Method Article
Monitoring Pulmonary Nodule Progression Using Micro-Computed Tomography and Blood Sampling in a Mouse Model
* These authors contributed equally
In This Article
Summary
This protocol describes an efficient, simple, and minimally invasive method for studying pulmonary nodules. Submaxillary vein blood collection and micro-CT imaging are used as investigative techniques.
Abstract
Micro-computed tomography (micro-CT) is a real-time, intuitive, sensitive, and minimally invasive technique for monitoring changes from pulmonary nodules (PN) to lung cancer (LC). The integration of submandibular vein blood sampling enables rapid, stable, and straightforward detection of imaging and key target alterations during the progression of PN to LC. In this study, we administered a dosage of 100 mg/kg of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mice to develop a lung adenocarcinoma model. Disease progression in the experimental animals was then monitored through submandibular vein blood sampling and micro-CT assay. Experimental results showed the presence of nodular foci in the lungs of some animals by the 10th week, with the development of lung adenocarcinoma images becoming evident by the 21st week. In conclusion, micro-CT can effectively observe pathological changes in the lungs of mice and, when combined with submandibular vein blood sampling, can dynamically monitor changes in blood, protein, and targets. This method provides a highly specific, simple, and sensitive approach for drug screening, pharmacokinetic testing, toxicological experiments, and safety studies.
Introduction
Lung cancer (LC) is a severe neoplasm originating in the bronchial mucosa or lung glands. According to 2021 statistics, LC causes approximately two million fatalities globally each year, with incidence and mortality rates on the rise1. Early diagnosis and intervention in LC contribute to higher cure rates, reduced mortality, and lower treatment costs. Pulmonary nodules (PN) are specific precursors to LC, characterized by localized, round, and denser solid or subsolid shadows ≤30 mm in diameter on radiological exams, without evidence of lung collapse, mediastinal lymph node enlargement, or pleural effusion2. The National Comprehensive Cancer Network (NCCN) in 2022 categorized PN by number, diameter, and density, identifying combinations such as a 5 mm isolated ground-glass nodule in the right lung3. However, the NCCN guidelines indicate that the risk of malignancy in PN increases with the nodules' diameter and quantity. The widespread application of low-dose computed tomography has dramatically increased PN diagnoses, with millions of new cases identified each year4.
The combination of A/J mice with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is the most commonly used animal model for lung cancer (LC)5,6. The use of micro-CT alongside submandibular vein blood sampling is an effective approach for real-time monitoring of changes from pulmonary nodules (PN) to LC. Chemical carcinogen induction, particularly with NNK and A/J mice, is the most prevalent method for lung cancer modeling and has proven to be an efficacious approach for establishing carcinoma in situ7,8. This modeling method more accurately simulates the progression of PN to LC compared to the axillary inoculation method.
Previous studies have focused on statistical analysis of nodule morphology and pathological staining of tissue samples post-euthanasia9. However, these methods lack the capacity for real-time monitoring of the dynamic progression from PN to LC10. Micro-CT, as a non-invasive imaging technique, provides accurate longitudinal data with high resolution, fast imaging, a low radiation dose, and safety, making it suitable for detecting lung images in real time11,12. Submandibular vein blood sampling is the latest, simplest, and fastest method for obtaining blood samples from mice13. This non-invasive technique requires minimal animal handling and allows for rapid recovery, aligning with the 3R principles that aim to reduce the number of animals used in research, minimize discomfort, and promote ethical treatment. The collected blood volume, approximately 0.2-0.5 mL, is sufficient for monitoring blood parameters with moderate requirements14.
The concurrent use of micro-CT and submandibular vein blood sampling allows for dynamic, real-time observation of PN-to-LC progression in imaging and the real-time detection of key targets within the bloodstream15. Additionally, this approach enables real-time investigation of metabolites and other biochemicals, which, when combined with techniques such as high-performance chromatography, advances our understanding of LC16,17.
In this study, A/J mice combined with NNK were used to create an in-situ lung cancer mouse model. Micro-CT scans were performed at 4, 10, and 20 weeks post-model induction to capture lung images, while blood was collected via submandibular vein sampling throughout the experiment. This study aims to establish a foundation for PN and LC research by combining submandibular vein blood sampling with micro-CT.
In oncology, micro-CT is a highly effective tool for detecting tumor growth, offering a high-resolution technique for measuring local shadow-focus changes at any time during such studies18,19. However, it is essential to recognize that micro-CT alone does not provide information on shadow-focus characteristics, the physiological status of the animal, or levels of key biological factors. Therefore, submandibular vein sampling was utilized as a complementary method in this study.
Protocol
All animal experiments described in this study were approved by the Experimental Animal Welfare Ethics Committee of Chengdu University of Traditional Chinese Medicine and were conducted in accordance with relevant laws and ethical standards for animal research (review number: 2024035). Female inbred A/JGpt mice (7-8 weeks old) were maintained at a temperature of 20-24 °C with a relative humidity of 40%-70%. They were provided with standard animal feed and purified water ad libitum throughout a 12-h light-dark cycle. Before the experiment, each animal was acclimated to this environment for 7 days. Details of the reagents and equipment used are listed in the Table of Materials.
1. Reagents and animal preparation
- Chemicals and reagents
- Dissolve NNK in saline to form a 10 mg/mL master mix20. Administer a single intraperitoneal injection of 0.2 mL with a concentration of 100 mg/kg to the NNK group, while providing an equal volume of normal saline to the blank group.
NOTE: Follow Jang et al.21 to determine the timing for micro-CT scanning and blood sampling.
- Dissolve NNK in saline to form a 10 mg/mL master mix20. Administer a single intraperitoneal injection of 0.2 mL with a concentration of 100 mg/kg to the NNK group, while providing an equal volume of normal saline to the blank group.
- Blood collection
NOTE: To ensure the health of the mice, limit blood collection to no more than 0.2 mL at each time, and allow one week for recovery. Due to the abundance of hair in the submental region, take care to avoid contamination of the blood sample by hair during collection.- Remove the animal's facial hair the day before the experiment using an appropriate shaver.
- Grasp the skin on the posterior aspect of the mouse's head firmly with the left hand to prevent any movement and to keep the mouse's head in a fixed position.
- Insert the blood collection needle swiftly into the submaxillary artery from the mandibular area behind the oblique eye socket. Keep the needle in place for at least 3 s to allow optimal blood flow. Collect 50-200 µL of blood.
- Collect the blood into an EDTA tube. Use a cotton swab to apply gentle pressure to the skin to stop bleeding. Once the bleeding has stopped, release the mouse and observe it for 30 s.
- Gently agitate the test tube to ensure that the blood is thoroughly mixed with the coagulant.
- For routine blood testing, place the collected blood in the veterinary blood routine testing instrument, press the collection button, and allow the instrument to collect the blood. Record the results displayed. Dispose of any remaining blood safely.
2. In vivo imaging by micro-CT
NOTE: Always remove metal objects, such as ear tags, from the test animal before using the micro-CT scan. Metal objects can cause severe artifacts in the image. Micro-CT emits a certain amount of radiation; ensure that other experimental results are not affected.
- Start the unit, launch the micro-CT software, and perform the probe calibration and warm-up. Use the mouse-specific tool bed for scanning.
- Create a new database and name it for this scan, or connect it to an existing database.
- Modify the parameters in the software setup window. Set the X-ray filter to Cu0.06 + Al0.5, with a voltage of 70 kV, a current of 80 µA, a field of view of 36 mm × 36 mm, 360° rotational scanning, and a scanning time of 4 min22.
- Anaesthetise the mice with 3% isoflurane before scanning23 (following institutionally approved protocols). Open the micro-CT viewing screen and secure the mice to the tool bed with adhesive tape. Maintain anesthesia continuously using a nasogastric tube placed within the micro-CT instrument on the tool bed.
- Carefully guide the animal into the apparatus and monitor its position in real time. Use the appropriate buttons to adjust the mouse's position, ensuring that its chest is fully visible within the field of view.
- Rotate the tool bed 90° to position the mouse. Use the buttons to adjust the position of the mouse, ensuring that the lung region is centrally located within the field of view. Then, return the tool bed to its original position.
- To initiate the scan, select the Scan button. Allow the system to complete the scan without interruption, and avoid opening the viewing screen during the process. Observe the transaxial, coronal, and sagittal slices of the reconstruction through the software.
- Evaluate the image quality immediately after the scan. If artifacts or blurred images appear, repeat the scanning procedure.
- Remove the mice from the apparatus and monitor their health to ensure they are in stable condition before returning them to their cages.
- At the end of the experiment, remove the adhesive tape from the tool bed, then clean the bed. Save the data and turn off the instrument.
- Transfer the mice back to their cages gently to minimize stress. Ensure that the cages are clean and have appropriate bedding.
- Monitor the mice for signs of recovery from anesthesia. Observe their behavior, mobility, and appetite. Provide food and water as needed.
- Maintain a warm environment for the mice to prevent hypothermia after anesthesia. Use heating pads or blankets if necessary.
- Conduct daily health checks for the next week. Look for signs of distress, unusual behavior, or any injuries. Document observations for each mouse.
- If any mouse shows signs of illness or distress, consult with a veterinarian for appropriate intervention and care.
- Ensure that the mice are returned to their normal housing conditions after they have fully recovered and are stable.
3. Data processing and analysis
- Use statistics and graphing software as a valuable tool for data analysis and creating tables to present results.
- Open the software. Select XY graphs from the newly created data table, input the weekly data for the NNK and control groups, and generate a chart displaying the changes in the weight of the mice.
- Open the software again, select the contingency diagram from the newly created data table, input the blood routine data for the NNK group and the control group, and generate an icon.
- Select the analysis options in software. Analyze the overall data using one-way ANOVA, followed by verification of the data through the implementation of a t-test.Mark significant differences.
- Save the micro-CT data in SimpleViewer format or DICOM. Open the SimpleViewer software, and observe the imaging data under the guidance of a professional imaging physician. Label the nodules and quantify the shadow volume using the provided measurement tools.
Results
This study demonstrated the construction of a stable lung cancer model using NNK in combination with A/J mice. The experimental design is illustrated in Figure 1. The objective was to observe the real-time process of the transition from pulmonary nodules (PN) to lung cancer (LC) in mouse lungs, utilizing micro-CT and submandibular vein blood sampling. Accordingly, micro-CT scanning and blood sampling from the mouse lungs were conducted at the fourth, tenth, and twentieth weeks.
The experimental results showed that the modeling approach of NNK in conjunction with A/J mice effectively mimicked the pathological process from PN to LC. Firstly, it can be stated that the assay used in this study did not significantly impact the well-being of the experimental animals. As illustrated in Figure 2, the body weights of the experimental animals over the 20-week feeding period did not exhibit notable differences compared to the control group. Secondly, the results of the routine blood tests conducted on samples taken from the experimental animals revealed a significant increase in the number of leukocytes and platelets in the model group, whereas the numbers of red blood cells and hemoglobin remained unchanged (Figure 3). This indicates that the transformation process from PN to LC is also associated with a gradual increase in chronic inflammation. Importantly, both micro-CT scanning and submandibular vein blood sampling did not damage the hematopoietic function of the experimental animals, consistent with findings from numerous previous studies. Additionally, close observation of the behavior, coat condition, respiration, diet, and water intake of the experimental animals throughout the study revealed no abnormalities.
Following the initial administration of NNK to the experimental animals, we conducted micro-CT scanning of the lungs on the first day of the fourth, tenth, and twentieth weeks24. The results indicated that, compared to the control group, the lung texture of the model group exhibited a gradual thickening. By the 10th week, the formation of minute nodular foci was observable, and by the 20th week, the nodules had developed into discernible shadow foci. In light of these findings, it can be postulated that the formation of shadow foci in the lungs is associated with the chronic inflammation induced by NNK25. However, since this study was designed to observe the safe, efficient, and harmless development of PN to LC without involving pathological studies in animals, subsequent studies must be conducted in accordance with specific experimental protocols26. Figure 4 illustrates the alterations in lung imaging observed in experimental animals at weeks 4, 10, and 20.

Figure 1: Experimental design for NNK treatment in A/J mice. Five female A/J mice were injected with the compound NNK at a single time point, while another five were injected with saline as a control. Blood samples were collected at weeks 4, 10, and 20 using micro-CT scans of the mouse lungs and submaxillary vein blood collection. The obtained data were cross-validated to evaluate disease progression in the mice. (A) Overview of the experimental design. (B) Diagram of submaxillary artery blood sampling. (C) Schematic representation of the micro-CT imaging setup, showing the mouse positioned on the animal bed (blue) and the yellow frame as the viewfinder, which should completely cover the lung tissue of the mouse. Please click here to view a larger version of this figure.

Figure 2: Weight changes in mice over 20 weeks. Weight trends indicated that NNK treatment in A/J mice did not significantly reduce body weight. Micro-CT scanning and submaxillary vein blood extraction may cause some stress to the mice; however, they recovered quickly. Data are expressed as mean ± SEM, (n = 5). Please click here to view a larger version of this figure.
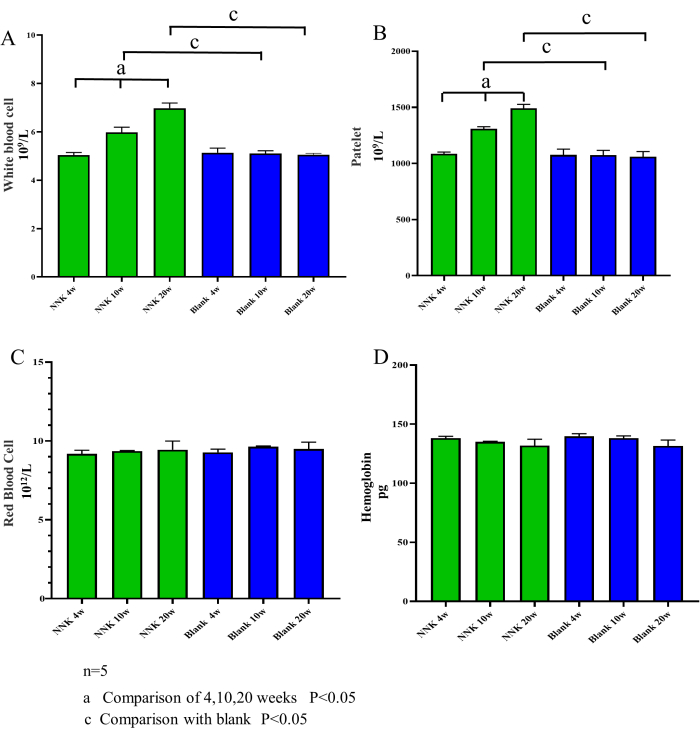
Figure 3: Blood cell counts over time. The contents of white blood cells, platelets, red blood cells, and hemoglobin were measured in mice at weeks 4, 10, and 20. Compared to the control group, the NNK group showed an increasing trend in the number of white blood cells and platelets, while hemoglobin and red blood cell levels did not change significantly. These findings suggest that the transformation process of PN-LC is associated with increased inflammation and that the submaxillary vein blood collection method, performed at intervals of more than 4 weeks, does not cause infection or damage to hematopoietic function in mice. (A) White blood cells. (B) Platelets. (C) Red blood cells. (D) Hemoglobin. Data are expressed as mean ± SEM, (n = 5). Please click here to view a larger version of this figure.
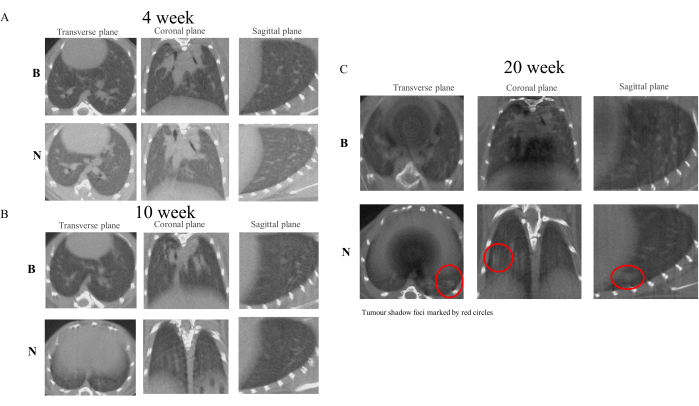
Figure 4: Micro-CT scan images of mice at weeks 4, 10, and 20. Micro-CT imaging results demonstrate that NNK treatment in A/J mice effectively simulates the transformation process of PN-LC. Compared to the control group, the NNK group began to exhibit characteristics of thickening and altered texture in lung images by week 10. At week 20, robust shadow foci were discernible within the lung tissue. (A) Images from week 4. (B) Images from week 10. (C) Images from week 20. Please click here to view a larger version of this figure.
Discussion
It is important to reiterate several key points from this study. Firstly, although submandibular vein blood collection is a relatively low-injury procedure, it may still result in some degree of harm to the animals. Therefore, it is necessary to conduct multiple procedures to reduce the burden on the mice and complete the process in a timely manner27. Secondly, the removal of hair prior to blood sample collection ensures the purity of the sample. Thirdly, it is imperative to use appropriate blood collection vessels. In the present study, blood collection vessels containing EDTA were employed for routine blood tests. If serum were to be utilized, vessels specifically designed for the collection of pure blood would be required28. Fourth, all anesthetics have a certain level of lethality; thus, minimizing anesthesia and imaging time can effectively safeguard the health of the mice. Fifth, since micro-CT can observe a variety of tissues and organs, the specific parameter settings in micro-CT software used during PN imaging can be referenced from this study but may not be applicable to other tissues29,30.
Previous studies were more inclined to euthanize animals at fixed time points and examine the process of pulmonary nodule transformation through pathological staining31. This approach resulted in a considerable number of deaths among experimental animals and hindered real-time tracking of lung changes. In comparison to conventional techniques, submandibular blood sampling and micro-CT offer several advantages, including minimal damage, real-time monitoring, intuitive operation, and versatility. In this study, submandibular blood collection was chosen as the preferred method for obtaining blood samples for routine tests32. Furthermore, the blood can be used for proteomic, serum pharmacological, and blood biochemical analyses.
Similarly, micro-CT was employed in this study to observe the dynamic growth of PN in experimental mice without the need to euthanize them. This approach facilitates a more intuitive and accurate assessment of the drug's inhibitory effect on PN while significantly reducing the number of animals required for the experiment, thereby enhancing the accuracy of the experimental outcomes. Notably, the combination of these two technologies allows for comprehensive tracking of the processes of nodule formation, development, and carcinogenesis in experimental animals, as well as the localization of alterations in key targets (such as TNF-α)33. This presents a unique concept for this research on PN and even lung cancer.
Nevertheless, several issues require further consideration to enhance the quality of future research. Given the lengthy experimental period required for the animal model of NNK combined with A/J mice, it is imperative that early drug injections are conducted with the utmost precision34. Secondly, the standard method for producing lung adenocarcinoma in mice involves NNK in coordination with female A/J mice, with the underlying mechanism linked to estradiol. Therefore, it is essential to consider the specific mechanisms of action of the therapeutic drugs involved35. Additionally, micro-CT was not used to determine the nature of the shadow foci, necessitating the use of hematoxylin staining and fluorescence staining, which still require euthanizing the mice to obtain lung tissue samples. Finally, although micro-CT has the advantage of low radiation exposure, it can still cause certain damage to the human body, necessitating the avoidance of proximity to unrelated personnel36. To address these issues, the differentiation and labeling of various tissues, airways, and blood vessels can be effectively achieved through tail vein injection of a contrast agent. Furthermore, micro-CT combined with novel material drugs (e.g., nanoparticles) can be employed for more precise treatment. Finally, micro-CT technology has gradually been integrated with pathology, such as spatial and imaging histology, to more dynamically track changes in pulmonary nodules36,37.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Professor Cong Huang of the School of Basic Medical Sciences and Professor Yan Huang of the School of Pharmacy, Chengdu University of Traditional Chinese Medicine, for their support. We would also like to thank Dr. Binjie Xu and Dr. Pengmei Guo. (Innovative Institute of Chinese Medicine and Pharmacy, Chengdu
University of Traditional Chinese Medicine) for providing instrument and Technical support.
Materials
| Name | Company | Catalog Number | Comments |
| A/J mice | GemPharmatech LLC. | N000018 | |
| 0.5 mL EDTA tubes | Labshark | 130201070 | |
| 1-Butanone,4-(methylnitrosoamino)-1-(3-pyridinyl) | Gu Shi Gong Yuan Medical Equipment Co. | N589770 | |
| 75% ethanol | ChengDu Chron Chemicals Co,.Ltd | 2023052901 | |
| Animal shaver | Codos | BM010220 | |
| Isoflurane | Shenzhen Reward Life Technology Co. | R510-22-16 | |
| medical tricorder | MedChemexpress | 69652 | |
| Quantum GX2 microCT imaging system | PerkinElme | 2020166501 | |
| Saline (medicine) | Beijing Biolabs Technology Co. | GL1736 |
References
- Sung, H., et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71 (3), 209-249 (2021).
- Baum, P., et al. Incidental pulmonary nodules: Differential diagnosis and clinical management. Dtsch Arztebl Int. , (2024).
- Ajani, J. A., et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 20 (2), 167-192 (2022).
- Kondo, K. K., et al. Lung cancer diagnosis and mortality beyond 15 years since quit in individuals with a 20+ pack-year history: A systematic review. CA Cancer J Clin. 74 (1), 84-114 (2024).
- Ray, E., et al. Inhalable chitosan-coated nano-assemblies potentiate niclosamide for targeted abrogation of non-small-cell lung cancer through dual modulation of autophagy and apoptosis. Int J Biol Macromol. 279 (Pt 4), 135411 (2024).
- Ali, N. A., et al. Chia seed (Salvia hispanica) attenuates chemically induced lung carcinomas in rats through suppression of proliferation and angiogenesis. Pharmaceuticals (Basel). 17 (9), 1129 (2024).
- Kiran, A., Kumari, G. K., Krishnamurthy, P. T. Preliminary evaluation of anticancer efficacy of pioglitazone combined with celecoxib for the treatment of non-small cell lung cancer. Invest New Drugs. 40 (1), 1-9 (2022).
- Marshall, K., Twum, Y., Gao, W. Proteome derangement in malignant epithelial cells and its stroma following exposure to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Arch Toxicol. 97 (3), 711-720 (2023).
- Li, B., et al. LNCRNA XIST modulates miR-328-3p ectopic expression in lung injury induced by tobacco-specific lung carcinogen NNK both in vitro and in vivo. Br J Pharmacol. 181 (15), 2509-2527 (2024).
- Liang, F., et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct Target Ther. 7 (1), 311 (2022).
- Ding, R., et al. The effect of immunotherapy PD-1 blockade on acute bone cancer pain: Insights from transcriptomic and microbiomic profiling. Int Immunopharmacol. 142 (Pt A), 113100 (2024).
- Kayı Cangır, A., et al. Microcomputed tomography as a diagnostic tool for detection of lymph node metastasis in non-small cell lung cancer: A decision-support approach for pathological examination "a pilot study for method validation"). J Pathol Inform. 15, 100373 (2024).
- Packialakshmi, B., et al. A clinically-relevant mouse model that displays hemorrhage exacerbates tourniquet-induced acute kidney injury. Front Physiol. 14, 1240352 (2023).
- Sørensen, D. B., et al. Time-dependent pathologic and inflammatory consequences of various blood sampling techniques in mice. J Am Assoc Lab Anim Sci. 58 (3), 362-372 (2019).
- Wu, G. L., Li, T. Y., Pu, X. H., Yu, G. Y. Effect of prescriptions replenishing vital essence, tonifying qi and activating blood on TNF-alpha, IL-1beta expressions in serum and submaxillary gland of nod mice with Sjogren's syndrome. Zhongguo Zhong Yao Za Zhi. 38 (3), 413-416 (2013).
- Schroeder, J. A., et al. Thromboelastometry assessment of hemostatic properties in various murine models with coagulopathy and the effect of factor VIII therapeutics. J Thromb Haemost. 19 (10), 2417-2427 (2021).
- Guo, K., et al. Integration of microbiomics, metabolomics, and transcriptomics reveals the therapeutic mechanism underlying Fuzheng-Qushi decoction for the treatment of lipopolysaccharide-induced lung injury in mice. J Ethnopharmacol. 334, 118584 (2024).
- Luo, T., Zhang, S., Li, X., Huang, M. Challenges in the differential diagnosis of pulmonary tuberculosis vs. lung cancer: A case report. Oncol Lett. 28 (4), 494 (2024).
- Mascalchi, M., et al. Large cell carcinoma of the lung: LDCT features and survival in screen-detected cases. Eur J Radiol. 179, 111679 (2024).
- Peng, M., et al. P27 specifically decreases in squamous carcinoma and mediates NNK-induced transformation of human bronchial epithelial cells. J Cell Mol Med. 28 (15), e18577 (2024).
- Jang, H. J., et al. Tobacco-induced hyperglycemia promotes lung cancer progression via cancer cell-macrophage interaction through paracrine IGF2/IR/NPM1-driven PD-L1 expression. Nat Commun. 15 (1), 4909 (2024).
- Zhang, Y., et al. Curcumin analogue EF24 prevents alveolar epithelial cell senescence to ameliorate idiopathic pulmonary fibrosis via activation of PTEN. Phytomedicine. 133, 155882 (2024).
- Li, M., et al. Isoflurane anesthesia decreases excitability of inhibitory neurons in the basolateral amygdala leading to anxiety-like behavior in aged mice. Exp Ther Med. 28 (4), 399 (2024).
- Xiong, R., et al. Hypermethylation of the ADIRF promoter regulates its expression level and is involved in NNK-induced malignant transformation of lung bronchial epithelial cells. Arch Toxicol. 97 (12), 3243-3258 (2023).
- Shaikh, Z. M., et al. Thearubigins/polymeric black tea polyphenols (PBPs) do not prevent benzo[a]pyrene (B[a]P) induced lung tumors in A/J mice. Am J Transl Res. 15 (9), 5826-5834 (2023).
- Li, M. Y., et al. Targeting CD36 determines nicotine derivative NNK-induced lung adenocarcinoma carcinogenesis. iScience. 26 (8), 107477 (2023).
- Arlt, E., et al. A flow cytometry-based examination of the mouse white blood cell differential in the context of age and sex. Cells. 13 (18), 1583 (2024).
- Su, Z., et al. Feasibility of using serum, plasma, and platelet 5-hydroxytryptamine as peripheral biomarker for the depression diagnosis and response evaluation to antidepressants: Animal experimental study. Clin Psychopharmacol Neurosci. 22 (4), 594-609 (2024).
- Zhou, X., et al. Mechanosensitive lncRNA H19 promotes chondrocyte autophagy, but not pyroptosis, by targeting miR-148a in post-traumatic osteoarthritis. Noncoding RNA Res. 10, 163-176 (2025).
- Chen, C., et al. Cardamonin attenuates iron overload-induced osteoblast oxidative stress through the HIF-1α/ROS pathway. Int Immunopharmacol. 142 (Pt A), 112893 (2024).
- Yan, F., et al. Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol Ther. 25 (1), 2304161 (2024).
- Inoue, S., et al. Utility of ultrasound imaging in monitoring fracture healing in rat femur: Comparison with other imaging modalities. Bone Rep. 23, 101807 (2024).
- An, Y., et al. Quercetin through miR-147-5p/CLIP3 axis reducing Th17 cell differentiation to alleviate periodontitis. Regen Ther. 27, 496-505 (2024).
- Tian, J., et al. Diallyl disulfide blocks cigarette carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis via activation of the Nrf2 antioxidant system and suppression of NF-κB inflammatory response. J Agric Food Chem. 71 (46), 17763-17774 (2023).
- Han, L., et al. Wutou decoction alleviates arthritis inflammation in CIA mice by regulating Treg cell stability and Treg/Th17 balance via the JAK2/STAT3 pathway. J Ethnopharmacol. 334, 118463 (2024).
- Napieczyńska, H., et al. µCT imaging of a multi-organ vascular fingerprint in rats. PLoS One. 19 (10), e0308601 (2024).
- Liu, H., et al. Using broadly targeted plant metabolomics technology combined with network pharmacology to explore the mechanism of action of the Yishen Gushu formula in the treatment of postmenopausal osteoporosis in vivo. J Ethnopharmacol. 333, 118469 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved