Method Article
A Data-Driven Approach to Quantifying Immune States in Sepsis
In This Article
Summary
This study investigates the immune condition in sepsis by analyzing the quantitative relationships among white blood cells, lymphocytes, and neutrophils in sepsis patients and healthy controls using data visualization analysis and three-dimensional numerical fitting to establish a mathematical model.
Abstract
In sepsis, understanding the interplay among white blood cells, lymphocytes, and neutrophils is crucial for assessing the immune condition and optimizing treatment strategies. Blood samples were collected from 512 patients diagnosed with sepsis and 205 healthy controls, totaling 717 samples. Data visualization analysis and three-dimensional numerical fitting were performed to establish a mathematical model describing the relationships among white blood cells, lymphocytes, and neutrophils. Self-organizing feature map (SOFM) was employed to automatically cluster the sepsis sample data in the three-dimensional space represented by the model, yielding different immune states.
Analysis revealed that white blood cell, lymphocyte, and neutrophil counts are constrained within a three-dimensional plane, as described by the equation: WBC = 1.098 × Neutrophils + 1.046 × Lymphocytes + 0.1645, yielding a prediction error (RMSE) of 1%. This equation is universally applicable to all samples despite differences in their spatial distributions. SOFM clustering identified nine distinct immune states within the sepsis patient population, representing different levels of immune status, oscillation periods, and recovery stages.
The proposed mathematical model, represented by the equation above, reveals a basic constraint boundary on the immune cell populations in both sepsis patients and healthy controls. Furthermore, the SOFM clustering approach provides a comprehensive overview of the distinct immune states observed within this constraint boundary in sepsis patients. This study lays the foundation for future work on quantifying and categorizing the immune condition in sepsis, which may ultimately contribute to the development of more objective diagnostic and treatment strategies.
Introduction
Sepsis, a life-threatening organ dysfunction caused by a dysregulated host response to infection, remains a significant challenge in critical care medicine1. Despite advances in understanding the pathophysiology of sepsis, the complex interplay between the immune system and pathogens continues to pose difficulties in diagnosing and treating this condition effectively2. Current clinical approaches often focus on monitoring infection indicators, organ function, cytokines, microbial detection, and gut microbiome3. However, there is a growing recognition of the crucial role played by immune cells, particularly white blood cells, lymphocytes, and neutrophils, in the progression and resolution of sepsis4.
During the course of sepsis, the immune system undergoes a complex series of changes, characterized by an initial hyperinflammatory phase followed by a prolonged immunosuppressive phase5. The early phase is marked by a surge in neutrophil counts and a concomitant decrease in lymphocyte populations, reflecting the activation of innate immune responses and the suppression of adaptive immunity6. As the condition progresses, neutrophil levels may oscillate or become exhausted while lymphocyte counts continue to decline, leading to a state of immunosuppression that renders patients vulnerable to secondary infections7. Understanding the dynamic interplay among these immune cell populations is crucial for accurately assessing the immune status of sepsis patients and devising targeted interventions.
Traditional approaches to analyzing immune cell counts in sepsis have relied on univariate or bivariate analyses, which fail to capture the complex relationships among multiple immune parameters8. Recent advances in data visualization and machine learning techniques have opened up new possibilities for exploring high-dimensional immunological data9. In particular, three-dimensional scatter plot visualization and self-organizing feature maps (SOFM)10 have shown promise in uncovering hidden patterns and identifying distinct immune states in various disease contexts.
This study aims to investigate the immune condition in sepsis patients by analyzing the quantitative relationships among white blood cells, lymphocytes, and neutrophils using advanced data visualization and clustering techniques. The hypothesis is that these immune cell populations are constrained within a three-dimensional space governed by an underlying mathematical relationship. By uncovering this relationship and identifying distinct immune states using SOFM, the study seeks to provide a framework for understanding the immune dynamic states in sepsis and facilitating clinical decision-making.
The approach involves collecting blood samples from 512 sepsis patients admitted to the intensive care unit (ICU) and 205 healthy individuals, totaling 717 samples. The study population included both male (54.3%) and female (45.7%) participants, with ages ranging from 35 to 100 years (mean age: 73.5 years). Three-dimensional scatter plot visualization and numerical fitting are applied to establish a mathematical model describing the interplay among white blood cells, lymphocytes, and neutrophils in both sepsis patients and healthy controls. SOFM is then employed to automatically cluster the sepsis sample data in the three-dimensional space, yielding different immune states. By comparing the immune profiles and spatial distributions of sepsis patients with those of healthy individuals within the constraint boundary represented by the mathematical model, the study aims to gain insights into the pathophysiological mechanisms underlying sepsis and identify potential targets for immunomodulatory therapies.
By providing a quantitative method for assessing the immune condition of sepsis patients, the approach could enable more precise staging of the disease and guide the selection of appropriate interventions. Furthermore, the identification of distinct immune states using SOFM may lay the foundation for future research on personalized immunotherapy approaches tailored to the specific immune profiles of individual patients.
In summary, this study presents an approach to understanding the immune condition in sepsis by leveraging advanced data visualization and machine learning techniques. By uncovering the mathematical relationship between key immune cell populations in sepsis patients and healthy controls and identifying distinct immune states in sepsis patients, the study provides a new perspective on the complex immune dynamics in sepsis. This approach enables a more precise assessment of the disease state (Different Clusters) and can guide the selection of appropriate interventions, ultimately contributing to developing more effective diagnostic and therapeutic strategies.
Protocol
This study explores the immune condition in sepsis patients by investigating the relationships among white blood cells, lymphocytes, and neutrophils. The patients were enrolled in the intensive care unit (ICU) of Dongzhimen Hospital in Beijing, China, and underwent standard blood tests after providing informed consent. The study was conducted as per the guidelines of the institutional human research ethics committee. Data grouping and detailed data content can be found in Supplementary Table 1. The software tools utilized in this study are enumerated in the Table of Materials.
1. Data collection and preparation
NOTE: White blood cells, neutrophils, and lymphocytes were selected as key indicators of the immune condition in sepsis patients. This choice is based on the well-established clinical observations that lymphocyte counts tend to be suppressed and gradually decrease, while neutrophil counts often oscillate in sepsis patients. These two cell types have been empirically recognized as important markers of the immune status in sepsis patient populations. However, the precise quantitative relationship among these parameters has not been clearly reported in the literature. Therefore, white blood cells, neutrophils, and lymphocytes were chosen as a starting point for quantifying the immune condition in sepsis patients.
- Installing MATLAB Spreadsheet Link for Excel Add-In.
- Open Microsoft Excel and navigate to the Insert tab on the ribbon.
- Click on Get Add-ins in the Add-ins section.
- In the Office Add-ins dialog box, search for MATLAB Spreadsheet Link in the search bar.
- Locate the MATLAB Spreadsheet Link for Excel add-in in the search results and click on the Add button.
- Read and accept the terms and conditions, then click Continue to proceed with the installation.
- If prompted, log in with a MathWorks account or create a new account to access the add-in.
- Once the installation is complete, the MATLAB Spreadsheet Link tab will appear on the Excel ribbon.
- Click on the MATLAB Spreadsheet Link tab to verify that the add-in is installed and ready to use.
- Send data into MATLAB workspace.
- Open the spreadsheet containing the sepsis patient data, including white blood cell counts, lymphocyte counts, and neutrophil counts.
- Ensure that the data is organized in a structured format, with each variable (white blood cell count, lymphocyte count, and neutrophil count) in a separate column and each patient in a separate row.
- Select the range of cells containing the white blood cell counts, lymphocyte counts, and neutrophil counts.
- Click on the MATLAB Spreadsheet Link tab in the Excel ribbon.
- In the MATLAB Spreadsheet Link tab, click the Send Data to MATLAB button.
- In the Send Data to MATLAB dialog box, choose the appropriate MATLAB instance from the dropdown menu if multiple instances are running.
- Specify the variable name for the data in the Variable name field. For example, sepsis_immune_data.
- Select the desired data type for the imported data in MATLAB (e.g., numeric matrix).
- Click OK to send the data to the MATLAB workspace.
- Switch to the MATLAB application and verify that the data has been successfully imported by checking the workspace for the specified variable name (e.g., sepsis_immune_data).
- Checking the data in MATLAB
- After sending the sepsis patient data (white blood cell counts, lymphocyte counts, and neutrophil counts) to MATLAB using the MATLAB Spreadsheet Link add-in, switch to the MATLAB application.
- To check the contents of the imported data, type the variable name in the MATLAB command window and press Enter.
2. Three-dimensional visualization of white blood cells, lymphocytes, and neutrophils
- Generating the plot using the Immune_scatter3 function
- The variable A stores the immune data of sepsis patients. Call the function Immune_scatter3(A) to obtain a graphical user interface (GUI) displaying the three-dimensional scatter plot of the samples, as shown in Figure 1.
NOTE: The fitting error between the three-dimensional plane and the sample distribution in the plot is very small. Section 3 will provide the exact formula.
- The variable A stores the immune data of sepsis patients. Call the function Immune_scatter3(A) to obtain a graphical user interface (GUI) displaying the three-dimensional scatter plot of the samples, as shown in Figure 1.
- The GUI usage
- Explore and analyze the three-dimensional scatter plot using the interactive features provided by the generated GUI.
- Rotate: Click and drag the plot to rotate it in 3D space, allowing viewing the sample distribution from different angles.
- Pan: Right-click and drag the plot to move it horizontally or vertically, adjusting the visible area of the plot.
- Zoom: Use the mouse wheel or the zoom controls in the toolbar to zoom in or out of the plot, focusing on specific regions or samples.
- Data cursor: Click on individual samples to display their corresponding values for white blood cells, lymphocytes, and neutrophils.
NOTE: By utilizing these interactive features, clinicians can gain insights into the relationships and patterns among white blood cells, lymphocytes, and neutrophils in sepsis patients, facilitating the exploration and analysis of the immune data.
- Explore and analyze the three-dimensional scatter plot using the interactive features provided by the generated GUI.
3. The formula
- In the MATLAB workspace, assign the neutrophil count, lymphocyte count, and white blood cell count to the variables X, Y, and Z, respectively.
- To obtain the precise mathematical expression (formula 1; WBC = 1.098 × Neutrophils + 1.046 × Lymphocytes + 0.1645 (RMSE: 1%)) of the three-dimensional plane in Figure 1, invoke the following command:
[fitresult, gof, output] = fit ([X, Y], Z, 'poly11')
NOTE: This formula quantitatively describes the relationship among white blood cells, neutrophils, and lymphocytes in sepsis patients, providing a concise and accurate representation of the immune data. - To evaluate the goodness of fit, calculate the normalized root mean square error (NRMSE) using the following command:
gof.rmse / (max(Z) - min(Z))
NOTE: The resulting NRMSE value of 1% indicates that the fitted plane (formula 1) closely approximates the actual sample distribution in the three-dimensional space. This low error level underscores the reliability and validity of the obtained mathematical expression in capturing the intricate relationships among the immune parameters in sepsis patients.
4. Immune condition in sepsis
NOTE: Self-Organizing Feature Maps (SOFM) were employed for unsupervised clustering to identify immune conditions in sepsis patients.
- By invoking the Immune_Condition function, generate clusters of sample points on the three-dimensional plane represented by formula 1, as depicted in Figure 2.
- Use the interactive visualization features for Figure 2 as described in Step 2.2.
NOTE: Figure 2 showcases nine automated clusters, labeled Cluster1 through Cluster9, derived from SOFM's unsupervised machine learning approach. This clustering technique takes into account both the spatial topology and the density of the samples, enabling the identification of distinct immune conditions within the sepsis patient population.
5. Typical immune oscillation trajectories in sepsis
- Based on Figure 2, use the hold on command to maintain the figure in an overlayable state, then use the following commands to create a three-dimensional plot of the typical patient's trajectory data.
hold on
for i=1: size(p,1)-1
pause (3); plot3(p (i: i+1,2), p (i: i+1,3), p (i: i+1,1),'k','Linewidth',3);
end
Results
The progression of sepsis involves a complex interplay between the human immune system and invading pathogens. In clinical diagnosis and treatment, much attention is focused on infection indicators, organ function markers, cytokines, microbial detection, and even the gut microbiome. However, this study emphasizes the importance of three common immune indicators: white blood cells, neutrophils, and lymphocytes, which are not without basis. Research has demonstrated that the pathological process of sepsis is accompanied by the suppression and depletion of lymphocyte populations, while neutrophil levels either become exhausted or overcome pathogens after repeated fluctuations5. Nevertheless, the mathematical constraints and dynamics underlying this process remain to be further quantified and elucidated.
When exploring the distribution of 512 samples (represented by blue dots) and 205 healthy controls (represented by green plus signs) in three-dimensional space (Figure 1), it is readily apparent that the sample points of white blood cells, neutrophils, and lymphocytes lie on a plane with a very small error. The distinct colors and markers used in the scatter plot clearly demonstrate the significant differences between the sepsis and healthy control groups within the constraint boundary. This observation suggests that these immune cell populations follow an objective law or constraint, regardless of the health status. Further analysis reveals the following formula: WBC = 1.098 × Neutrophils + 1.046 × Lymphocytes + 0.1645 (formula 1), with a root mean square error (RMSE) of 1%.This equation indicates that the plane slope of neutrophils is greater than that of lymphocytes, implying that changes in neutrophil counts have a more pronounced effect on white blood cell counts compared to changes in lymphocyte counts. Although simple, this formula represents a novel finding that uncovers an objectively existing mechanism or constraint governing the interplay among white blood cells, neutrophils, and lymphocytes, regardless of their individual variations. Importantly, this constraint boundary applies to both sepsis patients and healthy individuals, suggesting that it reflects a fundamental property of the immune system, while the distinct spatial distributions within this boundary reveal the immunological differences between sepsis and health.
To further distinguish different immune conditions, Figure 2 employs SOFM for automatic sample point clustering. SOFM was specifically chosen for this analysis because it is a well-established unsupervised clustering algorithm that excels at preserving topological relationships while considering both the distance between samples and their density distributions. The algorithm's neighborhood control parameter was set to 1, ensuring minimal overlap between adjacent clusters while maintaining clear boundary definitions. The choice of nine categories was determined through careful parameter optimization, balancing the need for detailed state subdivision with the practical consideration of maintaining clinically meaningful distinctions. This approach allows for both fine-grained immune state discrimination and the potential to merge adjacent states when biologically appropriate, thereby avoiding both over-segmentation and state omissions.
In Figure 2, SOFM clustering was employed to automatically identify distinct immune states among sepsis patients, with the aim of providing a comprehensive characterization of the immune landscape and minimizing the risk of overlooking any important categories. Cluster1, Cluster2, and Cluster4 represent higher levels of immune activity, characterized by relatively high counts of both lymphocytes and neutrophils. Cluster3, Cluster5, Cluster6, and Cluster9 depict the immune oscillation period, during which lymphocyte populations have already undergone suppression, while neutrophil levels may fluctuate. Cluster 8 represents a state of reduced immune activity, which may reflect either immunosuppression or a period of immune recovery following the resolution of the infection. Cluster7 likely represents a small proportion of patients who are gradually recovering and showing signs of improvement in their immune status.
It is worth noting that the use of multiple SOFM clusters allows for a more granular representation of the diverse immune states observed in sepsis patients. This approach aims to capture the full spectrum of immune profiles and minimize the chances of missing any critical categories. However, through practical validation and further analysis, it is possible to merge clusters that represent similar immune states, streamlining the classification scheme while maintaining its comprehensiveness. This refinement process ensures that the identified immune states are both biologically meaningful and clinically relevant, providing a robust framework for understanding the complex immune dynamics in sepsis.
Figure 3 illustrates the trajectory of the immune states of a typical sepsis patient who experienced a complex course of immune dysregulation before ultimately improving. The patient's journey began in Cluster 2, which represents a state of heightened immune activity, and then progressed through various immune states, including Clusters 5, 7, 9, and 8. These transitions reflect the dynamic interplay between the patient's immune system and the invading pathogens, with periods of oscillation and apparent immunosuppression.
Notably, the patient's sequential organ failure assessment (SOFA) score had already started to improve, decreasing from an initial value 7 to 2, before the patient entered Cluster 7. This suggests that the patient's immune system had made significant progress in clearing the infection, and the subsequent transition to Cluster 7 may represent a period of immune recovery rather than complete immune exhaustion.
Following the passage through Cluster 8, the patient's immune state gradually progressed towards Cluster 7, which is associated with a healthier immune profile. This recovery phase was characterized by an increase in both neutrophil and lymphocyte counts, with values approaching those observed in healthy individuals.
In clinical practice, patients generally exhibit immune oscillation trajectories similar to the typical red line shown in Figure 3. When the trajectory oscillates in Clusters 3, 5, 6, and 9, which are far from the center of healthy samples, the patient's condition deteriorates. When the patient's trajectory returns to Clusters 7 and 8, which are closer to the center of healthy samples, the condition improves, and some patients even regain healthy status. Clusters 1, 2, and 4 are commonly observed in the early stages of the disease, characterized by heightened immune activity. These patterns of state transitions provide an immune-based perspective for early recognition and disease course management in sepsis. From a patient state management perspective, it is evident that as patients trend toward Cluster 6, their condition worsens, while progression toward the healthy state indicates clinical improvement.
This protocol provides a quantitative approach for assessing immune states in sepsis patients through a mathematical model and clustering analysis, enabling both early recognition of sepsis through distinct immune state visualization and precise monitoring of disease progression through immune trajectory tracking, which can guide clinical decision-making and personalized treatment strategies.
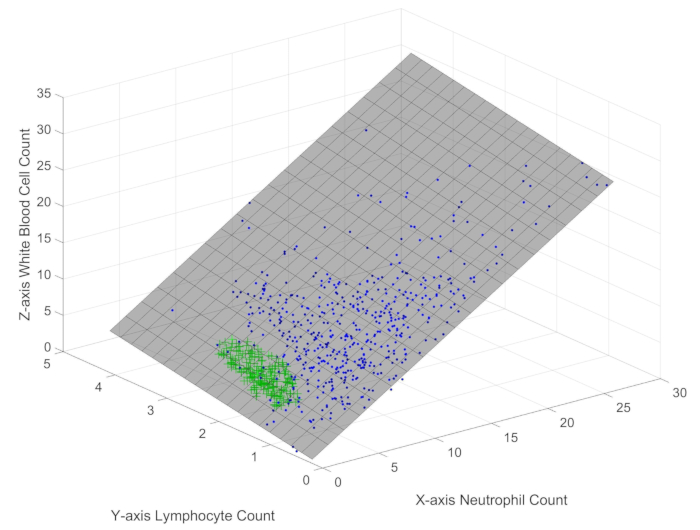
Figure 1: Three-dimensional scatter plot of white blood cells, lymphocytes, and neutrophils in sepsis patient samples. The figure depicts the spatial distribution of the main immune indicators in sepsis patients. Please click here to view a larger version of this figure.
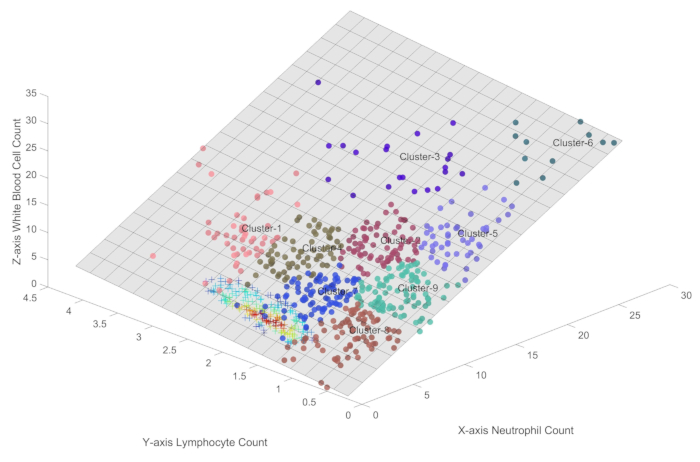
Figure 2: Different immune conditions in sepsis. This figure provides a precise quantification of the immune states in sepsis within the three-dimensional plane represented by formula 1. Please click here to view a larger version of this figure.
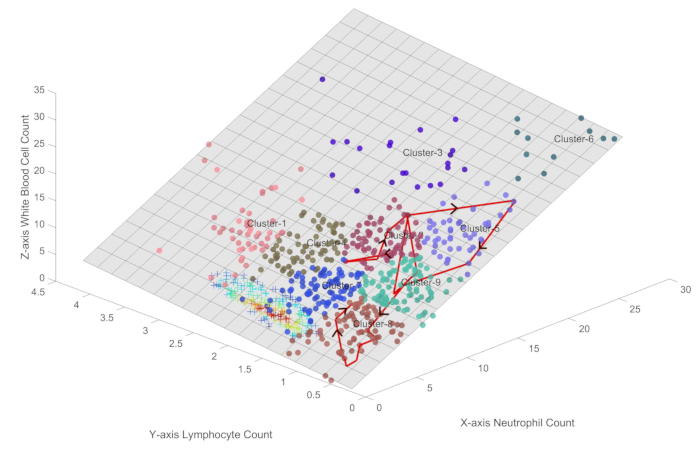
Figure 3: Visualization of the immune state trajectory for a sepsis patient undergoing clinical improvement. The figure illustrates the trajectory of the immune states of a typical sepsis patient who experienced a complex course of immune dysregulation before ultimately improving. Please click here to view a larger version of this figure.
Discussion
This study presents an approach to understanding the immune condition in sepsis by leveraging advanced data visualization and machine learning techniques. By uncovering the mathematical relationship among key immune cell populations and identifying distinct immune states, the study provides a new perspective on the complex immune dynamics in sepsis and contributes to the development of more effective diagnostic and therapeutic strategies11,12. The key findings include the discovery of a mathematical relationship (section 3) constraining these immune cell populations within a three-dimensional space (WBC = 1.098 × Neutrophils + 1.046 × Lymphocytes + 0.1645) and the identification of nine distinct immune states (section 4) using self-organizing feature maps (SOFM). These results provide a quantitative framework for assessing the immune status of sepsis patients and offer a potential method to triage early sepsis patients13.
This research presented here combined data visualization and machine learning techniques to uncover hidden patterns and relationships in high-dimensional immunological data in sepsis. By moving beyond traditional univariate and bivariate analyses, this study captures the complex interplay among multiple immune parameters and provides a more comprehensive understanding of the immune response (different clusters in Figure 2) in sepsis. The identification of distinct immune states using SOFM10 may lay the foundation for future research on personalized immunotherapy approaches tailored to the specific immune profiles of individual patients.
Future studies with even larger and more diverse cohorts will be necessary to further validate and refine the findings presented here. Additionally, the relationship between the identified immune states and other clinically relevant biochemical markers, such as procalcitonin (PCT), C-reactive protein (CRP), and interleukin-6 (IL-6), remains to be explored in depth14,15. Integrating these markers into the analysis could provide a more comprehensive understanding of the immune-inflammatory response in sepsis and its association with clinical outcomes, potentially enhancing the predictive power and clinical utility of the proposed approach. Furthermore, longitudinal studies that track the dynamics of immune states over time in individual patients could offer valuable insights into the trajectory of sepsis and inform personalized treatment strategies.
On one hand, the three-dimensional visualization method presented in Figure 1 demonstrates a striking distinction between the immune states of sepsis patients and healthy controls, providing a novel quantitative immunological perspective for rapid early recognition of sepsis. On the other hand, the discussion of typical patient immune trajectories shown in Figure 3 presents a new approach to patient state management, offering a quantitative method for monitoring and managing disease progression.
The importance and potential applications of the method proposed in this study extend far beyond the specific context of sepsis. The ability to precisely quantify disease states using data-driven approaches has the potential to become a paradigm shift in clinical research, with profound implications for personalized medicine16. For instance, tracking the immune states of sepsis patients can provide a clear assessment of the stages of the patient's battle against the infection, which is crucial for evaluating prognosis and improving treatment plans. By leveraging the power of data visualization and machine learning, researchers can uncover novel insights into the pathophysiology of complex diseases and develop more targeted diagnostic and therapeutic strategies17.
To build upon the findings of this study, several future directions can be envisioned. First, increasing the sample size and diversity of the sepsis patient population will be crucial for validating and refining the mathematical relationship and immune states identified here. This will require collaboration among multiple centers and the establishment of standardized protocols for data collection and analysis. Second, exploring the relationship between the identified immune states and other biochemical markers, such as PCT, CRP, and IL-6, could provide a more comprehensive understanding of the immune-inflammatory response in sepsis18. Additionally, investigating the complex, non-linear relationships between immune states, organ dysfunction (as measured by SOFA scores), and infection severity markers could help elucidate the varying pathways through which different patients progress from infection to organ failure. By integrating these infection severity indicators with our immune state quantification approach, future research could enhance our understanding of disease progression patterns, potentially leading to more precise and personalized treatment strategies that consider both the infection severity and immune status of individual patients. Finally, the research approach proposed in this study could be extended to other medical scenarios, such as autoimmune diseases, cancer, and transplantation, where the precise quantification of disease states could have significant clinical implications.
In conclusion, this study presents an approach to understanding the immune condition in sepsis by leveraging advanced data visualization and machine learning techniques. By uncovering the mathematical relationship among key immune cell populations and identifying distinct immune states, this study provides a new perspective on the complex immune condition in sepsis. The limitations of the study, such as the sample size and the need for further exploration of the relationship between immune states and other biochemical markers, should be addressed in future research. The potential applications of the method in specific research areas, such as personalized medicine, and the future directions, including increasing sample size and extending the approach to other medical scenarios, highlight the importance and promise of this line of research.
Disclosures
The software tool for Probabilistic Scatter Plots for Immune States V1.0 is developed and owned by Beijing Intelligent Entropy Science & Technology Co., Ltd. All intellectual property rights of this software are held by the company. The authors declare no conflicts of interest.
Acknowledgements
This study received support from two sources: the seventh batch of the Master-Apprentice Inheritance Project organized by the National Administration of Traditional Chinese Medicine of China (Project number: [2021] No. 272) and the 2024 Chinese Medicine Research Capacity Enhancement Project of Municipal-level Chinese Medicine Hospital (SZY-NLTL-2024-003) from the Shaanxi Provincial Administration of Traditional Chinese Medicine.
Materials
| Name | Company | Catalog Number | Comments |
| MATLAB | MathWorks | 2022B | Computing and visualization |
| Probabilistic Scatter Plots for Immune States | Intelligent Entropy | Immune States V1.0 | Beijing Intelligent Entropy Science & Technology Co Ltd. Modeling for CT/MRI fusion |
References
- Jarczak, D., Kluge, S., Nierhaus, A. Sepsis-pathophysiology and therapeutic concepts. Front Med (Lausanne). 8, 628302 (2021).
- Cheung, G. Y. C., Bae, J. S., Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 12 (1), 547-569 (2021).
- Adelman, M. W., et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. 24 (1), 278 (2020).
- Kumar, V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 11, 1722 (2020).
- Nakamori, Y., Park, E. J., Shimaoka, M. Immune deregulation in sepsis and septic shock: Reversing immune paralysis by targeting PD-1/PD-L1 pathway. Front Immunol. 11, 624279 (2021).
- Van der Poll, T., Shankar-Hari, M., Wiersinga, W. J. The immunology of sepsis. Immunity. 54 (11), 2450-2464 (2021).
- Chaturvedi, V., et al. T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis. Blood. 137 (17), 2337-2346 (2021).
- Reyes, M., et al. An immune-cell signature of bacterial sepsis. Nat Med. 26 (3), 333-340 (2020).
- Pant, A., Mackraj, I., Govender, T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J Biomed Sci. 28 (1), 6 (2021).
- Kohonen, T. Essentials of the self-organizing map. Neural Netw. 37, 52-65 (2013).
- Yang, X., et al. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood. 135 (14), 1087-1100 (2020).
- Zhang, Y. Y., Ning, B. T. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 6 (1), 407 (2021).
- Baghela, A., et al. Predicting sepsis severity at first clinical presentation: The role of endotypes and mechanistic signatures. EBioMedicine. 75, 103776 (2022).
- Yao, R. Q., et al. Publication trends of research on sepsis and host immune response during 1999-2019: A 20-year bibliometric analysis. Int J Biol Sci. 16 (1), 27-37 (2020).
- Owen, A. M., et al. TLR agonists as mediators of trained immunity: Mechanistic insight and immunotherapeutic potential to combat infection. Front Immunol. 11, 622614 (2021).
- Jung, E., et al. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med. 25 (4), 101146 (2020).
- Bruno, M., et al. Transcriptional and functional insights into the host immune response against the emerging fungal pathogen Candida auris. Nat Microbiol. 5 (12), 1516-1531 (2020).
- Barichello, T., et al. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. 26 (1), 14 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved