Method Article
A Cardiac Microphysiological System for Studying Ca2+ Propagation via Non-genetic Optical Stimulation
* These authors contributed equally
In This Article
Summary
Using light to control cardiac cells and tissue enables non-contact stimulation, thereby preserving the natural state and function of the cells, making it a valuable approach for both basic research and therapeutic applications.
Abstract
In vitro cardiac microphysiological models are highly reliable for scientific research, drug development, and medical applications. Although widely accepted by the scientific community, these systems are still limited in longevity due to the absence of non-invasive stimulation techniques. Phototransducers provide an efficient stimulation method, offering a wireless approach with high temporal and spatial resolution while minimizing invasiveness in stimulation processes. In this manuscript, we present a fully optical method for stimulating and detecting the activity of an in vitro cardiac microphysiological model. Specifically, we fabricated engineered laminar anisotropic tissues by seeding human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) generated in a 3D bioreactor suspension culture. We employed a phototransducer, an amphiphilic azobenzene derivative, named Ziapin2, for stimulation and a Ca2+ dye (X-Rhod 1) for monitoring the system's response. The results demonstrate that Ziapin2 can photomodulate Ca2+ responses in the employed system without compromising tissue integrity, viability, or behavior. Furthermore, we showed that the light-based stimulation approach offers a similar resolution compared to electrical stimulation, the current gold standard. Overall, this protocol opens promising perspectives for the application of Ziapin2 and material-based photostimulation in cardiac research.
Introduction
The use of light for stimulating living cells and tissues is emerging as a significant game-changer in biomedical research, offering touchless stimulation capabilities with precise temporal and spatial resolution1,2,3,4,5,6. One of the leading techniques used to make cells sensitive to light is optogenetics, which involves genetically modifying cells to express light-sensitive ion channels or pumps7,8. This approach has demonstrated impressive effectiveness in regulating cells within living tissue; however, its reliance on viral gene transfer has hindered its widespread adoption in research and clinical applications.
To overcome this limitation, organic and inorganic materials have been used as light-sensitive transducers to develop non-genetic, material-based light-mediated stimulation techniques9,10. Organic nanostructured phototransducers11,12,13,14,15 have recently demonstrated remarkable success in triggering cellular responses across diverse applications, including neurons, cardiomyocytes, and skeletal muscle cells.
Herein, we propose Ziapin216,17,18, an azobenzene derivative, for investigating Ca2+ propagation in engineered laminar cardiac tissues. The amphiphilic structure of the molecule allows for precise targeting of the cell plasma membrane, while the azobenzene core enables light-induced isomerization, leading to its conformational change16,17,18. In cardiac cells, this trans-to-cis isomerization alters the plasma membrane thickness, inducing a cascade of effects that generates an action potential, which in turn triggers the excitation-contraction process19,20,21.
Additionally, we describe the fabrication process of an engineered platform for the anisotropic growth of cardiac tissue22 and detail the experimental setup used for optically triggering and monitoring its activity, with a particular focus on acquiring Ca2+ dynamics within the tissue23,24. Finally, we compare the acquired signals with those obtained through electrical stimulation, which is considered the reference standard. Overall, this protocol highlights the application of a novel light-responsive transducer in advancing our understanding of cardiac cellular behavior, especially in the context of engineered tissues.
Protocol
The human pluripotent stem cell (hiPSC) culture used is a wild-type human male iPSC line that harbors a doxycycline (Dox)-inducible CRISPR/Cas9 system, created by introducing CAGrtTA::TetO-Cas9 into the AAVS1 locus (Addgene: #73500). The study was conducted in accordance with protocols approved by the Boston Children's Hospital Institutional Review Board. Informed consent was obtained from patients prior to their participation in the study. The generation of hiPSC-derived cardiomyocytes (hiPSC-CMs) was induced as previously described25,26. The protocol will be briefly summarized in the following section:
1. Generation and preparation of human induced pluripotent stem cell-derived cardiomyocytes
- Wash hiPSCs being cultured in T75 flasks once with PBS. Detach the cells by incubating with the chelating agent Versene for 10-15 min and seed into bioreactor vessels pretreated with 1% solution of non-ionic surfactant at a density of 50 million IPSCs/vessel in 100 mL of E8 medium supplemented with 10 µM ROCK inhibitor Y-27632.
- Set the following bioreactor parameters: agitation 60 rpm, temperature 37 °C, pH 7, and overlay gassing (O2 and CO2) at 3 standard L/h.
- After 1 day in culture, confirm that the embryoid body (EB) diameter has reached 100-300 µm and treat the cells with basic RPMI medium (RPMI 1640 medium supplemented with B27 minus Insulin), containing 7 µM CHIR99021 for 24 h, followed by media change to RPMI for another 24 h. Add basic RPMI medium containing 5 µM IWR-1-endo for another 48 h to start the differentiation process towards the cardiac lineage. After 48 h, change the medium back to basic RPMI.
- On day 7 of differentiation, culture cells in a basic RPMI medium supplemented with 1:1,000 (v/v) human insulin. Refresh the media every 2 days or every day with a 50% medium change if oxygen consumption reaches more than 30%.
- On day 15, enzymatically dissociate the bioreactor cells for 3 h using a Collagenase II solution containing HBSS, Collagenase II (200 units/mL), HEPES (10 mM), ROCK inhibitor Y-27632 (10 µM), and N-benzyl-p-toluenesulfonamide (BTS, 30 µM). To do this, first wash the differentiated hiPSC-CM EBs 2x with prewarmed HBSS and incubate them in Collagenase II solution (200 µl volume of EBs / 12 ml of Collagenase II solution) at 37 °C in 5% CO2 until single cells appear from full EB dissociation.
- Stop the dissociation reaction by adding an equal volume of blocking buffer (RPMI-1640 without B27 and DNase II, with 6 µL of DNase II per mL of RPMI-1640) to the single-cell suspension. Count the differentiated hiPSC-CMs, centrifuge (200 × g, 5 min), and prepare for downstream applications by freezing the cells at -80 °C in isopropanol for 24 h and moving them to liquid nitrogen tanks till seeding is performed.
2. Engineered laminar tissue fabrication
- Adhere two layers of laboratory tape, one white and one blue, to a 1 mm-thick clear, scratch- and UV-resistant acrylic sheet. Using a CO2 laser engraver, cut the acrylic sheet in circles (20 mm diameter to fit the 12 MW) and the chip pattern (3 x 7.5 mm squares, three per chip) on the tape as designed in vector graphics software (e.g., CorelDraw); acrylic cutting parameters: 100 power, 20 speed, and 1,000 PPI; tape cutting parameters: 8 power, 6 speed, and 1,000 PPI. Remove the two layers of tape inside the innermost line using tweezers, soak the chips in pure bleach for 30 min to 1 h to remove thick lines and dark spots from cutting, leaving a sharp line, and rinse the chips in a beaker with running deionized (DI) water overnight or for at least 3 h.
NOTE: Do not bleach for longer than 2 h, as this will etch the adhesives on the sidewalls, preventing gelatin from adhering properly. - Sonicate the chips for 10 min and the polydimethylsiloxane (PDMS, Sylgard 184) stamps with line groove features (25 µm ridge width, 4 µm groove width, and 5 µm groove depth) for 30 min in clean 70% ethanol. Transfer the chips and stamps to a clean area under a hood and allow them to dry under airflow for ~1-2 h.
- Weigh 1 g of gelatin from porcine skin (gel strength ~175 g Bloom, Type A) into a 50 mL tube. Add 5 mL of phosphate-buffered saline (PBS) and mix thoroughly to ensure immediate dissolution of the gelatin. Place the tube in a 65 °C water bath for 30 minutes to complete the dissolution process. Similarly, weigh 1 g of microbial transglutaminase (MTG) into a separate 50 mL tube. Add 12.5 mL of PBS, mix thoroughly, and place the tube in a 37 °C water bath for 30 minutes to ensure full dissolution of the MTG.
- Sonicate the gelatin tube for 15 min, then return it to the 65 °C water bath before use. Place the MTG tube in a desiccator with the cap slightly loosened and turn on the vacuum slowly. Observe the bubbles being removed from the MTG solution under vacuum. After degassing, return the MTG tube to the 37 °C water bath.
NOTE: Monitor the process and adjust the vacuum to avoid vigorous boiling. - Cover a grid sheet with clean parafilm, place the chips on the grid, and keep the stamp nearby, ready for use. Add 5 mL of MTG to the 5 mL of gelatin solution, pipetting carefully to avoid bubbles; then, quickly pipette the gelatin onto the chip, using enough to cover the chip area (approximately 0.5 mL each). Then, place the line-patterned PDMS22 (line width 25 µm, height 5 µm, spacing 4 µm) stamp on top and apply a 200 g weight to ensure the gelatin is patterned parallel to the tissue's longitudinal axis. Once all chips are molded, cover them with a glass jar to avoid environmental disturbance and let them crosslink overnight.
NOTE: Use the mixed solution gelatine-MTG within 5 min; otherwise, the target pattern will be altered due to a higher crosslinked state of the gelatine. - Transfer the chip and PDMS stamp sandwich to a new P150 dish filled with PBS and hydrate the gelatin for 30 min to 1 h to facilitate the separation of the PDMS stamp from the chip. Remove any excess unmolded gelatin around the chip and transfer the clean chip to a new P150 dish filled with PBS. Store the PDMS stamps in 70% ethanol.
- Sterilize the chips by soaking them in ethanol for 10 min under the hood.
NOTE: Do not exceed 10 min in ethanol, as longer exposure may deform the gelatin. - Transfer the chips to PBS, soak for 10 min, and rinse 3x. Prepare coating solutions by mixing 20 µg/mL fibronectin with 1:100 diluted basement membrane matrix in culturing media (approximately 0.5 mL per well). Coat the chips for 2 h in the incubator at 37 °C and 5% CO2 or at 4 °C overnight.
- Thaw and seed cells (8 × 105 hiPSC-CMs per cm2) in RPMI medium with Y-27632 (10 µM), then replace it with RPMI without Y-27632 after 24 hours.
- Three days after cell seeding, remove the white tape using tweezers.
3. Synthesis and application of the phototransducer
NOTE: Ziapin2 was synthesized according to a previously published procedure16,18 and was administered to hiPSC-CMs directly in the culturing medium.
- Add 25 µM of Ziapin2 to the cell culture and incubate at 37 °C and 5% CO2 for 7 min.
- Gently wash out excess molecules and rinse with fresh culturing medium.
4. Viability assay
NOTE: Alamar Blue is a resazurin-based assay that can permeate cells and act as a redox indicator to monitor cell viability. Resazurin dissolves in physiological buffers, resulting in a deep blue solution that is added directly to cells in culture. Viable cells with active metabolism reduce resazurin to resofurin, which is pink and fluorescent.
- Plate cells in a 96-well plate previously coated with 20 µg/mL fibronectin and 1:100 diluted basement membrane matrix in culture media. Mix by shaking and then aseptically add Alamar Blue to each well in an amount equal to 10% of the well volume.
- Incubate cultures with Alamar Blue for 4 h.
- Treat cells with the phototransducer and vehicle (DMSO) as previously described. Apply light stimulation (1 Hz pulsed light for 1 min) through an optical fiber (cyan, 470 nm) to assess the effect of Ziapin2 internalization and light exposure on hiPSC-CM viability.
- Read fluorescence with a plate reader at excitation 560 nm and emission 590 nm.
5. Assessment of engineered laminar cardiac tissue anisotropy
NOTE: This protocol outlines a systematic approach for assessing the anisotropy of engineered laminar cardiac tissue using immunostaining, confocal microscopy, and nuclei analysis27.
- Wash the substrate with PBS several times before fixation with 4% (v/v) paraformaldehyde containing 0.05% (v/v) Triton X-100 in PBS for 10 min.
- Block non-specific binding by incubating samples with 5% (w/v) bovine serum albumin (BSA) in PBS for 30 min.
- Incubate samples with Hoechst (1:500) at room temperature for 1 h.
- Wash with PBS before mounting onto microscope slides with an anti-fade agent. Allow slides to dry overnight and store at 4 °C until imaging.
- Image samples using a spinning disk confocal microscope at 20x magnification. Determine the orientation of the cells using OrientationJ Measure28, a FiJi plugin.
6. Optical mapping recordings
NOTE: Optical mapping was performed after 5 days in culture on hiPSC-CMs seeded on gelatin-molded tissue chips.
- To follow this protocol, ensure that the optical mapping apparatus consists of a modified tandem-lens microscope equipped with a high-speed camera and the excitation light source, a 200 mW Mercury lamp. Place a dichroic mirror in front of the designated Ca2+ imaging camera to ensure that the preparation is exposed to excitation light and to collect the emitted fluorescence coming from the sample.
- Incubate the sample with 2 µM X-Rhod 1 added to the culturing medium for 30 min at 37 °C.
NOTE: Expose the sample to light only during image acquisition to avoid photobleaching of the dye. - Incubate the phototrasducer as previously described in section 3.
- Wash with fresh culturing medium and transfer the chips to phenol red-free RPMI 1640 medium supplemented with B27 minus Insulin.
- Place the tissue chips in a temperature-controlled dish and start recordings at physiological temperature (37 °C), acquiring images at a frame rate of 2.5 frames/s.
- For optical pacing, apply the optical point stimulation at one end of the tissue using an LED light source (465/25 nm) allowing Ziapin2 stimulation. Pace the tissues at 0.5 or 1 Hz frequency via a temporally regulated optical fiber positioned 1 mm away from the tissue. For electrical pacing, apply a point stimulation using a bipolar platinum electrode positioned at the center of the most distal end of the rectangular tissue. This placement ensures that Ca2+ wave propagation originates from the midpoint of the tissue's width, which corresponds to the shorter side of the rectangle. The stimulation should be applied at a frequency of 0.5 Hz, with an amplitude of 10 V and a pulse duration of 100 ms.
- After the recordings, apply a spatial filter with a 3 x 3 pixel size to improve the signal-to-noise ratio.
- Determine the Ca2+ wave conduction velocity at each pixel for every pulse, calculating the rate of change along both the x- and y-directions. To do this, measure the spatial gradient of the Ca2+ signal intensity in these directions and relate it to the time delay of the signal's propagation. By combining these directional change rates, quantify how quickly the wave travels across the cellular field in both dimensions.
7. Data export and handling
- Extract and analyze the data with the referenced software.
- Apply a spatial filter of 3 x 3 pixels to improve the signal-to-noise ratio.
- Extract the Ca2+ transients (CaT) traces as the mean of a specific manually selected region of interest (ROI).
- Measure the CaT parameters in a central region of interest (ROI) of 50 x 50 pixels (≈ 25 mm2).
- For each CaT, analyze CaT amplitude, rise time (tpeak), time of maximum decay slope (Max Decay Slope), and time to 90% transient decay (Decay Time90).
8. Statistical analysis
- Assess the normality of the distribution using an appropriate statistical test, such as a normality test, to determine whether parametric or non-parametric methods should be applied.
- Evaluate statistical significance between two conditions using the Student's t-test or Mann-Whitney U-test for continuous or categorical data, respectively. When comparing more than two groups, use one-way ANOVA or the Kruskal-Wallis test depending on data assumptions, followed by appropriate post-hoc tests to identify significant pairwise differences.
Results
A multistep process was developed and implemented for the fabrication of engineered laminar cardiac tissue using a combination of laser patterning, gelatin molding, and cell seeding techniques. Originally established by McCain et al.22 and Lee et al.24, this technique was re-implemented, following their protocols to construct the engineered laminar microtissues. The process integrates precise laser-based patterning for structural guidance, gelatin as a scaffold material, and controlled cell seeding to create a biomimetic tissue environment. (Figure 1).
The deposition of paper substrates involved adhering two layers of laboratory labeling tape, one white and one blue, to a 1 mm-thick acrylic substrate, providing a robust base for subsequent steps and enabling precise visualization and manipulation during the fabrication process (Figure 1A). Laser patterning was employed to achieve the desired chip geometry on the substrates, with optimized parameters ensuring precise ablation without damaging the acrylic base (Figure 1B). The selective removal of tape layers exposed specific regions of the substrate for further processing. A gelatin solution, prepared from porcine skin and crosslinked with microbial transglutaminase, was then deposited onto the patterned acrylic surface to create a biocompatible layer suitable for cell adhesion (Figure 1C). This gelatin layer was molded using a PDMS stamp featuring line groove patterns, ensuring alignment with the tissue's longitudinal axis, and crosslinked overnight to produce a stable, patterned substrate (Figure 1D and Supplemental Figure S1).
Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were seeded onto the pre-coated substrate at a density of approximately 8 × 105 cells/cm2 (Figure 1E). Culturing conditions were optimized with daily media refreshment to support cell growth. After 3 days, the white tape layer was removed to expose the patterned gelatin surface (Figure 1F), ensuring precise confinement of cells to specific substrate regions, facilitating the development of bioengineered laminar tissue with desired geometric configurations. The viability and alignment of the hiPSC-CMs in the bioengineered tissue were subsequently evaluated.
The impact of Ziapin2 internalization followed by light exposure on cell viability was assessed using the AlamarBlue Assay. The results reported in Figure 2A indicated that Ziapin2, when internalized at a concentration of 25 µM and followed by 470 nm light stimulation, did not adversely affect cell viability, confirming the compatibility of this phototransducer approach with living cells.
Anisotropy within the bioengineered laminar tissue was achieved as intended, with fluorescence imaging showing significant cellular alignment along the patterned grooves of the micromolded gelatin substrate (Figure 2B). The cell orientation, evident from the fluorescence images was quantitatively assessed employing the FiJi plugin OrientationJ28. The orientation was evaluated by processing the Hoechst fluorescence images resulting in a mean angle equal to θmean = 13.39 ± 2.67°. The angle indicates the orientation normalized to the pattern direction, showing a high degree of anisotropy in the tissue. The measured angular distribution, as well as the low measured value of the standard deviation, suggests a strong directional preference in the tissue structure (Figure 2C). This demonstrated the formation of anisotropic cardiac tissue, which is critical for mimicking the natural architecture necessary for proper functional performance, such as coordinated contraction and electrical conduction.
After 5 days in culture, optical mapping was performed on Ziapin2-loaded hiPSC-CMs seeded on gelatin-molded tissue chips (Figure 3). The optical mapping provided clear and detailed imaging of Ca2+ dynamics within the engineered cardiac tissue, facilitated by a high-speed camera and precise light stimulation. Ziapin2 stimulation was effectively achieved using an LED light source, and the tissue samples responded well to pacing at both 0.5 Hz (Figure 4A) and 1 Hz (Figure 4B) frequencies.
Photostimulation at these frequencies induced propagating Ca2+ waves across the tissue and longitudinal conduction velocities were calculated, showing uniform propagation of electrical signals throughout the tissue at both stimulation frequencies and resulting equal to 4.5 ± 0.5 cm/s (Figure 4A,B).
Finally, we compared our methodology with the gold standard reference of electrical stimulation. A detailed analysis of Ca2+ transients (CaTs), as illustrated in Figure 5, revealed that key parameters, including CaT amplitude (Figure 5A), rise time (Figure 5B), maximum decay slope (Figure 5C), and decay time (Figure 5D), were similar between the two stimulation cues. Overall, the optical mapping approach provided high-resolution data on the physiological properties of the bioengineered laminar cardiac tissue, demonstrating the effectiveness of Ziapin2 in modulating its behavior through light stimulation.

Figure 1: Sketch of the laminar cardiac tissue preparation. (A) Deposition of two paper tapes on an acrylic substrate. (B) Patterning of both the paper layers by laser ablation. (C) Deposition of the gelatin on the patterned substrate. (D) Molding and sintering of the gelatin layer by a line-patterned PDMS stamp. (E) Cell seeding on the prepared substrate. (F) Removal of the first paper layer achieving the required geometry. Abbreviation: PDMS = polydimethylsiloxane. Please click here to view a larger version of this figure.

Figure 2: Cell viability and alignment in the bioengineered laminar cardiac tissue. (A) Effect of Ziapin2 internalization and light exposure on hiPSC-CMs viability measured with the Alamar Blue Assay. Cell viability of Ctrl in the dark was set to 100%. Data are represented as mean ± standard error of the mean (SEM) n = 16 per each condition. (B) Anisotropic bioengineered laminar tissue on micromolded gelatin. Fluorescence images of anisotropic hiPSC tissue; scale bar = 100 µm. Cells are stained with Hoechst (blue).(C) Average orientation (normalized to pattern direction) of the cells seeded on gelatine substrates (N = 45 ROI coming from 4 different samples, average angle of the distribution, θmean = 13.39° ± 2.67°). Abbreviations: hiPSC-CM = human-induced pluripotent stem cell-derived cardiomyocyte; ROI = region of interest. Please click here to view a larger version of this figure.
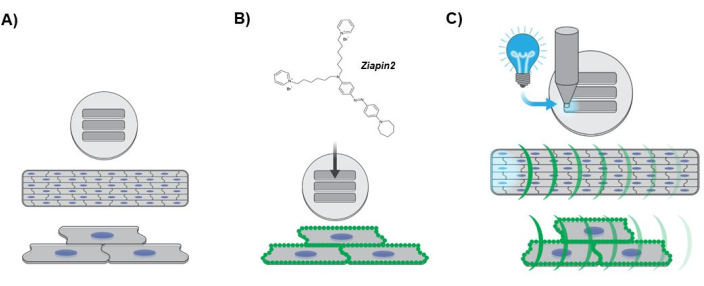
Figure 3: Diagram depicting the experimental protocol. (A) Anisotropic cardiac tissue formation. hiPSC-CMs nuclei are depicted in blue. (B) Phototransducer internalization into the engineered laminar tissue. The green dots along the sarcolemma indicates Ziapin2 membrane partitioning. (C) Photostimulation protocol and Ca2+ dynamics optical mapping. The cyan light indicates the optical point stimulation applied at one end of the tissue using an LED light source. The green curvatures represent the propagation of the Ca2+ wave along the engineered laminar tissue. Abbreviations: hiPSC-CMs = human-induced pluripotent stem cell-derived cardiomyocytes; LED = light-emitting diode. Please click here to view a larger version of this figure.
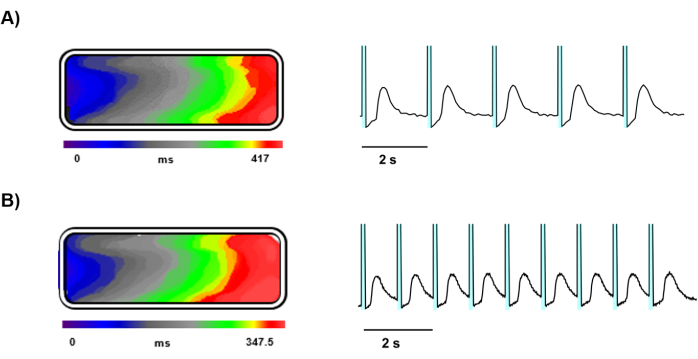
Figure 4: Representative recordings of light-triggered Ca2+ waves. Time course representing the Ca2+ wavefront propagation, isochrone map and Ca2+ signal traces of a light stimulated tissue at (A) 0.5 Hz and (B) 1 Hz. In the representative traces the cyan shaded area before each Ca2+ transient represents the photoexcitation. Please click here to view a larger version of this figure.
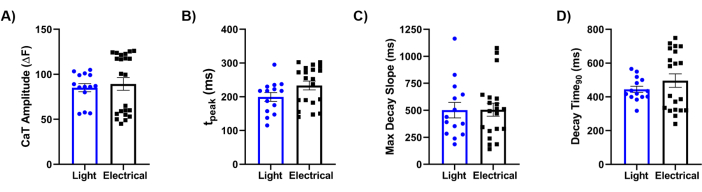
Figure 5: Comparison between electrical and optical stimulation. Quantification of light-induced and electrically evoked Ca2+ transient (CaT) parameters recorded on chips seeded with hiPSC-CMs at 0.5 Hz. The CaT features analyzed were (A) CaT amplitude; (B) rise time (tpeak); (C) time of maximum decay slope (Max Decay Slope); and (D) time to 90% transient decay (Decay Time90). Paired measurements were carried out at 37 °C for each condition. Light stimulation N = 5 chips, n = 14 tissues; electrical stimulation N = 8 chips, n = 22 tissues. Data are represented as mean ± standard error of the mean. Abbreviations: CaT = Ca2+ transient; hiPSC-CMs = human-induced pluripotent stem cell-derived cardiomyocytes. Please click here to view a larger version of this figure.
Supplemental Figure S1: Micropatterning evaluation on PDMS and gelatine substrates. (A) Optical image of a pattern realized on a PDMS stamp; scale bar =100 µm. (B) Optical image of a micromolded gelatine substrate; scale bar =100 µm. (C) Pattern profile of a PDMS stamp (black line) and micromolded gelatine film (red line). Please click here to download this File.
Discussion
This approach provides a robust platform for advancing cardiac research, providing insights into the complex dynamics of cardiac tissue opening up new possibilities for long term in vitro cardiac mechanistic studies that could potentially lead to new therapeutic strategies. To ensure the success of this methodology, it is crucial to reproduce a microphysiological environment that closely mimics in vivo conditions of the human heart. Therefore, careful attention must be given to designing and aligning the tissue to replicate the natural anisotropy of cardiac architecture, which is vital for proper electrical conduction and contractile function. Critical factors include the precise laser cutting of the chip pattern, the timing of bleach treatment, and the control of gelatin solution viscosity, all of which are necessary for creating uniform tissue molds. Additionally, accurately coating the chips with fibronectin and GelTrex (the reduced growth factor basement membrane matrix) is essential for successful cell attachment and subsequent tissue formation.
Furthermore, the incubation time and concentration of the photoactuator (i.e., Ziapin216,18) must be carefully controlled to ensure adequate internalization. Thorough washing of excess molecules is essential to prevent non-specific effects. It is always recommended to perform viability assays to evaluate whether the molecule has any detrimental effects on the cells. If internalization of the photoactuator is inadequate, consider extending the incubation time or using a higher concentration.
For the functional experiments, the proper setup of the optical mapping apparatus, including the precise positioning of the optical fiber and the calibration of the stimulation frequency, is crucial for capturing high-quality data. If recordings show high noise levels, refine the spatial filter size or check for any misalignment in the optical setup.
The approach methodology presents several significant advantages; the development and application of aligned hiPSC-CMs represent a notable improvement in replicating native cardiac tissue characteristics. By cell alignment, researchers can closely mimic the anisotropic structure of native cardiac tissue, which is essential for maintaining proper electrical and mechanical function. This ensures that the cardiomyocytes interact in a manner that closely resembles in vivo conditions, thereby exhibiting physiological behaviors that are more representative of the native heart.
Unlike electrical stimulation, which requires wiring and the presence of electrode into the electrolyte, light-based modulation offers a non-invasive and precise method to control the electrical properties of specific regions. The ability to adjust light in terms of intensity, wavelength, and timing facilitates targeted interventions without disrupting adjacent areas. Furthermore, contrary to optogenetics, which necessitates the genetic modification of cells to express light-sensitive channels or proteins, does not require viral-gene transfer. These aspects are crucial for applications where genetic modification is not feasible or desired. Furthermore, the photostimulating ability of materials disentangles the cell or procedure specificity making this stimulation approach easily transferable to different cell types.
Dynamic and real-time control over cardiac tissue is another advantage of this methodology. The use of light enables real-time modulation of the electrical properties, providing a powerful tool for investigating how different stimuli affect cardiac function. Integration with Ziapin2 allows for direct conversion of light into electrical signals, enabling precise control over action potentials and rhythm in hiPSC-CMs.
This approach also holds promise for advancing research applications, laying the groundwork for creating accurate disease models, particularly for cardiac arrhythmias and other electrical disorders, allowing researchers to study cellular responses to various stimuli or drugs in a controlled setting. Moreover, it offers a robust platform for drug screening and toxicity testing, providing a more human-relevant context compared to traditional animal models.
In this regard, ethical considerations are also addressed, as this technology reduces the reliance on animal models, aligning with the principles of the 3Rs (Replacement, Reduction, and Refinement). By providing a more ethical and potentially predictive model for human cardiac responses, it supports a shift towards more humane research practices.
The method's scalability and customization further enhance its utility. Researchers can tailor tissue constructs to specific research needs, adjusting factors such as cell type, alignment, and phototransducer placement. Additionally, the methodology could be potentially scaled for larger experiments or high-throughput studies, making it a versatile tool for both basic research and preclinical applications.
Finally, the potential for personalized medicine is significant. By deriving hiPSC-CMs from individual patients, researchers can create patient-specific cardiac tissue models, enabling the study of personalized responses to drugs or interventions. This paves the way for tailored treatment approaches in cardiology, offering new avenues for therapeutic development.
Disclosures
CB, GL, and FL are inventors of “PHOTOCHROMIC COMPOUNDS" Patent No. EP 3802491 (02/07/2020).
Acknowledgements
The authors gratefully thank Michael Rosnach for the illustrations in Figure 1 and Figure 3, and Prof. William T. Pu for hiPSC supply. This work was supported by the NCATS Tissue Chips Consortium (UH3 TR003279) to KKP, the Italian Ministry of Universities and Research through the PRIN 2022 project (ID 2022-NAZ-0595) to FL, the PRIN 2020 project (ID 2020XBFEMS) to CB and GL, and the Fondo Italiano per la Scienza project (ID FIS00001244) to GL.
Materials
| Name | Company | Catalog Number | Comments |
| alamarBlue Cell Viability Reagent | Thermo Fisher Scientific | DAL1025 | Cell Viability Assay |
| B-27 Supplement, minus insulin | Thermo Fisher Scientific | A1895601 | For cell culture |
| Bovine Serum Albumin | Sigma-Aldrich | A9056-50G | For cell staining |
| BrainVision Analyzer software | Brain Products | https://www.brainproducts.com/downloads/analyzer/ | Data export and handling |
| BTS | Sigma | 203895-5MG | |
| CHIR99021 | Stem Cell Technologies | 72054 | |
| Clear Scratch- and UV-Resistant Acrylic Sheet, 12" x 12" x 0.01 inch | McMaster Carr | 4076N11 | Tissue chip fabrication |
| Collagenase Type II | Worthington | CLS-2 / LS004176 | |
| DNase II | VWR | 89346-540 | |
| Essential 8 Medium | Thermo Fisher Scientific | A1517001 | For cell culture |
| Fibronectin | VWR | 47743-654 | Coating |
| Gelatin from porcine skin gel strength 175 Type A | Sigma-Aldrich | G2625-100G | Tissue chip fabrication |
| Geltrex LDEV-Free, hESC-Qualified, Reduced Growth Factor Basement Membrane Matrix | Thermo Fisher Scientific | A1413302 | Coating |
| HBSS | Thermo Fisher | 14175-095 | |
| HEPES (1 M) | Thermo Fisher Scientific | 15630080 | |
| Hoechst 33342 | Life technologies | H1399 | For cell staining |
| Insulin solution human | Sigma Aldrich | I9278-5ML | |
| IWR-1-endo | Stem Cell Technologies | 72564 | |
| Paraformadehyde 16% Aqueous Solution (PFA) | VWR | 100503-917 | For cell staining |
| PBS, sterile, 500 mL | Thermo Fisher Scientific | 10010049 | Tissue chip fabrication |
| phosphate buffered saline | Thermo Fisher Scientific | 10010049 | |
| Pluronic F-127 (20% Solution in DMSO) | Thermo Fisher Scientific | P3000MP | Non-ionic surfactant |
| ROCK inhibitor Y-27632 | Stem Cell Technologies | 72304 | |
| RPMI 1640 Medium, GlutaMAX Supplement | Thermo Fisher Scientific | 61870127 | For cell culture |
| RPMI 1640 Medium, no phenol red | Thermo Fisher Scientific | 11835030 | Optical mapping |
| Versene Solution | Thermo Fisher Scientific | 15040066 | chelating agent |
| VWR General-Purpose Laboratory Labeling Tape | VWR | 89098-058 | Tissue chip fabrication |
| X-Rhod-1 AM | Thermo Fisher Scientific | X14210 | Optical mapping |
References
- Di Maria, F., Lodola, F., Zucchetti, E., Benfenati, F., Lanzani, G. The evolution of artificial light actuators in living systems: from planar to nanostructured interfaces. Chem Soc Rev. 47 (13), 4757-4780 (2018).
- Manfredi, G., et al. The physics of plasma membrane photostimulation. APL Mater. 9 (3), 030901 (2021).
- Bareket-Keren, L., Hanein, Y. Novel interfaces for light directed neuronal stimulation: advances and challenges. Int J Nanomed. 9 Suppl 1 (Suppl 1), 65-83 (2014).
- Ford, S. M., Watanabe, M., Jenkins, M. W. A review of optical pacing with infrared light. J. Neural Eng. 15 (1), 011001 (2018).
- Antognazza, M. R., et al. Shedding light on living cells. Adv Mater. 27 (46), 7662-7669 (2015).
- Zhang, J., Wang, J., Tian, H. Taking orders from light: progress in photochromic bio-materials. Mater Horiz. 1 (2), 169-184 (2014).
- Deisseroth, K. Optogenetics. Nat Methods. 8 (1), 26-29 (2011).
- Ambrosi, C. M., Entcheva, E. Optogenetics' promise: pacing and cardioversion by light. Future Cardiol. 10 (1), 1-4 (2014).
- Hopkins, J., et al. Photoactive organic substrates for cell stimulation: Progress and perspectives. Adv Mater Technol. 4 (5), 1800744 (2019).
- Vurro, V., Venturino, I., Lanzani, G. A perspective on the use of light as a driving element for bio-hybrid actuation. Appl Phys Lett. 120 (8), 080502 (2022).
- Bruno, G., et al. All-optical and label-free stimulation of action potentials in neurons and cardiomyocytes by plasmonic porous metamaterials. Adv Sci. 8 (21), 2100627 (2021).
- Ronchi, C., et al. Nongenetic optical modulation of pluripotent stem cells derived cardiomyocytes function in the red spectral range. Adv Sci. 11 (3), 2304303 (2023).
- Li, P., et al. Monolithic silicon for high spatiotemporal translational photostimulation. Nature. 626 (8001), 990-998 (2024).
- Jiang, Y., et al. Nongenetic optical neuromodulation with silicon-based materials. Nat Protoc. 14 (5), 1339-1376 (2019).
- Rotenberg, M. Y., et al. Living myofibroblast-silicon composites for probing electrical coupling in cardiac systems. Proc Natl Acad Sci USA. 116 (45), 22531-22539 (2019).
- DiFrancesco, M. L., et al. Neuronal firing modulation by a membrane-targeted photoswitch. Nat Nanotechnol. 15 (4), 296-306 (2020).
- Paternò, G. M., et al. Membrane environment enables ultrafast isomerization of amphiphilic azobenzene. Adv Sci. 7 (8), 1903241 (2020).
- Vurro, V., et al. Molecular design of amphiphilic plasma membrane-targeted azobenzenes for nongenetic optical stimulation. Front Mater. 7, 631567 (2021).
- Vurro, V., et al. Optical modulation of excitation-contraction coupling in human-induced pluripotent stem cell-derived cardiomyocytes. iScience. 26 (3), 106121 (2023).
- Vurro, V., et al. Light-triggered cardiac microphysiological model. APL Bioeng. 7 (2), 026108 (2023).
- Florindi, C., et al. Role of stretch-activated channels in light-generated action potentials mediated by an intramembrane molecular photoswitch. J. Transl. Med. 22 (1), 1068 (2024).
- McCain, M. L., Agarwal, A., Nesmith, H. W., Nesmith, A. P., Parker, K. K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 35 (21), 5462-5471 (2014).
- Park, S. -. J., et al. Insights into the pathogenesis of catecholaminergic polymorphic ventricular tachycardia from engineered human heart tissue. Circulation. 140 (5), 390-404 (2019).
- Lee, K. Y., et al. An autonomously swimming biohybrid fish designed with human cardiac biophysics. Science. 375 (6581), 639-647 (2022).
- Lian, X., et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 8 (1), 162-175 (2013).
- Prondzynski, M., et al. Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems. Nat Commun. 15 (1), 5929 (2024).
- Pasqualini, F. S., Sheehy, S. P., Agarwal, A., Aratyn-Schaus, Y., Parker, K. K. Structural phenotyping of stem cell-derived cardiomyocytes. Stem Cell Rep. 4 (3), 340-347 (2015).
- Fonck, E., et al. Effect of aging on elastin functionality in human cerebral arteries. Stroke. 40 (7), 2552-2556 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved