a final concentration of either 1% Luxol Fast Blue or 0.002% fluorescein are compatible dyes to use.
CAUTION: Use the dye at its optimum concentration in the dye and candidate drug mixture. When the concentration of dye in the mixture is too low, it may not be visible to track the injection. When the concentration of dye is too high, it may result in a mixture that is too thick and has high viscosity, which will block the injection needle.
4. Loading the candidate drug and dye mixture into the tubing
- Connect a 100 µL glass syringe with the plunger removed to a 27 G needle.
- Connect one end of approximately 45 cm of sterilized polyethylene tubing to the 27 G needle.
- Take a 3 mL plastic syringe and fit it with a 27 G needle. Load the syringe with about 0.5 mL of 0.9% normal saline.
- Fill the polythene tubing with the 0.9% normal saline from the open end to fill the full length of the tubing and the whole barrel of the glass syringe until it overflows out.
NOTE: It is critical to avoid air bubbles getting trapped inside the tubing. - Remove and discard the 3 mL syringe and needle.
- Insert the syringe plunger into the barrel of the glass syringe and push it slightly forward up to the middle of the barrel.
- Place the glass syringe in the syringe pump.
- Pull the plunger slightly back using the syringe pump to create an air space of about 1.5 cm at the open end in the length of the tubing.
- Pipette at least 5 µL of dye and candidate drug mixture with a 10 µL micropipette and drop it onto a sterilized piece of parafilm.
- Withdraw the candidate drug mixture into the tubing by pulling the plunger back via the syringe pump. Maintain the air bubble between the 0.9% saline and the injection solution, but otherwise, avoid trapping any air bubbles within the injection solution. Mark the level of the injection mixture in the tubing with a marker.
NOTE: The air gap between the candidate drug-dye mixture and the sterile saline within the tubing is critical to prevent the mixing of the two solutions. - Fit the 35 G needle with the tubing. Fix the needle onto the clean surface of the surgical stage with tape so that the needle is not loose, does not move and touch anything else, and remains sterilized.
5. Priming of the injection needle
- Set the syringe pump program to dispense 0.5 µL/min.
- Start the syringe pump for about 10-15 s, until the injection solution starts to come out of the tip of the needle. A tiny amount of the mixture at the tip of the needle without a leak at the joint of the tubing and injection needle (or anywhere else along the length of the syringe, tubing, or needle) indicates that the setup is correct.
NOTE: If a tiny droplet at the tip of the needle is not seen, that indicates a blockage of the needle. Needle blockage may occur due to the high viscosity of the candidate drug and dye mixture, excess air trapped within the system, and the glass syringe is not properly seated within the syringe pump. See Figure 1 for an example of leakage at the junction between the needle and tubing. Priming is critical because it helps to identify if the needle is unobstructed and the injection inside the nerve would pass smoothly.
6. Preparation of rat for surgery
- Obtain the body weight of the rat to calculate the necessary dose of analgesics. For example, carprofen is used at the dose rate of 5 mg/kg subcutaneously in rats.
NOTE: Carprofen is recommended to store at 4 °C before injection. - Anesthetize the rat with an isoflurane vaporizer using a flow rate of 3%-4% for induction of anesthesia for about 2-3 min. Reduce the isoflurane flow rate to about 1.75%-2% for maintenance of anesthesia.
- Transfer the rat to a separate table with a setup for hair removal and site preparation. Ensure this table is close to the surgical space.
- Apply artificial tearing ointment in both eyes of the rat to prevent excessive dryness.
- Administer analgesics per the approved institutional animal protocol to control pain when the rat comes back into consciousness.
- Shave the surgical area with a hair clipper at the ventral side of the neck at the cervical region. Shave up to about 2 cm perpendicular distance from the midline on both sides from chin to sternum.
- Sterilize the area with 70% ethyl alcohol and 10% betadine, wiping the area three times alternatively with an alcohol swab and povidone-iodine. Wipe from the center of the area outward in a circular pattern.
- Transfer the rat to the surgical stage under the microscope in a supine position. Adjust the focus of the microscope to visualize the area of surgery at the cervical region.
7. Performing rat surgery to inject the candidate drug directly into the left vagus nerve (Figure 2, Figure 3, and Figure 4)
NOTE: This portion of the method will require a second person to assist the surgeon.
- Place the rat on a heating pad in a supine position under the dissecting microscope associated with a mounted light.
- Hook the front upper jaw teeth of the rat with a piece of non-absorbable suture and fix the open end of the suture inside the nose cone so that the nostril of the rat is always inside the nose cone throughout anesthesia.
- Position the rat so its head lies on the left side of the right-handed surgeon (or the right side of a left-handed surgeon). In this position, the front side of the surgeon is perpendicular to the body of the rat. Place a cushion of sterilized gauge under the neck and adjust the angle of the cervical region of the rat so that the cervical region becomes straight. This will make it easier to locate the left vagus nerve and perform the injection.
- Drape the whole body of the rat with a sterilized drape, keeping the surgical space open.
- Fix four retractor pins individually with 4 magnetic fixators with elastomers and place the fixators at the four corners of the surgical stage.
- Inject local anesthetics epidermally at the midline of the cervical region where the incision is made. For example, a mixture of lidocaine and bupivacaine is usually used.
- Make about a 2 cm long longitudinal skin incision at the midline between the chin and sternum with a scalpel blade in the ventral side of the neck at the cervical region (Figure 3A).
- Blunt separate apart the cut edges of the skin with sterilized cotton tips.
- Retract the skin edges in opposite directions from the site of the incision with the help of retractor tips.
- Separate the facia with cotton tips to go deeper. Push the salivary glands towards the lateral side.
- Separate the sternomastoid muscles with cotton tips and retract them aside with the pins. As the sternomastoid muscle separation advances wide, the trachea appears in the middle, covered with sternohyoid muscle (Figure 3B).
NOTE: Be careful not to exert pressure on the trachea. - Advance the tissue separation on the rat's left side of the trachea until the carotid sheath appears, which contains the common carotid artery running together with the left vagus nerve.
- Carefully make a small point hole into the carotid sheath with fine forceps, paying attention so as not to injure the carotid artery under the microscope. Separate the left vagus nerve from the common carotid artery with fine forceps.
NOTE: The surgical area is anatomically critical. The wall of the carotid artery is thick, slippery, and not injured easily, but the sharp tip of fine forceps may injure it, and bleeding may happen. At the same time, pay attention so as not to injure the nerve. - Once the nerve is separated from the artery by the forceps, use a 25 G curved needle fitted to a 1 mL syringe from that hole to "hook" and hold the nerve by the non-dominant hand (Figure 4A).
NOTE: The tip of the curved needle is made blunt so as not to injure the nerve and artery. The angle of curvature of the needle tip is custom-made to about 90° using a hemostat (Figure 2). - With the dominant hand, hold the needle loaded with the candidate drug-dye mixture and gently prick the nerve in the same direction that the nerve runs so that the bevel of the needle is facing up.
- For an easy prick, keep the nerve straight by gently pulling using the 25 G hook. Insert the needle into the nerve and advance forward, more than 0.5 cm, while keeping the needle parallel to the vagus nerve. Then, pull slightly back so that about 0.4-0.5 cm needle remains inside the nerve.
NOTE: The angle of the needle should be shallow enough to penetrate inside the nerve without going through to the other side.
- For an easy prick, keep the nerve straight by gently pulling using the 25 G hook. Insert the needle into the nerve and advance forward, more than 0.5 cm, while keeping the needle parallel to the vagus nerve. Then, pull slightly back so that about 0.4-0.5 cm needle remains inside the nerve.
- There might be occasions when the needle is unobstructed outside the nerve at the time of priming, but the candidate drug-dye mixture does not pass smoothly inside the nerve. To avoid this situation, limit the number of nerve pricks by a single needle not more than 3 times. If the needle gets blunted, the chances of blocking or otherwise poor injection will increase.
- Make sure the needle is completely inside the nerve. Retain the position immobile and turn the syringe pump on.
NOTE: Check carefully at the site of injection to see that the candidate drug-dye mixture is not back-flowing out of the nerve. - While the injection is proceeding, constantly examine that the infusion is remaining inside the nerve and flowing smoothly. Use the mark made on the infusion tubing to monitor the movement of the drug-dye mixture from the starting point.
- If the candidate drug-dye mixture is not flowing smoothly, pull the needle out and repeat the priming step (step 5.2). Then re-insert the needle into the nerve and resume the infusion.
- The nerve starts to develop the color of the dye (Figure 4B). Continue the infusion at 0.5 µL/min for 10 min to infuse all 5 µL of the candidate drug-dye mixture.
NOTE: It is recommended to irrigate the exposed tissues with a small amount of warm 0.9% sterile saline as needed to prevent excessive dryness and damage. - After the 5 µL has been infused and the syringe pump stopped, retain the needle in place for an additional 1 min to allow all of the candidate drug-dye mixture to spread from the injection site. Remove the needle.
- Remove the 25 G curved needle. Use sterile cotton tips to gently move tissues back into their original location. Remove the retractor tips.
- Close the wound with a non-absorbable suture (absorbable suture can also be used). Apply a tiny amount of tissue glue between the stitches if necessary to close the wound perfectly.
8. Post-operative care of a rat
- Infuse about 3 mL of 0.9% warm saline subcutaneously after the wound is closed. This helps the rats to rehydrate immediately.
- Place the rat in a heat source until it recovers fully from anesthesia to normal condition. Note anything observed that is abnormal during the process of recovery.
NOTE: the recovery duration may be longer if the nerve injection procedure takes a long time. - Transfer the rat into its home cage with moist chow on the floor of the cage. Make sure water is available all the time. Observe the rat post-operatively for about 2 h to ensure the rat is not feeling cold.
NOTE: Place the rat in a heat source for additional hours if it feels cold. - Monitor the rat at 12 h, 24 h, 36 h, 48 h, and 72 h post-surgery with written notes of observations. Repeat analgesics as per the approved institutional animal protocol to control pain. For example, carprofen is dosed at the rate of 5 mg/kg subcutaneously two times at the interval of 24 h. Consult a veterinarian if pain remains in the rat for more than 48 h.
- Repeat the subcutaneous injection of about 3 mL 0.9% warm saline at 24 h based on the conditions of the rat.
- Continue to evaluate the wound. Ensure the wound is dry and is in the process of healing.
NOTE: Redness, swelling, and painful condition of the wound with inactivity and dullness of the rat can be an indication of wound inflammation and may warrant additional veterinarian consultation. A rat with pain will show a hunched back, ruffled coat, red eyes, and inactive condition. It is usually found in a corner of the cage. - Remove the non-absorbable sutures within 2 weeks once the wound is healed.
Results
Six adult rats (3 males and 3 females) were used in the present study. Out of six, one rat was injected multiple times to demonstrate a condition of failure injection that shows a diffusion of dye around the injection site Figure 5A. All the other rats showed smooth injection and nerve staining, as shown in Figure 4B and Figure 5B.
Figure 1 demonstrates a situation where leakage of dye between the needle and tubing has occurred when the setting of the injection needle with the tubing is not correct. The 1% dye used in this experiment is not too thick, but the passing of dye through the injection needle has failed due to a leaky seal. This kind of problem can easily be identified in the process of priming (see step 5.2). To solve this condition, check if the connection between the needle and tubing is correctly made. Make sure about 3 mm of needle has been inserted into the tubing, and make sure that the size of the tubing and needle are correct. Repeat the priming after the faulty connection has been corrected, especially if air bubbles are introduced (repeat step 5.2).
Figure 2 shows a setup of a 25 G curved, blunted needle fitted to a 1 mL plastic syringe. The tip of the needle is blunted and bent to about 90° angle by a needle holder. It is sterilized before use and fitted to the 1 mL sterilized syringe at the time of surgery. This syringe-fitted needle is used to separate the left vagus nerve from the common carotid artery, as described in step 7.14, hook and hold it at the time of injection, as shown in Figure 4A.
Figure 3 shows a setup of an aseptic procedure of midline incision with a scalpel blade at the ventral neck region of a rat to cut the skin layer. After the rat is covered with a sterilized drape, about 2 cm long horizontal incision is made to cut the skin layer, Figure 3A. Once the skin is cut, the two edges of the cut skin are pulled opposite sides with the help of pins connected with elastomers. The traction of the cut edges to the opposite side creates a wider space to go deeper. The layer of facia beneath the skin and the sternomastoid muscles are bluntly separated by sterilized cotton tips, as shown in Figure 3B. Once the left vagus nerve appears on the left side of the trachea within the common carotid sheath, as described in step 7.12, it is separated by fine forceps and hooked like in Figure 4A.
Figure 4 demonstrates a situation of the left vagus nerve separation from the common carotid artery and its holding ready for injection. The surgeon is holding the injection needle with his dominant hand (right hand), and the other hand (left) is holding the left vagus nerve of the rat with the help of a curved needle fitted to a 1 mL syringe, Figure 4A. The left vagus nerve is well separated from the common carotid artery and stretched enough to inject into it. The dotted rectangle on the right side of the nerve shows the area of the trachea covered with sternohyoid muscle. Figure 4B shows the left vagus nerve colored with the color of the blue dye after successful injection without diffusion of the dye. The white dotted rectangle is the area of the trachea. Figure 4B is stretched more than Figure 4A to show a clear picture of the nerve and its surroundings after injection.
Figure 5 shows examples of both failed and successful injections into the left vagus nerve of a rat. These injections used 5 µL of 1 % dye at a flow rate of 0.5 µL/min into the nerve. Figure 5A shows a failure condition of injection because the dye is not limited to only inside the nerve (see inset). Rather, the dye has diffused all over the area around the nerve. In addition, the actual injection site in the nerve is not clear. This suggests that the injection was not inside the nerve, and a significant amount of injection volume leaked out and diffused to the surrounding area.
A similar situation can be seen in conditions when the needle needs to be re-inserted or repositioned in the nerve. This can be necessary when the needle clogs and does not pass the mixture or if the needle gets blunt and cannot easily pass into the nerve. In the example shown in Figure 5A, the small amount of blood accumulated in the area shows the accidental rupture of tiny blood vessels in the area. This happens when blood vessels in the area are injured during nerve separation.
Figure 5B demonstrates a successful injection into the nerve. It shows a clear point of injection and well-stained nerve, distinctive from the surrounding area. A tiny dye stain area outside the nerve is due to a small amount of the dye solution at the tip of the needle that is present after priming (see inset). Priming is done to test the obstruction of the needle just before pricking the nerve.

Figure 1: Leakage of dye (arrow) due to a faulty seal between the needle and the tubing. This type of error occurs if the tubing is not correctly fitted with the tip of the needle. To prevent this kind of leaking, about 3 mm or more of the needle tip is inserted into the tubing, and the correct size of the needle and tubing are used. Please click here to view a larger version of this figure.

Figure 2: A 25 G blunted needle custom curved at about 90° angle and fitted to 1 mL plastic syringe. Please click here to view a larger version of this figure.
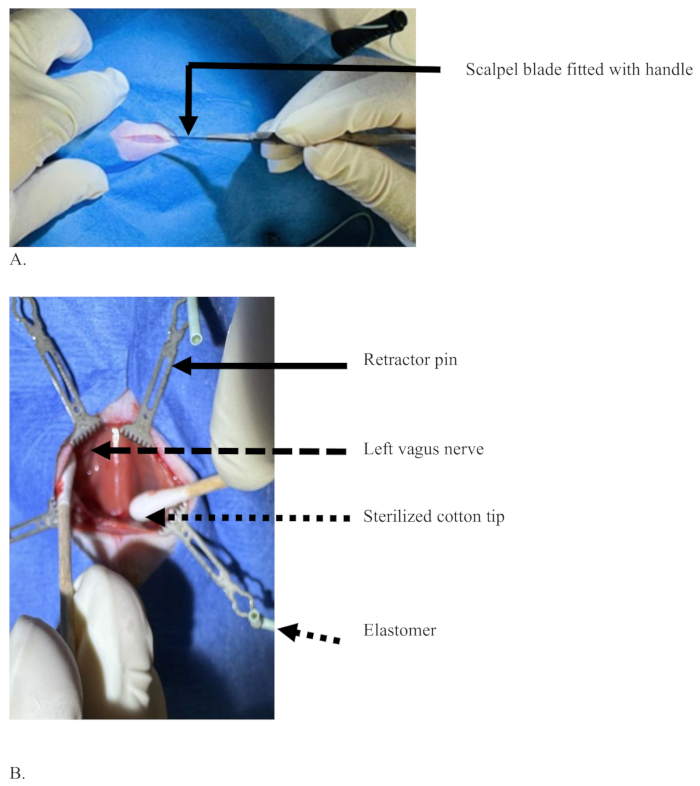
Figure 3: A midline incision with a scalpel blade at the ventral neck region of a rat to cut the skin layer. (A) An incision with only the skin cut but without blood, and a scalpel blade with a handle held with the dominant hand of the surgeon. (B) The sterilized cotton tips (dotted arrow) separating tissues apart to expose the left vagus nerve (dashed arrow), tissues pulled apart with the help of pins (solid arrow) supported by elastomer (short dotted arrow). Please click here to view a larger version of this figure.
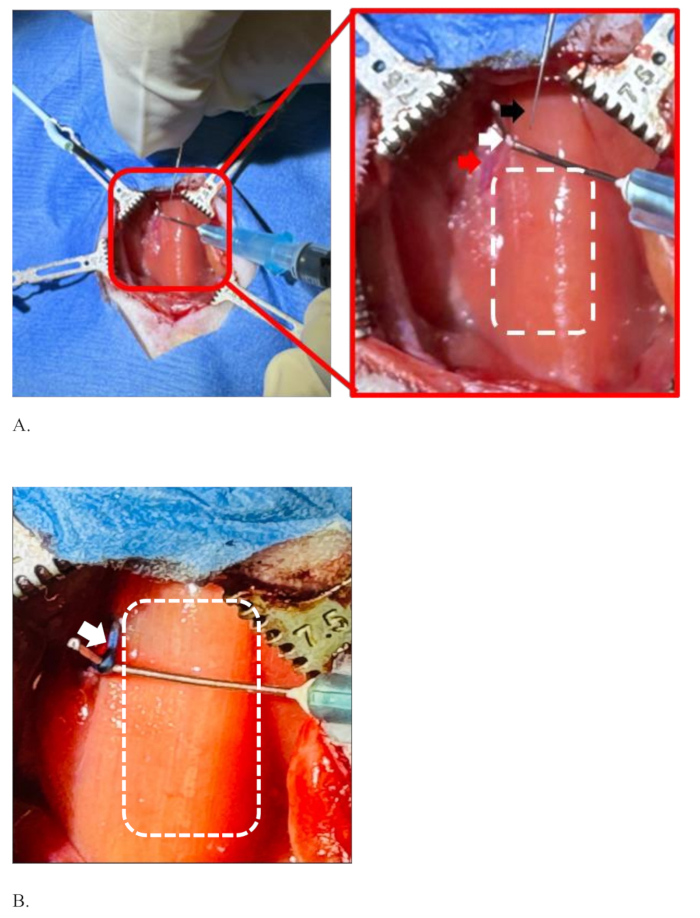
Figure 4: Illustration of separation of the left vagus nerve from the common carotid artery, holding it before and after injection. (A) The injection needle (black arrow), the left vagus nerve (white arrow), the common carotid artery (red arrow), and the dotted rectangle showing the area of the trachea covered with sternohyoid muscle. (B) The left vagus nerve in the blue color, the color of the dye after successful injection without diffusion of the dye (white arrow), and the area of the trachea (dotted rectangle). Please click here to view a larger version of this figure.
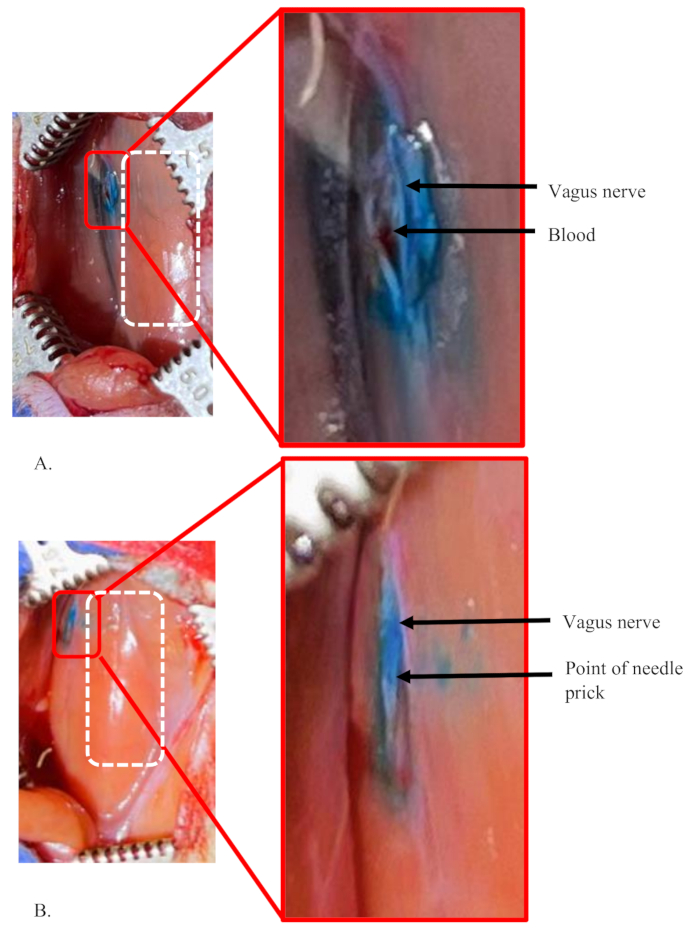
Figure 5: Examples of a failed injection and a successful injection into the left vagus nerve of a rat. (A,B) 5 µL of dye at 1% concentration was injected into the left vagus nerve of rats. The white dashed rectangle shows the area of the trachea in both panels. (A) A failure condition showing the dye is not limited to only inside the nerve, suggesting a significant amount of dye solution loss during the procedure. The small amount of blood accumulated in the area shows an accidental rupture of a tiny blood vessel in the area, which was damaged during nerve separation. (B) A successful injection into the nerve. It shows a clear point of single injection and well stained nerve, distinctive from the surrounding area. Please click here to view a larger version of this figure.
Discussion
The method for direct injection into the left vagus nerve can be done safely and without post-surgery complications in rats. Drug delivery to the vagus nerve can be used to target the autonomic nervous system (ANS). This involves certain critical steps that require practice and a moderate to high degree of surgical skill.
This surgical procedure requires balanced general anesthesia in rats. The surgeon aims to finish the surgery within a shorter duration to limit the exposure of anesthetics for a better recovery, especially in old rats. This method also assumes a trained surgeon with a certain level of aseptic surgical skills to make the injection and post-operative recovery successful. The anatomical location of the vagus nerve is deeply seated in a critical location13. The left vagus nerve runs side-by-side with the left common carotid artery, which poses a risk of injury to both the artery and the nerve at the time of separation and injection. A high degree of practice and skill is required to correctly separate the nerve from the common carotid artery. It should be held at the appropriate angle during the procedure while keeping the needle inside the nerve.
While there are only two published examples of a direct vagus nerve procedures in rats5 and mice6 by the same group of researchers, both studies lack a detailed description of how to conduct the procedure. The use of a 35 G needle to inject the nerve was convenient (i.e., no special equipment was needed to prepare needles), and the results remained consistent compared to using the pulled glass micropipette used by the other group. Compared to the injections in other nerves, such as the sciatic nerve, the vagus nerve is relatively smaller in size and can only hold a smaller volume of drug-dye mixture at a time. In a published study injecting a 10 µL dye solution into the sciatic nerve, the dye diffusion reached to a total length of 2.3 cm within the nerve from the site of injection without reaching into the DRG1. The vagus nerve can absorb up to 5 µL volume of drug-dye mixture safely using this injection protocol.
This injection requires a dissecting microscope. While the use of a dye in the injection solution is not absolutely required, it is strongly recommended to enable visualization of injection fidelity in real-time during the procedure. At the same time, if the concentration of dye is too high in the candidate drug and dye mixture, it may cause the needle to become blocked at the time of injection. Limiting the number of injection pricks by a single needle is also critical in this method. It is suggested not to use the same needle for more than 3-5 nerve pricks (equivalent to 3 rats per needle) to prevent blunting of the needle. Re-use of the needle (optional) requires it to be sterilized between animals by wiping it three times with 70% alcohol.
Examples of studies in the literature utilizing direct vagus nerve injection are extremely limited, especially for rodents5,6. A few studies reported direct DRG and other nerve injections with modifications in methods and quantity of volume to be injected in mice and rats1,2,9. The present method is highly significant as an approach to deliver the candidate drugs or other agents through the vagus nerve to target aspects of the ANS. The vagus nerve is the longest cranial nerve and innervates almost all visceral organs to regulate their physiological functions. Thus, the application of this approach has present and future importance in preclinical studies, including but not limited to gene therapy using rats. In addition, this method can be extrapolated to other nerve injections, such as sciatic nerve, with relatively minor modifications.
In short, the direct vagus nerve injection method is a potential route of drug administration for ANS-related rat model studies. Our experience is that the procedure itself does not cause any long-term adverse effects, as animals that have been maintained over a year post-injection have not developed any long-term complications. However, it also involves several critical steps and demands practices in rodent surgery. All those critical points have been cautiously included in this detailed step-by-step protocol in the text. Specific attention is drawn to several critical points: 1) separation of the vagus nerve from the common carotid artery, 2) holding the nerve at a stable position at and after needle insertion within the vagus nerve throughout the injection period, and 3) a smooth delivery of drug-dye mixture without needle blockage. Extensive surgical practice is the best way to overcome all those surgical issues.
Disclosures
Authors NR and XC have no conflicts of interest. RMB and SJG are inventors of intellectual property related to gene transfer to the ANS via vagus nerve injections (United States patent #11,753,655). SJG and RMB have received royalty income from Taysha Gene Therapies, and SJG has received consulting income from Taysha Gene Therapies.
Acknowledgements
We would like to thank the UT Southwestern Animal Resource Center Facility for arranging rat surgical space. Funding for this work was provided by the following sources to SJG: NIH/NINDS R01 NS087175, Hannah's Hope Fund, and Taysha Gene Therapies.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL BD Tuberculin Syringe with Detachable 25 G x 5/8". Needle | Becton, Dickinson and Company | SKU:309626 | Used to connect with curved needle to pull the vagus nerve and hold it at the time of injection. |
| 0.5% Bupivacaine Hydrochloride Injection | Hospira | NDC 0409-1162-19 | Local anesthetics used to anesthetize local tissue. |
| 100 mL 0.9% Sodium Chloride Irrigation USP | Stericare Solutions | Item #6240 | Normal saline, used to rehydrate rat and tissue. |
| 20 Blunt, Retractor Tips, 7.5 mm | Kent Scientific Corporation | Surgi 5018 | Used to pull apart and hold tissues at the time of surgery. |
| 3 mL BD-Luer-Lok Syringe, Sterile, Single Use | Becton, Dickinson and Company | SKU # 309657 | Used to inject saline in rat and fill the saline into the Polythene tubing. |
| AK-Fluor10% | Akorn | NDC 17478-253-10 | Fluorescein dye visible within the nerve. Used to track injection fidelity. |
| Animal Weighing Scale | Kent Scientific Corporation | SCL 4000 | Used to measure body weight of rat. |
| Ansell ENCORE Perry Style 42 PF Surgical Gloves | Ansell | ASTM D3577 | Sterilie glove, it is used at the time of surgery by a surgeon. |
| Artificial Tears Ointment 3.5g | Pivetal | NDC 46066-753-55 | Used in eyses to prevent excessive dryness of eyes. |
| Baby-Myxter Hemostat | Fine Science Tools | 13013-14 | Used to stop bleeding in case of emergency. Also used to bend the 25 G x 5/8" in needle. |
| BD Intramedic PE Tubing | Becton, Dickinson and Company | 14-170-12A | Used in the injection set up system to connect with Hamilton needle and NanoFil Needles. It also holds the injection mixture. |
| BD Precison Glide Needle, 25 G x 5/8" | Becton, Dickinson and Company | REF#305122 | Used to inject saline in rat, and to make a curved needle. |
| BD Precison Glide Needle, 27 G x ½" | Becton, Dickinson and Company | REF#301629 | Used to fill sterile saline into the BD Intradermic tubing. |
| Benchmark Accuris ”NextPette” Variable Volume Pipette Micro Starter Setincludes 4 pipettes: 10/20/200/1000 μL, plus stand | MilliporeSigma | BMSP7700S1 | Used to pippette sterile solution. |
| Betadine, Povidine Iodine 10% | Honestmed | 67618015017 | Used to disinfect the surgical area. |
| Carprofen Injectable solution 50 mg/mL | Supplied by Covtrus (6451506845) | SKU 591149 | In our case, we used diluted carprofen at the dose rate of 5 mg/kg provided by the Animal Resource Center of University of Texas Southwestern Medical Center. |
| Curved needle (custom made) | Becton, Dickinson and Company | REF#305122 | BD PrecisionGlide 25 G x 5/8" in needle is curved to 90 degrees with the help of a hemostat. The tip of the needle is made blunt. It needs to be sterilized before use. It is used to hook the vagus nerve and hold it at the time of separation and injection. |
| Dissecting microscope | Motic | SMZ-171-BLED (Binocular with Lights) | Used to magnify the crifical anatomical area at the time of vagus nerve separation, injeciton, and to check injection leakage. |
| Drape sheet | Dynarex | Reorder#8122 | Used as drape after sterilization. |
| Dukal Cotton Tip Applicators, Non-Sterile | Dukal | Item 9003 | Used to blunt separation of tissue, needs to sterilize before use. |
| Dumont #7 - Fine Forceps | Fine Science Tools | 11274-20 | Used to separate the left vagus nerve from common carotid artery. It is curved so easy to use. |
| Ethicon PDS II Undyed Monofilament Suture - SUTURE, 4/0 18 PDS II CLR MONO PS | Ethicon | VA - Z682G | Used in suturing the wound. |
| Ethilon Nylon Suture Black Monofilament | Ethicon | 1856G | Used in suturing the wound if non-absorbale suture is used. Also used to hook the rat tooth to fix nose inside the nose cone. |
| Fine Forceps - Mirror Finish | Fine Science Tools | 11412-11 | Used at the time of vagus nerve separation from the common carotid artery. This is straight. |
| Fine Scissors - Sharp | Fine Science Tools | 14060-09 | Ued to cut tissue. |
| Hamilton cleaning solution | Hamilton | HT18311 | Used to clean the Hamilton after use. |
| Hamilton Needle, 27G, Small Hub RN Needle, 2”, PT3, 6/PK | Hamilton | 7762-01 | Used to connect BD Intramedic™ PE Tubing. |
| Hamilton Syringe , 710RN | Hamilton | 7638-01 | Used to hold drug at the time of vagus nerve injection. |
| Insulin Syringe | EXEL INT, Comfort point | REF 26027 | Used to inject carprofen and local anesthetics. |
| Lidocaine 2% Injection | Covetrus | Reorder#002468 | Used to mix with Bupivacaine and inject at the site of incision. |
| Luxol Fast Blue MBSN | Acros Organics | 212170250 | Dye visible within the nerve, used to mix with drug so that injection mixture is visible. |
| Micro Bead Sterilizer with Glass Beads | Fine Science Tools | Item No. 18090-46 | Used to sterilize surgical tools in between the rat surgery. |
| NanoFil Needles-NF35BV-2 | World Precision Instrument | NC9708956 | Used to inject drug - dye mixture inside the vagus nerve. |
| Olsen-Hegar Needle Holders with Suture Cutters | Fine Science Tools | 12002-12 | Used in wound suturing. |
| Parafilm M Laboratory Wrapping Film, 4 Inches x 125 Feet, 1 Roll per Box, 12 Count | Honestmed | PM#996 | Used to hold the aliquoted 5 uL of drug-dye mixture so that loading of drug-dye mixture into the BDTM intradermic tubing is accurate. |
| PDI Alcohol Prep Pads | Honestmed | NDC 10819-3914-2 | Used to disinfect the surgical area. |
| Premium Care Sterile Type VII Gauze Sponges, 8-Ply, 2" x 2" | Dukal | Item C5119 | Used as cushon under the neck of rat at the time of surgery. |
| Press’n Seal Cling Film | Glad | Used to cover a rat at the time of surgery like a drape. | |
| Rat Retractor Set | Kent Scientific Corporation | Surgi 5002 | Used to keep the incision open so that it is easy to separate the vagus nerve from the carotid artery. |
| RightTemp Jr. | Kent Scientific Corporation | 20.3 cm W x 25.4 cm L (8 in W x 10 in L), used to keep rat warm. | |
| S&T Forceps - SuperGrip Tips | Fine Science Tools | 00632-11 | Used at the time of suturing to hold tissue without damage. |
| S&T Suture Tying Forceps | Fine Science Tools | 00272-13 | Used to tight the suture. |
| Scalpel blade #15 | Fine Science Tools | 10015-00 | Used to make an incision in the skin at the ventral side of neck. |
| Scalpel Handle-#7 | Fine Science Tools | 10007-12 | Used to hold the scalpel blade. |
| Syringe Pump | KD Scientific | 78-81-8052GL | Serial #D107034, Model#LEGATO-180, is a programmable pump that can pump small volume of mixture under a program. |
| TipOne Filter Tip Refill Starter Systems | USA Scientific | Item #1120-3510 | Used to pipette the drug and dye mixture. |
| Vaporizer for Isoflurane, Funnel Filled | Kent Scientific Corporation | Vetflow 1231 | Used to anesthetize rats. |
| Vetbond Tissue Adhesives | 3M Science Applied to Life | ID B00016067 | Used to seal tissue at the site of cut wound if suturing is not perfect. |
| Wahl BravMini+ Professional Cordless Clipper Kit | Kent Scientific Corporation | CL7300-Kit | Used to cut hair of rat. |
References
- Fischer, G., et al. Direct injection into the dorsal root ganglion: Technical, behavioral, and histological observations. J Neurosci Methods. 199 (1), 43-55 (2011).
- O'donnell, M., Fontaine, A., Caldwell, J., Weir, R. Direct dorsal root ganglia (drg) injection in mice for analysis of adeno-associated viral (AAV) gene transfer to peripheral somatosensory neurons. J Neurosci Methods. 411, 110268 (2024).
- Puljak, L., Kojundzic, S. L., Hogan, Q. H., Sapunar, D. Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods. 177 (2), 397-402 (2009).
- Rueter, L. E., Kohlhaas, K. L., Curzon, P., Surowy, C. S., Meyer, M. D. Peripheral and central sites of action for a-85380 in the spinal nerve ligation model of neuropathic pain. Pain. 103 (3), 269-276 (2003).
- Ulusoy, A., et al. Neuron-to-neuron alpha-synuclein propagation in vivo is independent of neuronal injury. Acta Neuropathol Commun. 3, 13 (2015).
- Helwig, M., et al. Brain propagation of transduced alpha-synuclein involves non-fibrillar protein species and is enhanced in alpha-synuclein null mice. Brain. 139 (Pt 3), 856-870 (2016).
- Chiang, B., et al. Development of a novel suprachoroidal-to-optic-nerve (scone) drug delivery system. Drug Deliv. 31 (1), 2379369 (2024).
- Ferrari, L. F., Cunha, F. Q., Parada, C. A., Ferreira, S. H. A novel technique to perform direct intraganglionar injections in rats. J Neurosci Methods. 159 (2), 236-243 (2007).
- Yuan, X. M., et al. Rapid injection of lumbar dorsal root ganglia under direct vision: Relevant anatomy, protocol, and behaviors. Front. Neurol. 14, 1138933 (2023).
- Mason, M. R., et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 18 (4), 715-724 (2010).
- Arjomandnejad, M., Dasgupta, I., Flotte, T. R., Keeler, A. M. Immunogenicity of recombinant adeno-associated virus (AAV) vectors for gene transfer. BioDrugs. 37 (3), 311-329 (2023).
- Ertl, H. C. J. Immunogenicity and toxicity of AAV gene therapy. Front Immunol. 13, 975803 (2022).
- Wayman, C., et al. Performing permanent distal middle cerebral with common carotid artery occlusion in aged rats to study cortical ischemia with sustained disability. J Vis Exp. (108), e53106 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved