Method Article
Live/Dead Staining for Quantifying Viable but Not Culturable Cells in Manuka Honey-Treated Wound-Causing Bacteria
In diesem Artikel
Zusammenfassung
The presented protocol describes a procedure to quantify Viable But Not Culturable Cells (VBNC) in Manuka Honey-treated bacterial cultures.
Zusammenfassung
Antibiotic resistance and tolerance among bacteria pose a significant threat to global health. Mechanisms contributing to antibiotic resistance and tolerance include genetic mutations and, acquisition of resistance genes, and transition to Viable But Not Culturable (VBNC) and other dormancy states, respectively. Although genetically identical to their non-antibiotic-tolerant counterparts, VBNC cells evade antibiotic effects by remaining metabolically inactive. Antibiotics are effective only when their target processes, such as DNA replication or transcription, are active. Since environmental stressors, particularly antibiotics, can drive bacteria into dormancy, alternative antimicrobials are needed to minimize or prevent this response. The antimicrobial Manuka Honey (MH) is effective against many bacteria, with rare development of resistance. Its multifaceted antimicrobial mechanisms make it a valuable agent for treating bacterial infections. This research investigated MH recalcitrance to antibiotic resistance development by testing the hypothesis that MH induces fewer VBNC cells than conventional antibiotics. To investigate this, a protocol was developed to treat the wound-causing bacteria Staphylococcus aureus and Pseudomonas aeruginosa with minimum inhibitory concentrations of MH or the conventional antibiotics tobramycin or meropenem, that then used the viable plate count to identify metabolically active culturable cells and live/dead staining to identify all viable cells. The number of VBNC cells equaled the viable cell number minus the culturable cell number. In some experiments, the culturable cell number was higher than the viable cell number, giving a negative number of VBNC cells; thus, VBNC cell numbers were not directly compared. Instead, culturable and viable cell numbers were compared for each treatment. Only P. aeruginosa treated with tobramycin had significantly fewer culturable cells than viable cells, indicating a higher number of VBNC cells. This protocol is quick and easy and can be used to evaluate MH induction of VBNC cells in other pathogenic bacteria.
Einleitung
Bacteria in the Viable But Not Culturable (VBNC) dormancy state survive antibiotic treatment and cause recurring and sometimes deadly infectious diseases1,2,3. More familiar to us is antibiotic resistance, which occurs via a heritable genetic change4. The genetic change provides resistance to a specific antibiotic by several mechanisms, including limiting the uptake of an antibiotic, increasing the efflux of the antibiotic, modifying the antibiotic target, or inactivating the antibiotic4. By contrast, bacteria in dormancy states exhibit a more broad and non-heritable antibiotic tolerance by reducing their metabolism2,3. Antibiotics target specific metabolic processes, including DNA replication and cell wall synthesis, and are ineffective against metabolically inactive VBNC cells4. VBNC cells exist stochastically in low numbers alongside their genetically identical antibiotic-susceptible siblings in most bacterial populations5. However, environmental stressors, including antibiotic exposure, induce susceptible bacterial cells to enter the antibiotic-tolerant VBNC state3,6. Antibiotic treatment will kill antibiotic susceptible cells within the population, while dormant VBNC cells remain. As their name suggests, VBNC cells are not culturable on media suitable for the growth of their metabolically active siblings. However, their membranes and genetic material are undamaged, and they can reproduce7,8. When antibiotics are removed, specific stimuli such as nutrients, temperature, or host factors are required to wake VBNC cells from their dormancy state and restore growth3,9. VBNC cells have been documented in many pathogenic bacterial species, including P. aeruginosa and S. aureus, major etiological agents of wound infections10,11.
Honey has long been used in medical applications, including wound treatment, because of its antibacterial properties12. Manuka Honey (MH), derived from flowers of the Manuka bush (Leptospermum scoparium), has been noted for its broad-spectrum bactericidal activity (reviewed in13,14,15). It successfully kills the majority of human infection-causing bacteria tested, including bacterial strains such as Methicillin Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus that exhibit heritable resistance to traditional antibiotics16,17. Additionally, induced bacterial resistance to MH is rare, being detected only in honey-exposed P. aeruginosa biofilms and Escherichia coli cultured in increasing concentrations of a subset of the honey tested18,19,20,21. This suggests that the antimicrobial mechanisms of MH are distinct from conventional antibiotics and may induce fewer or no VBNC cells. This hypothesis was tested by determining how MH impacts VBNC populations compared to conventional antibiotics.
VBNC cells must be distinguished from their metabolically active culturable siblings and dead cells. Culturable cells are counted using the viable plate count technique, where cells are transferred to routine culture media and incubated for a period sufficient for enough cell divisions to result in observable colonies. Several methods for distinguishing VBNC cells from dead cells have been developed based on their intact membranes and low-level transcription. Substrate and fluorescent dye uptake, combined with microscopy or flow cytometry, allows for direct counting of cells by microscopy or flow cytometry with a color that indicates intact membranes22,23. Respiration assays that require the electron transport chain to be present in an intact cell membrane have also been used to distinguish between viable and dead cells24. Quantitative PCR (qPCR) methods combined with DNA modifying dyes that prevent amplification have also been developed; only DNA from viable cells will be amplified as the intact membranes exclude the dye25. Though VBNC cells are dormant, they still transcribe genes at a low level, and this can also be used to distinguish them from dead cells using reverse transcription PCR26.
In this work, an assay was developed to quantify VBNC cells in two wound-causing pathogenic bacteria, S. aureus and P. aeruginosa, treated with MH. It describes the treatment of the bacteria with MH and conventional antibiotics and the use of the viable plate count and live/dead staining to detect culturable and viable cells. This easy and inexpensive protocol will allow for analysis of MH's ability to induce antibiotic-resistant VBNC cells in many bacterial pathogens.
Protokoll
Since P. aeruginosa (ATCC9721) and S. aureus (ATCC29213) are classified as Biosafety Level 2 agents, the rooms where the work is conducted must have limited access and be equipped with a biosafety cabinet for manipulations that could create splashes or aerosols, such as agitating and centrifuging cultures. In all steps of this protocol, wear personal protective equipment (PPE), including a lab coat, protective glasses, and gloves. All reagents and equipment used in the experiments are listed in the Table of Materials.
1. Preparation of sterile broth and agar culture media
- Prepare Luria Bertani and Mueller Hinton, NaCl (0.85%), antibiotics, and glassware.
- Prepare the broth media according to the manufacturer's instructions, adding agar powder to 1.5% for the agar media. Add 0.85 g of NaCl to 100 mL of dH20 to prepare 0.85% NaCl. Apply autoclave tape on the vessels, place them in an autoclave-safe container, and autoclave them on a liquid cycle appropriate for the volumes used.
- When the autoclave cycle is complete, cool the agar to approximately 60 °C before pouring it into sterile Petri dishes.
- Prepare 100 μg/mL stock solutions of the antibiotics tobramycin and meropenem in dH2O for dilution and treatment of bacteria. Sterilize the antibiotic stocks using a 5 mL sterile syringe connected to a 0.2 μm sterile filter.
- Store the sterilized antibiotic stocks at -20 °C in multiple small aliquots to prevent loss of activity due to multiple freeze-thaw cycles.
2. Retrieving the archived Staphylococcus aureus and Pseudomonas aeruginosa strains from -80 °C (Day 1)
- Gather the materials for streaking a small aliquot of the frozen bacterial strains from their 80 °C freezer vials to Luria Bertani (LB) agar. This includes multiple sterile wooden sticks and an LB agar plate labeled with the organism and the date.
- Open the -80 °C freezer, quickly remove the vials of interest, and close the freezer door. Open each vial sequentially and use a sterile stick to scrape a small amount of frozen bacteria onto the appropriately labeled LB agar plate. Replace the vial cap and quickly return it to the -80 °C freezer.
- Incubate the inoculated LB agar plates, agar side up, at 37 °C under aerobic (atmospheric) conditions for 18-24 h. After the incubation period, seal the inoculated LB agar plates with grafting tape and store them at 4 °C for no longer than one week. Inoculate fresh bacterial cultures from the -80 °C frozen stock weekly as needed.
3. Preparation of S. aureus and P. aeruginosa for treatment with Manuka Honey (MH) and the antibiotic (Day 2)
- Gather the materials required for starting an LB broth culture of each strain, including small sterile test tubes, 5 mL serological pipets, sterile LB broth, and an inoculating loop. Also, gather a manual or electric pipettor for use with serological pipets, a Bunsen burner, and a shaking incubator.
- Working next to the Bunsen burner flame, use a serological pipet to transfer 2 mL of LB broth to two sterile test tubes. Sterilize the inoculating loop in the Bunsen burner flame and then transfer a quarter loop full of bacteria from the LB agar plates to the appropriately labeled test tube with LB broth.
- Incubate the test tube under aerobic conditions in a shaking (~250 rpm) incubator at 37 °C for 18-24 h.
4. Preparation of MH and set up MH and antibiotic treatments of S. aureus and P. aeruginosa (T = 0) (Day 3)
- Gather the materials needed for the experiment, including Mueller Hinton broth and agar plates, the LB broth bacterial cultures from Day 2, sterile test tubes, the antibiotic stocks on ice, pipets (P20, P200, and P1000) and tips, 15 mL tubes, serological pipets and a pipettor, and microfuge tubes.
- Prepare the 25% and 50% MH stock solutions fresh on the day the experiment is set up. Based on the density of MH (1.47 g/mL), weigh the appropriate amount of MH in a sterile 15 mL tube and add the appropriate amount of sterile Mueller Hinton broth. Resuspend the MH by placing the tubes in warm water and inverting them as needed.
- Prepare a 1:1000 dilution (approximately 106 CFU/mL) of the overnight bacterial cultures in sterile 15 mL tubes by adding 10 μL of the bacterial culture to 10 mL of Mueller Hinton broth.
- Use the diluted cultures to prepare three samples for each bacterial species: an untreated control, a MH-treated sample (at the minimum inhibitory concentration, MIC), and an antibiotic-treated sample (tobramycin for S. aureus or meropenem for P. aeruginosa, at the MIC).
- Add the diluted bacterial culture, MH, and appropriate antibiotic to the samples in the volumes indicated in Table 1 and Table 2.
- Transfer 0.1 mL and 0.5 mL of the untreated sample to sterile microcentrifuge tubes for the viable plate count (step 5) and live/dead staining (step 6), respectively, for the T = 0 samples.
- Incubate the untreated control, MH-treated, and antibiotic-treated (tobramycin or meropenem) samples under aerobic conditions at 37 °C in a shaking incubator (250 rpm) for 24 h. Conduct the viable plate count (step 5) and live/dead staining (step 6) techniques on the T= 0 untreated samples.
5. Determining the culturable cells in samples using the viable plate count method (Day 3 - 5)
- On Day 4, following the 24 h sample incubation, collect 0.1 mL and 0.5 mL of each sample in microcentrifuge tubes for the viable plate count and live/dead staining (step 6), respectively. These will be the T = 24 h samples.
- Conduct the viable plate count on T = 0 and T = 24 h samples on the day they are collected by preparing 10-fold dilutions of the viable plate count samples in LB broth to obtain a countable number of cells (~25-150).
- Spread 50 μL of each diluted sample on ½ of an LB agar plate. Incubate the LB agar plates under aerobic conditions at 37 °C for 24 h.
- The following day, remove the LB agar plates from the incubator and identify the dilution plate that has ~25-150 colonies. Count the exact number of colonies on the plate and use the colony forming units (CFU) formula (CFU/mL= # colonies counted/ (volume plated, mL) (dilution used)) to determine the number of culturable cells per mL.
NOTE: The T = 0 sample will be plated on Day 3 and analyzed on Day 4; the T = 24 h samples will be plated on Day 4 and analyzed on Day 5.
6. Determining the viable cells in the samples using live/dead staining and fluorescent microscopy (Day 3 or 4)
- Gather the materials required for live/dead staining, including 0.85% sterile NaCl to maintain a favorable osmotic environment for cells, pipets (P20, P200, and P1000), and sterile pipet tips, a live/dead staining kit for bacteria, and a disposable hemocytometer. Obtain access to a fluorescent microscope with a fluorescein isothiocyanate (FITC) filter set to detect the SYTO 9 dye, which stains live cells.
- Add 0.5 mL of 0.85% NaCl to 0.5 mL of each of the following live/dead samples: all undiluted T = 0 samples, undiluted T = 24 h MH, and antibiotic-treated samples, and a 1:1000 dilution of the T = 24 h untreated control sample.
- Ensure to multiply the final live cell counts of all samples by two and multiply the diluted T = 24 h untreated control by 1000. Combine 1.5 μL of SYTO 9 and 1.5 μL of propidium iodide per sample, and then add 3 μL of the mixture to each sample. Incubate the samples at 21 °C in the dark for 15 min.
- Transfer 6 μL of each stained, gently mixed sample to a disposable hemocytometer and view it using a fluorescent microscope with the FITC filter and 40x objective lens. Take a photo that shows both the hemocytometer grid lines and the live green cells.
- Count the green cells in six fields per sample, noting the dimensions of the grid so the number of green cells per volume can calculated.
NOTE: The field dimensions in the current experiments are 1.05 mm x 0.6 mm x 0.1 mm, which corresponds to 0.063 mm3 or 6.3 x 10-5 mL. To obtain the number of viable cells per mL, divide the live cell number counted by 6.3 x 10-5 mL, and then multiply them by two (or 2000 if it is the T = 24 h untreated control).
- Count the green cells in six fields per sample, noting the dimensions of the grid so the number of green cells per volume can calculated.
7. Determining the number of viable but not culturable cells (VBNCs)
NOTE: Use the culturable cell numbers obtained from the viable plate count and the viable cell number obtained from live/dead staining to determine the number of VBNCs.
- Calculate VBNCs/mL by subtracting the number of culturable cells/mL from the number of viable cells per mL.
- Use a Wilcoxon matched-pairs test to compare the VBNCs/mL or the culturable cells/mL to the viable cells/mL for each treatment.
Ergebnisse
Viable but not culturable (VBNC) bacterial life forms are induced by stressors, including antibiotic treatment, and cause recurring infections due to their antibiotic tolerance. Because MH is a broad-spectrum antimicrobial to which resistance has rarely been detected, it was hypothesized that MH induced fewer VBNCs than conventional antibiotics. The method described here was used to quantify VBNCs formed by two wound-causing bacteria, S. aureus and P. aeruginosa. The number of VBNCs equals the number of viable cells determined by live/dead staining minus the number of culturable cells determined by the viable plate count. Table 3 shows the average number of VBNCs detected in S. aureus and P. aeruginosa, respectively, with indicated time points and treatments. One challenge faced in these experiments is when the viable number of cells is less than the culturable number of cells and gives a negative number of VBNCs. All culturable cells are viable, based on their ability to reproduce and form colonies. This was addressed by determining if the culturable cell number was significantly different from the viable cell number for each treatment. The number of culturable cells for antibiotic-treated S. aureus and P. aeruginosa is clearly less than the number of viable cells, but only the meropenem-treated P. aeruginosa had significantly fewer culturable cells than viable cells (Figure 1).
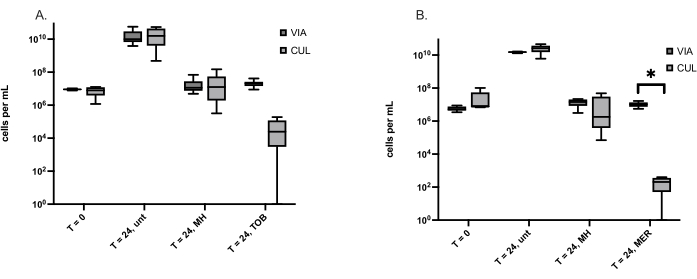
Figure 1: Culturable (CUL) and viable cells (VIA) determined using the viable plate count and live/dead staining, respectively. (A) S. aureus cells before treatment (T = 0) and at 24 h, untreated (unt), treated with Manuka Honey (MH) or the antibiotic tobramycin (TOB). The box plots represent six independent experiments, each with one replicate. (B) P. aeruginosa cells before treatment (T = 0) and at 24 h, untreated (unt), treated with Manuka Honey (MH), or the antibiotic meropenem (MER). The box plots represent five independent experiments, each with one replicate. A Wilcoxon matched-pairs test was used to compare VIA and CUL cell numbers, *p < 0.05. Please click here to view a larger version of this figure.
| Item | Untreated sample | MH sample, final concentration = 5% | Tobramycin sample, final concentration = 10 μg/mL |
| S. aureus culture diluted 1:1000 | 4 mL | 2 mL | 2 mL |
| Treatment | ________ | 0.5 mL of 25% MH solution | 0.250 mL of 100 μg/mL solution |
| Sterile LB broth | 1 mL | -------- | 0.25 mL |
| Final volume | 5 mL | 2.5 mL | 2.5 mL |
Table 1: Reagents and volumes necessary to prepare S. aureus antimicrobial treatment samples.
| Item | Untreated sample | MH sample, final concentration = 18% | Meropenem sample, final concentration = 6 μg/mL |
| P. aeruginosa culture diluted 1:1000 | 4 mL | 2 mL | 2 mL |
| Treatment | ________ | 1.44 mL of 50% MH solution | 0.24 mL of 100 μg/mL solution |
| Sterile LB broth | 4 mL | 0.56 mL | 1.76 mL |
| Final volume | 8 mL | 4 mL | 4 mL |
Table 2: Reagents and volumes necessary to prepare P. aeruginosa antimicrobial treatment samples.
| Time and treatment | S. aureus VBNCs | Time and treatment | P. aeruginosa VBNCs |
| T = 0 | 1.30 x 106 | T = 0 | -2.05 x 107 |
| T = 24 unt | 1.30 x 106 | T = 24 unt | -1.09 x 1010 |
| T = 24 MH | -1.37 x 107 | T = 24 MH | 1.78 x 106 |
| T = 24 TOB | 2.15 x 107 | T = 24 TOB | 1.04 x 107 |
Table 3: The average number of VBNCs detected in S. aureus and P. aeruginosa. VBNCs detected in S. aureus and P. aeruginosa samples by subtracting the number of culturable cells from the number of viable cells. Abbreviations are as follows: untreated (unt), Manuka Honey (MH), Tobramycin.
Diskussion
The protocol described here allows for the detection of VBNC cell populations in wound-causing bacteria treated with minimum inhibitory concentrations (MICs) of MH and conventional antibiotics. Critical steps in the protocol included preparing the MH dilutions on the day of the experiment, preventing sample loss during live/dead staining, and calculating the analyzed volume correctly. For this and other studies, the MH MICs for S. aureus and P. aeruginosa were experimentally determined27. In preparation for testing MH percentages between 1%-20% MH, stock solutions of 25% or 50% were made in the culture media used in the experiments. Interestingly, as the diluted samples age, their effectiveness slightly increased, decreasing the MIC (Carlson, unpublished observations). The age of the 100% MH did not appear to impact its effectiveness, although this was not carefully tested. To avoid variation in MH effectiveness, the 25% and 50% dilutions were prepared on the day of the experiment.
In multiple experiments, a negative number of VBNC cells was calculated due to the number of viable cells being less than the number of culturable cells. Because all culturable cells are viable, it is not possible to have fewer viable cells than culturable cells. It was hypothesized that some viable cells were lost during centrifugation of the post-treatment samples collected for live/dead cell analysis. So, instead of centrifuging 1 mL of the samples and resuspending them in 0.85% NaCl, 0.5 mL of the sample was collected and added to 0.5 mL of 0.85% NaCl. Although this improved the detection of viable cells by, on average, 47%, in some samples, the viable cell number was still lower than the culturable cell number. Since this precluded comparison of VBNC cell number among treatments, culturable and viable cells per sample were compared instead and showed that treatment of P. aeruginosa with meropenem, but not MH, produced significantly fewer culturable than viable cells (Figure 1). No significant difference between culturable and viable cells suggested that many VBNC cells were not present, while significantly fewer culturable cells indicated a high number of VBNC cells.
This protocol combines a suite of microbiology techniques with live/dead staining and detection by fluorescent microscopy to assess VBNC cell populations in MH-treated bacteria. Compared to other techniques and protocols for detecting VBNC cells, it is relatively inexpensive, and it does not require enzymes for qPCR or reverse transcription PCR, nor a real-time PCR machine25,26. This protocol will allow researchers to explore MH's impact on a wide range of bacterial pathogens to form VBNC cells and potentially reveal a mechanism for MH's recalcitrance to bacterial resistance.
Offenlegungen
The authors have no conflicts of interest to disclose.
Danksagungen
This work was funded by two Eastern Washington University grants, the Faculty Research and Creative Works Grant to A.R.C and the Undergraduate Research & Creative Activities Grant to L.T.B. Bill and Connie Cross donated funds to EWU to purchase the Leica DMIL inverted fluorescent microscope with a digital camera.
Materialien
| Name | Company | Catalog Number | Comments |
| Mueller Hinton broth | Fisher Bioreagents | B12322 | |
| Luria Bertani (LB) broth | Fisher Bioreagents | BP142602 | |
| BD Difco Agar | Fisher Scientific | DF0812-07-1 | |
| sodium chloride | Sigma | S3014-500g | |
| meropenem | TargetMol | T0224 | |
| tobramycin | Selleck Chemicals | S2514 | |
| Staphylococcus aureus | ATCC | ATCC29213 | |
| Pseudomonas aeruginosa | ATCC | ATCC9721 | |
| shaking incubator | Eppendorf | M13520000 | |
| incubator | Benchmark Scientific | H2200-H | |
| Manukaguard Manuka Honey | Amazon | NA | |
| DMIL inverted fluorescent microscope | Leica Microsystems | 11521265 | |
| Digital camera | Leica Microsystems | 12730522 | |
| 15 mL falcon tubes | Genesee Scientific | 28-103 | |
| serological pipets | Diagnocine | DP-LB0100005, DP-LB010010 | |
| Pipet Aid Pipettor | Drummond Scientific Company | 4-000-101 | |
| Bunsen burner | Fisher Scientific | S48108 | |
| Rainin pipets | Pipette.com | L-20, L-200, L-1000 | |
| balance | Mettler Toledo | XS603S | |
| 96-well plate | Falcon | 351172 | |
| inoculating loop | Fisher Scientific | 13-104-5 | |
| isopropanol | Fisher Scientific | BP2618-1 | |
| glass spreader | homemade | NA | |
| Live dead staining kit for bacteria | Invitrogen | L7012 | |
| microfuge tubes | Biologix Research Company | SKU 80-1500/P80-1500 | |
| tin foil | Costco | NA | |
| Figure making software | GraphPad | NA | GraphPad Prism was used for making figures and conducting statistical analyses. |
Referenzen
- Spoering, A., Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 183 (23), 6746-6751 (2001).
- Mulcahy, L. R., Burns, J. L., Lory, S., Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol. 192 (23), 6191-6199 (2010).
- Li, L., Mendis, N., Trigui, H., Oliver, J. D., Faucher, S. P. The importance of the viable but non-culturable state in human bacterial pathogens. F Microbiol. 5, 00258 (2014).
- Reygaert, W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4 (3), 482-450 (2018).
- Ayrapetyan, M., Williams, T. C., Baxter, R., Oliver, J. D. Viable but nonculturable and persister cells coexist stochastically and are induced by human serum. Infect Immun. 83 (11), 4194-4203 (2015).
- Cabral, D. J., Wurster, J. I., Belenky, P. Antibiotic persistence as a metabolic adaptation: Stress, metabolism, the host, and new directions. PHARH2. 11 (1), 11010014 (2018).
- Heidelberg, J. F., et al. Effect of aerosolization on culturability and viability of gram-negative bacteria. Appl Environ Microbiol. 63 (9), 3585-3588 (1997).
- Cook, K. L., Bolster, C. H. Survival of Campylobacter jejuni and Escherichia coli in groundwater during prolonged starvation at low temperatures. J Appl Microbiol. 103 (3), 573-583 (2007).
- Xu, H. S., et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbl Ecol. 8 (4), 313-323 (1982).
- Qi, Z., Huang, Z., Liu, C. Metabolism differences of biofilm and planktonic Pseudomonas aeruginosa in viable but nonculturable state induced by chlorine stress. Sci Total Environ. 821, 153374 (2022).
- Li, Y., et al. Study on the Viable but Non-culturable (VBNC) state formation of staphylococcus aureus and its control in food system. F Microbiol. 11, 599739 (2020).
- Oryan, A., Alemzadeh, E., Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability. 25 (2), 98-118 (2016).
- Lusby, P. E., Coombes, A. L., Wilkinson, J. M. Bactericidal Activity of different honeys against pathogenic bacteria. Arch Med Res. 36 (5), 464-467 (2005).
- Mandal, M. D., Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac J Trop. Biomed. 1 (2), 154-160 (2011).
- Carter, D. A., et al. Therapeutic Manuka Honey: No longer so alternative. F Microbiol. 7, 00569 (2016).
- Cooper, R. A., Molan, P. C., Harding, K. G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 93 (5), 857-863 (2002).
- George Narelle May, C., Keith, F. Antibacterial Honey: In-vitro activity against clinical isolates of MRSA, VRE, and other multiresistant Gram-negative organisms. HMP Global Learning Network. , (2007).
- Blair, S. E., Cokcetin, N. N., Harry, E. J., Carter, D. A. The unusual antibacterial activity of medical-grade Leptospermum honey: Antibacterial spectrum, resistance and transcriptome analysis. Eur Soc Clin Microbiol. 28 (10), 1199-1208 (2009).
- Cooper, R. A., Jenkins, L., Henriques, A. F. M., Duggan, R. S., Burton, N. F. Absence of bacterial resistance to medical-grade manuka honey. Eur Soc Clin Microbiol. 29 (10), 1237-1241 (2010).
- Lu, J., et al. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci Rep. 9 (1), 18160 (2019).
- Bischofberger, A. M., Pfrunder Cardozo, K. R., Baumgartner, M., Hall, A. R. Evolution of honey resistance in experimental populations of bacteria depends on the type of honey and has no major side effects for antibiotic susceptibility. Evol Appl. 14 (5), 1314-1327 (2021).
- Cunningham, E., O'Byrne, C., Oliver, J. D. Effect of weak acids on Listeria monocytogenes survival: Evidence for a viable but nonculturable state in response to low pH. Food Control. 20 (12), 1141-1144 (2009).
- Kogure, K., Simidu, U., Taga, N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 25 (3), 415-420 (1979).
- Albertini, M. C., et al. Use of multiparameter analysis for Vibrio alginolyticus viable but nonculturable state determination. Cytom J Int Soc Anal Cytol. 69 (4), 260-265 (2006).
- Nocker, A., Camper, A. K. Novel approaches toward preferential detection of viable cells using nucleic acid amplification techniques. FEMS Microbiol Lett. 291 (2), 137-142 (2009).
- Trevors, J. T. Viable but non-culturable (VBNC) bacteria: Gene expression in planktonic and biofilm cells. J Microbiol Methods. 86 (2), 266-273 (2011).
- Ankley, L. M., Monteiro, M. P., Camp, K. M., O'Quinn, R., Castillo, A. R. Manuka honey chelates iron and impacts iron regulation in key bacterial pathogens. J App Microbiol. 128 (4), 1015-1024 (2020).
Nachdrucke und Genehmigungen
Genehmigung beantragen, um den Text oder die Abbildungen dieses JoVE-Artikels zu verwenden
Genehmigung beantragenWeitere Artikel entdecken
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten