Method Article
Establishing a Swine Model to Study Uterus Dynamic Preservation and Transplantation
In This Article
Summary
A detailed and reproducible swine uterus model is described, from surgical procurement to the initiation of machine perfusion, allowing for the study of uterus preservation in transplantation.
Abstract
To date, uterus transplantation is the only option for women with absolute uterine infertility, such as those with Rokitansky syndrome, to experience pregnancy and give birth. Despite the growing interest in uterus transplantation in recent years, several issues still require further research, including ischemia-reperfusion injury and its impact on graft quality and rejection. Recent literature has highlighted a thrombotic complication rate of up to 20% following uterus transplantation. This type of complication may result from hypoxia-induced endothelial cell damage, often leading to uterine graft rejection. Hypoxia is induced during static cold storage, which remains the gold standard for graft preservation in solid organ transplantation. Recently, dynamic preservation using machine perfusion has been shown to improve the long-term storage of conventional and marginal organs by reducing ischemic and hypoxic injury. In this protocol, we aim to describe every surgical step involved in porcine uterus procurement and dynamic preservation, based on both uterine pedicles, to enable the connection and initiation of the machine perfusion protocol.
Introduction
Uterus transplantation (UTx) has significantly developed over the last ten years, with several teams starting clinical research programs. To date, the main indication of UTx is absolute uterine infertility due to uterine agenesis, including the Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. MRKH syndrome is a congenital disorder with a prevalence of one in 5,000 female live births1. UTx could potentially address additional causes of infertility, including those resulting from hysterectomy due to malignant disease, postpartum hemorrhage, uterine fibroids, infectious sequelae, and various congenital malformations. This suggests that approximately 1 in 500 women may be eligible for UTx.
The first-ever clinical UTx occurred in 2000 in Saudi Arabia2, but vascular complications led to a hysterectomy three months later. Since then, several cases of UTx have been performed, based on both living and deceased donors, resulting in more than 80 live births3,4. Similar to the realm of solid organ transplantation and vascularized composite allotransplants (VCA), immune rejection is a significant challenge in UTx.5 Several factors can lead to graft rejection, including microcirculatory failure and venous stasis, both of which can lead to thrombotic complication. In a recent review studying uterine vascularization in transplantation, Kristek et al. reported up to 15% arterial thrombosis and 5% venous thrombosis6. Additionally, cold and warm ischemia are critical factors that must be addressed for successful transplantation, as ischemia-reperfusion injury (IRI) can lead to graft dysfunction and acute rejection7,8. Myocytes respond to ischemic stress by producing lactate for up to 6 h9, after which muscle cell damage is irreversible. The impact of cold ischemia on the myometrium has been documented in clinical studies, and the use of intracellular-like University of Wisconsin solution during static cold storage (SCS) has been shown to improve preservation with better contractile response to prostaglandin and higher ATP concentrations when compared to Ringer's acetate solution10. However, the impact of warm and cold ischemia remains poorly explored in UTx.
SCS remains the gold standard for VCA preservation, including the uterus, and for most solid organ transplants. However, in recent years, significant advancements in machine perfusion systems and preservation solutions have led to a paradigm shift. There is now strong evidence supporting that dynamic machine perfusion can improve and prolong the preservation of healthy and marginal solid organs11,12,13,14,15. This technique is now commonly used in clinical practice for lung, heart, liver, and kidney transplantation14,16,17,18. Dynamic organ preservation demonstrated multiple benefits, including minimizing cold ischemia and hypoxia injuries by providing continuous oxygen and nutrient supply, clearing toxic metabolites, and improving graft quality and viability parameters12,19. Multiple modalities have been developed, ranging from hypothermic to normothermic machine perfusion (with or without oxygen carriers), with several perfusates available, but only a few have been tested on the uterus20. To ensure the substantial contribution of such research perspectives, relevant preclinical surgical models are of crucial importance.
In this work, subnormothermic machine perfusion (SNMP) is used as an oxygenated dynamic organ preservation method at room temperature (around 20 °C) by circulating a perfusate through a roller pump and an oxygenator. A porcine model is employed that is relevant for studies on UTx and preservation due to its similarities with the human reproductive system in terms of anatomy, physiology, and vessel size21,22. The uterus is procured following circulatory death, providing relevance for donation after cardiac death and suggesting the possibility for a procurement delay after all other relevant solid organs23,24. In addition, this model facilitates the development of uterus preservation studies within established transplant laboratories focusing on other organs, applying the "3Rs" principles25. The aim is to establish a new preservation model based on uterine pedicles and assess its reliability for dynamic preservation. All the procedure steps are detailed, from the hysterectomy to the preservation, encompassing highlighted key points on using SNMP.
The protocol described below preceded a preliminary experiment based on a single pump and a "Y-tubing" inflow system for both uterine arteries (Supplementary Figure 1). After 4 h-SNMP, the organ gained over 50% of its initial weight. Flow, pressure, resistance, and weight variation are shown in Supplementary Figure 2. A single perfusion system separated into two inflows did not allow modulation of each flow rate to each side's pressure. In this case, SNMP led to substantial edema in half of the organ (Supplementary Figure 3). This system proved unsuitable for the uterine model, partly because it should not be considered a perfectly symmetrical model. Therefore, two systems of machine perfusion were used in this protocol, one for each uterine artery.
Protocol
All animals received humane care following the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC). Overall, 6 female Yucatan minipigs weighing 30-40 kg were used for uterus procurement, with four uteri undergoing SNMP. All animals were heparinized with one full dose (100 IU/kg) before euthanasia. Organ procurement occurred post-mortem with less than 60 min of warm ischemia. Other organs could have been harvested from the same donor for different studies, according to the "3Rs" principles25. See the Table of Materials for details about all reagents and equipment used in the protocol.
1. Preoperative preparation (day before the surgery)
- Prepare the perfusate solution. For subnormothermic machine perfusion, a Steen+ solution optimized for VCA was used26,27. One liter of solution per uterus was used, and the composition is detailed in Table 1.
NOTE: A large quantity of sodium hydroxide is added to the perfusate with the aim of achieving a pH of around 7.5-7.6. This value is expressly high but necessary as the pH will tend to fall as the machine is circulated and oxygenated with a carbogen mixture (95% oxygen; 5% carbon dioxide). - Set up the machine perfusion system (Figure 1). Check for leaks and bubbles when perfusate is circulated.
2. Post-mortem uterus procurement
NOTE: To simulate donation after cardiac death and/or post-mortem procurement, the animal should be euthanized according to the local IACUC guidelines. Exsanguination should be preferred to intravenous Pentobarbital injection in order to avoid toxicity that could interfere with the study.
- Place the euthanized animal in the supine position. Scrub the abdominal area and place sterile drapes.
- Make a 10 cm median infra umbilical incision with a #20 blade.
- Open the subcutaneous tissue and aponeurosis with a monopolar electric scalpel.
NOTE: Attention must be paid to not damage intestines by opening the abdominal cavity. - Set aside the small intestine with a surgical gauze and expose the uterus.
NOTE: The uterine anatomy of the model used is shown in Figure 2A. - Proceed similarly for the left and right sides as follows:
- Identify the uterine vessels.
NOTE: The uterine vein is positioned laterally to the uterine artery (Figure 3). - Create an opening into the broad ligament laterally to the uterine vein with right-angle forceps.
- Through this opening, insert 2-0 silk tie sutures to ligate the ovarian vessels and release the uterus from the surrounding connective tissue in the broad ligament using cautery.
- Ligate the utero-ovarian vessels with 2-0 silk tie sutures and remove the ovary and tube.
- Skeletonize uterine vessels and divide them as close to the internal iliac vessels as possible.
NOTE: Attention must be paid to keeping the pedicle as long as possible to facilitate cannulation and anticipate pedicle retraction after it has been severed. - Repeat steps 2.5.1-2.5.5 on the opposite side.
- Identify the uterine vessels.
- Remove the uterus by cutting through the cervix with a monopolar electric scalpel.
NOTE: Use a long contact time to ensure proper cervix vessel coagulation, preventing leakage during perfusion.
3. Preparation for perfusion
- On a side table, dilate both uterine arteries using a microsurgical dilator and insert an angiocatheter. Secure the cannulation with 3-0 silk ties (Figure 2B).
NOTE: Here, an 18 G angiocatheter was used for all arteries. Care must be taken not to insert the catheter too far to avoid selective cannulation, as the bifurcation is relatively close. The uterine veins are not cannulated, as venous outflow is sufficient to keep the lumen of these vessels open, allowing easy collection. In addition to saving time, traumatic cannulation may lead to vascular damage and potentially affect venous flow. - Slowly manually flush both uterine arteries with 20 mL of heparin solution on each side until all the vessels are washed out and the outflows are clear.
NOTE: Attention must be paid not to flush with high pressure, which can lead to microvascular injuries and perfusion failure. - Weigh the uterus.
4. Subnormothermic machine perfusion
NOTE: For the uterus, two independent systems of machine perfusion are required. Each uterine artery is connected to a perfusion system composed of a roller pump, an oxygenator, a bubble trap, and a pressure sensor. The perfusate in a reservoir circulates through silicone tubes connected to the elements listed above before running through the organ via the uterine artery to the uterine vein on each side, where the perfusate exits and is released in the same reservoir.
- Connect the uterus to the machine perfusion systems by connecting the uterine artery cannulas into the inflow tubing (Figure 1).
- Using the roller pump, adjust the flow rate to low (2.5-4.0 mL/min) to maintain a constant arterial pressure between 25-35 mmHg.
- Assess viability parameters at each predefined time point in both inflow and outflow using a 1 mL syringe and analyzing samples with the blood gas system machine [e.g., blood gas metrics (pH, pCO2, pO2, lactate, base excess, bicarbonate), glucose, sodium, potassium, calcium, chloride].
NOTE: In this protocol, the perfusion lasts for 4 h, and samples from the inflow and the outflow are taken every 30 min. - Weigh the uterus at the end of perfusion.
Results
During perfusion, the system was connected to a pressure sensor that recorded the pressure during the experiment. The pressure was initially recorded for a uterus-free system, which was subtracted from pressure recordings during uterus perfusion to obtain the real organ pressure. The flow rate was adapted to maintain the pressure within the desired range and was controlled by the roller pump. The resistance was calculated using the formula R = P / Q (R: resistance (mmHg.mL.min-1); P: pressure (mmHg); Q: flow rate (mL/min)).
The evolution of perfusion parameters is shown in Figure 4. The average initial flow rate was 5.2 (min 2.4, max 10.0) mL/min on both sides and did not fluctuate much during the experiment. Based on literature and previous experiments, the target pressure was set at 25-35 mmHg. The average initial arterial pressure was 26 (min 10, max 36) mmHg on the left side of the uterus and 27 (min 16, max 39) mmHg on the right side. As flow and pressure were slightly stable and low, the resulting arterial resistance profile also showed a flat curve throughout the experiment. The mean initial vascular resistance was 8.5 (min 1, max 13.3) mmHg.mL.min-1 on both sides (Figure 4).
SNMP preservation was evaluated according to several metabolic parameters (Figure 5): lactate clearance (difference between lactate outflow and lactate inflow), weight change, the difference between the inflow glucose and the outflow glucose reflecting glucose consumption, and oxygen consumption. The oxygen consumption was calculated using the formula O2consumption = 0.0314 x Q x (pO2 inflow - pO2 outflow) / W (Q: flow rate (mL/min), pO2: partial oxygen pressure (mmHg), W: weight (g), and 0.0314 Henry's constant in water at 20 °C and 1 atm). The decrease in lactate levels during the first hour of perfusion indicates that the machine perfusion allowed lactate clearance following accumulation during the warm ischemic phase. The weight change showed that none of the uteri had edema. The curve highlighted that weight loss occurred mainly during the first two hours and that weight tended to stabilize afterward. The glucose difference between the inflow and the outflow decreased during the first hour of perfusion before stabilizing at low values. Oxygen consumption remained constant during perfusion.
After 4 h of SNMP, angiography was performed in each uterus, slowly flushing 20 mL of contrast agent in each uterine artery (Figure 6), which enabled assessment of the vascular tree after the preservation phase. In all organs, the microvasculature was well identified along the cervix, uterine body, and horns.
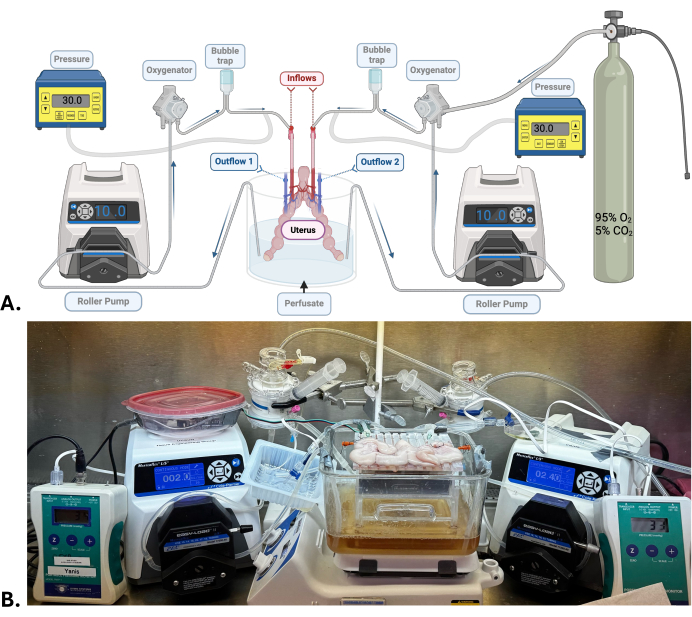
Figure 1: Surgical model and machine perfusion system. (A) Diagram of the machine perfusion system used for uterus SNMP. (B) Representative photograph of a porcine uterus undergoing SNMP. Please click here to view a larger version of this figure.

Figure 2: Diagram of human (left) and pig (right) uterine anatomy showing the main vascularization through the uterine artery. The anatomical characteristics of the porcine uterus are mainly due to the presence of a small body divided into two large uterine horns. Please click here to view a larger version of this figure.

Figure 3: Intra and post-operative views. (A) Intraoperative picture of the uterus after identifying uterine vessels. (B) Post-operative images of the uterus after procurement and heparin-saline flush. A: corpus; B: uterine horns; C: cervix; D: uterine arteries; E: uterine veins; F: tube; G: ovary. Please click here to view a larger version of this figure.
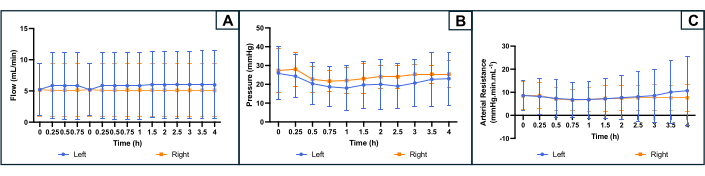
Figure 4: Profile of the perfusion parameters during 4 h machine perfusion on both uterine sides. (A) Flow rate (mL/min). (B) Pressure (mmHg). (C) Calculated Arterial Resistance (mmHg.min.mL-1). The curves indicate the average for each side of the uterus, and error bars indicate the standard deviation of the mean. Please click here to view a larger version of this figure.
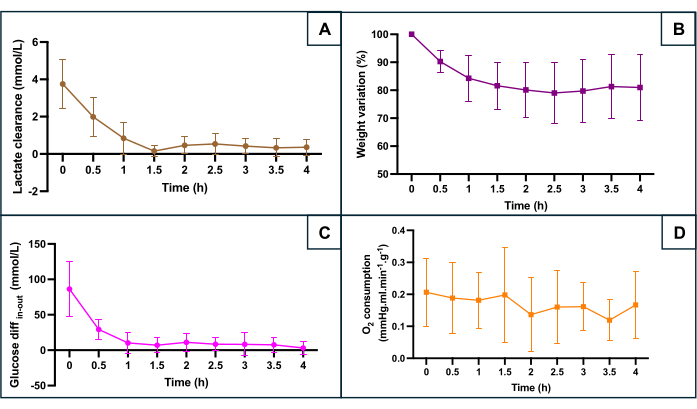
Figure 5: Monitoring of main hypoxia markers during machine perfusion. (A) Lactate clearance throughout the first 90 min of perfusion before stabilization. (B) Weight decreases throughout the perfusion. (C) Difference between inflow and outflow glucose concentration. (D) Calculated O2 consumption. The data represent the average for both sides of each uterus, and error bars show the standard deviation of the mean. Please click here to view a larger version of this figure.

Figure 6: Representative angiography of the uterine vascular tree after subnormothermic preservation. (A) Before injection of contrast agent. (B) After injection of contrast agent. Please click here to view a larger version of this figure.
| Component name | Quantity | Component name | Quantity |
| De-ionized water | 1L | Dextran low fraction (~75 kDa) | 5 g |
| NaCl | 5.026 g | Bovine Serum Albumin | 150 g |
| KCl | 0.343 g | Hydrocortisone [50 mg/mL] | 0.2 mL |
| CaCl2 . 2H20 | 0.221 g | Dexamethasone [1.6 g/100 mg] | 4 mL |
| NaH2PO4 | 0.187 g | Sodium hydroxide [1 mol/mL] | 16 mL |
| NaHCO3 | 1.26 g | Low molecular weight heparin | 200 IU |
| MgCl2 . 6H2O | 0.244 g | Insulin | 200 IU |
| D-Glucose | 4.3 g | Vancomycin | 1.5 g |
| PEG (35 kDa) | 5 g |
Table 1: Steen+ solution composition. NaCl: sodium chloride; KCl: potassium chloride; CaCl2. 2H20: calcium chloride dihydrate; NaH2PO4: sodium dihydrogen phosphate; NaHCO3: sodium bicarbonate; MgCl2 . 6H2O: magnesium chloride hexahydrate; PEG; polyethylene glycol.
Supplementary Figure 1: Single pump-based perfusion system. (A) Diagram of the machine perfusion "Y-system" used for the preliminary experiment. (B) Representative photograph of the set-up for the preliminary experiment. Please click here to download this File.
Supplementary Figure 2: Profile of perfusion parameters during 4.5h-SNMP for the preliminary experiment. (A) Flow rate (mL/min). (B) Pressure (mmHg). (C) Calculated Arterial Resistance (mmHg.min.mL-1). (D) Weight increasing throughout the perfusion. Please click here to download this File.
Supplementary Figure 3: Photograph of the uterus at the end of the preliminary experiment (4.5 h-SNMP). An asymmetrical distribution of edema is evident, mainly in the right horn (on the right of the image). Please click here to download this File.
Discussion
Uterus transplantation, often considered part of VCA, has rapidly developed in the last few years. In parallel, machine perfusion started to be explored in VCA since it demonstrated robust evidence in improving solid organ preservation. Hypothermic and subnormothermic machine perfusion has allowed up to 24 h preservation in swine models of myocutaneous and bone-containing VCA26,27,28. Since the uterus presents challenges comparable to bone- and skin-containing VCA, it is critical to explore similar techniques for UTx to enhance preservation quality and long-term outcomes29. Beyond improving organ quality, a potential benefit of dynamic preservation using machine perfusion is expanding the pool of donors30. Uterus retrieval from living donors imposes substantial risks, such as ureteral injury during the dissection of the uterine pedicle, but also hormonal disruption and all potential risks associated with early menopause due to the ovariectomy that may be performed31,32. The purpose of improving the quality of deceased donor organs would avoid the need for such surgery in living donor procurement procedures. The ultimate goal would be to enable uterus procurement in both living and deceased donors in order to meet the needs of women with absolute uterine infertility33.
This article provides a reproducible model for porcine uterus procurement and preservation using machine perfusion. Improving preservation quality and duration is relevant for donor pool expansion by enabling brain-dead donors to be reliable sources for uterus procurement. Clinically, most UTx were performed based on living donors, mostly family members or close friends. Ischemia time is a critical factor in the final organ viability, particularly in brain-dead donors. IRI is defined by cellular damage caused by a succession of ischemia (interruption of blood circulation in the organ vascular tree) and reperfusion (return of the oxygen supply), often associated with an increase in temperature, contributing to the cell damages. This cascade of events produces reactive oxygen species, eventually leading to chronic inflammation, apoptosis, antigen release, and graft rejection7,8,34. Warm (WIT) and cold ischemia times are critical factors in the IRI process, but only a few authors have studied it in the uterus. In a study exploring the effect of long-term SCS preservation in mice UTx, transplantation was performed after 24 h of SCS, with successful pregnancies developed in five out of six animals. However, after 48 h of SCS, all transplants demonstrated necrosis35. In another study comparing UTx in 14 ewes after 3 h of SCS (n = 7) and 24 h of SCS (n = 7), only 7 animals were alive 8 days post-transplant, with 35% of uteri considered viable regarding edema and necrosis, 2 uteri in the 3 h SCS group, and 3 uteri in the 24 h SCS group36. Interestingly, contraction of the myometrium was maintained in viable uteri, but the method assessment was not specified. This highlights the critical importance of considering that a successful uterine transplant involves not only a viable graft but also functional menstruation and a successful pregnancy. With this regard, decreasing IRI and their negative consequences is primordial to allow improvement in graft quality and better live birth success rates. To ensure consistency, we made sure to harvest all organs after comparable WIT. All vessels within the broad ligament were coagulated or ligated to ensure relevant pressure measurement and adequate capillary perfusion to the entire organ. Catheterization is another crucial point where attention must be paid to secure the cannula while avoiding damaging the vessels.
The preliminary experiment with a "Y-system" led to the optimization of two independent perfusion systems, addressing each uterine artery separately. Indeed, one of the critical parameters is the flow rate and the perfusion pressure26,27, which lead to adapting the flow rate, reflecting the overall vascular resistance of the organ. Optimized bilateral inflow allowed lactate clearance from each side of the organ, as highlighted in our results (Figure 5), as well as continuous arterio-venous difference measurements for all metabolic parameters. At subnormothermic temperature, cellular reoxygenation by machine perfusion allows the elimination of lactates accumulated during the anaerobic metabolic phase associated with hypoxia and hypoperfusion during procurement and cannulation, keeping metabolic activity low and avoiding lactate rebound. Similar results have been found in rat hindlimbs19. In another anatomical model, Dion et al. showed a different approach by harvesting the uterus with the aorta in their hypothermic perfusion study37. It would be of great interest for future studies to properly compare aorta-based single perfusion to the model developed in our protocol.
While a reproducible model for machine perfusion of the porcine uterus is described here, one major concern is the suitability for further transplantation. In order to confirm the efficacy of preservation protocols, transplantation is crucial since it reproduces the clinical setting. This requires arterial and venous microsurgical anastomoses. Several studies have shown that the utero-ovarian vein can be used in clinical UTx when the uterine vein is of insufficient caliber to ensure venous drainage38,39. One limitation in this model is the lack of preservation of the utero-ovarian vein. However, no major edema was recorded after 4 h SNMP in all included replicates, and continuous venous outflow was noted from all uterine veins. This suggests a difference between the human and porcine models with a potential simplification of UTx procedures in Yucatan minipigs. Further studies should include extending the perfusion time beyond 4 h to determine the limits of dynamic preservation. To conclude, this article describes a reproducible porcine uterus model for studying machine perfusion-based preservation and provides a valuable resource to target IRI with the goal of increasing the overall number of available organs for UTx.
Disclosures
All authors have no financial interest to declare.
Acknowledgements
This work was partially funded by the National Institute of Health under award No R01AR082825 (BEU) and Shriners Children's 84308 (YB). HO and YB received funding from the Fondation des Gueules Cassées. Support from Société Française de Chirurgie Plastique, Reconstructrice et Esthétique (SOFCPRE, France), and CHU de Rennes (France) to YB is greatly acknowledged.
Materials
| Name | Company | Catalog Number | Comments |
| Affinity Pixie Oxygenation System | Medtronic | BBP241 | Oxygenator |
| Bovin serum albumin | Sigma-Aldrich | A9647 | Perfusate component |
| Calcium chloride dihydrate | Sigma-Aldrich | 223506 | Perfusate component |
| Carbon Dioxide Oxygen | Airgas | UN3156 | Carbon Dioxide Oxygen mix gas |
| D-(+)-Glucose monohydrate | Sigma-Aldrich | 49159 | Perfusate component |
| Dexamethasone | Sigma-Aldrich | D2915 | Perfusate component |
| Dextran | Thermo scientific | 406271000 | Perfusate component |
| Heparin sodium injection | Eugia Pharma | 63739-953-25 | Perfusate component |
| Humulin Regular Insulin human | Lilly | 0002-8215-01 | Perfusate component |
| Hydrocortisone sodium succinate | Pfizer | 0009-0011-03 | Perfusate component |
| Magnesium chloride hexa-hydrate | Sigma-Aldrich | M9272 | Perfusate component |
| MasterFlex L/S | Cole-Parmer | 77200-32 | Roller pump |
| Polyethylene glycol 35000 | Sigma-Aldrich | 25322-68-3 | Perfusate component |
| Potassium chloride | Sigma-Aldrich | 7447-40-7 | Perfusate component |
| Pressure Monitor, Portable, PM-P-1 | Living Systems Instrumentation | PM-P-1 | Pressure sensor |
| Radnoti Bubble Trap Compliance Chamber | Radnoti | 130149 | Bubble trap |
| RAPIDPoint500 | Siemens | 500 | Blood Gas System |
| Sodium bicarbonate | Sigma-Aldrich | S5761 | Perfusate component |
| Sodium chloride | Sigma-Aldrich | S9888 | Perfusate component |
| Sodium hydroxide | Sigma-Aldrich | 72068 | Perfusate component |
| Sodium phosphate monobasique dihydrate | Sigma-Aldrich | 71505 | Perfusate component |
| Syringe 1 mL | BD | 309659 | Sample procurement |
| Vancomycine hydrochloride | Slate run pharmaceuticals | 70436-021-82 | Perfusate component |
References
- Ejzenberg, D., et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet. 392 (10165), 2697-2704 (2019).
- Fageeh, W., Raffa, H., Jabbad, H., Marzouki, A. Transplantation of the human uterus. Int J Gynaecol Obstet. 76 (3), 245-251 (2002).
- Lavoue, V., et al. Which donor for uterus transplants: brain-dead donor or living donor? A systematic review. Transplantation. 101 (2), 267-277 (2017).
- Brännström, M., et al. Registry of the International Society of Uterus Transplantation: First report. Transplantation. 107 (1), 172-181 (2023).
- Van Dieren, V., et al. Acute rejection rates in vascularized composite allografts: A systematic review of case reports. J Surg Res. 298, 33-44 (2024).
- Kristek, J., et al. Human uterine vasculature with respect to uterus transplantation: A comprehensive review. J Obs Gynaecol Res. 46 (11), 1999-2007 (2020).
- He, J., Khan, U. Z., Qing, L., Wu, P., Tang, J. Improving the ischemia-reperfusion injury in vascularized composite allotransplantation: Clinical experience and experimental implications. Front Immunol. 13, 998952 (2022).
- Ponticelli, C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol Dial Transplant. 29 (6), 1134-1140 (2014).
- Harris, K., et al. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 250 (2 Pt 2), H213-H220 (1986).
- Wranning Almen, C., et al. Short-term ischaemic storage of human uterine myometrium--basic studies towards uterine transplantation. Hum Reprod. 20 (10), 2736-2743 (2005).
- Bodewes, S. B., et al. Oxygen transport during ex situ machine perfusion of donor livers using red blood cells or artificial oxygen carriers. Int J Mol Sci. 22 (1), 235 (2020).
- Boncompagni, E., et al. Decreased apoptosis in fatty livers submitted to subnormothermic machine-perfusion respect to cold storage. Eur J Histochem. 55 (4), e40 (2011).
- Czigany, Z., et al. Hypothermic oxygenated machine perfusion reduces early allograft injury and improves post-transplant outcomes in extended criteria donation liver transplantation from donation after brain death: Results from a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann Surg. 274 (5), 705-712 (2021).
- Markmann, J. F., et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: The OCS Liver PROTECT randomized clinical trial. JAMA Surg. 157 (3), 189-198 (2022).
- Cypel, M., et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. New Eng J Med. 364 (15), 1431-1440 (2011).
- Roesel, M. J., Ius, F., Knosalla, C., Iske, J. The role of ex-situ perfusion for thoracic organs. Curr Opin Organ Transplant. 27 (5), 466-473 (2022).
- Michel, S. G., et al. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann Transplant. 20, 461-468 (2015).
- Ghoneima, A. S., Sousa Da Silva, R. X., Gosteli, M. A., Barlow, A. D., Kron, P. Outcomes of kidney perfusion techniques in transplantation from deceased donors: A systematic review and meta-analysis. J Clin Med. 12 (12), 3221 (2023).
- Charlès, L., et al. Effect of subnormothermic machine perfusion on the preservation of vascularized composite allografts after prolonged warm ischemia. Transplantation. 108 (5), e280-e290 (2024).
- Duru, &. #. 1. 9. 9. ;., et al. Review of machine perfusion studies in vascularized composite allotransplant preservation. Front Transplantation. 2, 103-111 (2023).
- Brännström, M., Diaz-Garcia, C., Hanafy, A., Olausson, M., Tzakis, A. Uterus transplantation: Animal research and human possibilities. Fertil Steril. 97 (6), 1269-1276 (2012).
- Wranning, C. A., et al. Auto-transplantation of the uterus in the domestic pig (Sus scrofa): Surgical technique and early reperfusion events. J Obstet Gynaecol Res. 32 (4), 358-367 (2006).
- Croome, K. P., et al. American Society of Transplant Surgeons recommendations on best practices in donation after circulatory death organ procurement. Am J Transplant. 23 (2), 171-179 (2023).
- Dickens, B. M. Legal and ethical issues of uterus transplantation. Int J Gynaecol Obstet. 133 (1), 125-128 (2016).
- Díaz, L., et al. Ethical considerations in animal research: The Principle of 3R's. Rev Invest Clin. 73 (4), 199-209 (2020).
- Goutard, M., et al. Machine perfusion enables 24-h preservation of vascularized composite allografts in a swine model of allotransplantation. Transpl Int. 37 (1), 102-111 (2024).
- Berkane, Y., et al. Towards optimizing sub-normothermic machine perfusion in fasciocutaneous flaps: A large animal study. Bioengineering (Basel). 10 (12), 1545 (2023).
- Brouwers, K., et al. 24-hour perfusion of porcine myocutaneous flaps mitigates reperfusion injury: a 7-day follow-up study. Plast Reconstr Surg Glob Open. 10 (2), e4123 (2022).
- Richter, O., et al. Extracorporeal perfusion of the human uterus as an experimental model in gynaecology and reproductive medicine. Human Reprod. 15 (6), 1235-1240 (2000).
- O'Neill, K., et al. Availability of deceased donors for uterus transplantation in the United States: Perception vs Reality. Transplantology. 5 (1), 27-36 (2024).
- Chan, J. K., Morrow, J., Manetta, A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 188 (5), 1273-1277 (2003).
- Rocca, W. A., et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: A population-based cohort study. Mayo Clin Proc. 91 (11), 1577-1589 (2016).
- Dion, L., et al. Procurement of uterus in a deceased donor multi-organ donation National Program in France: A scarce resource for uterus transplantation. J Clin Med. 11 (3), 730 (2022).
- Agarwal, A., et al. Clinicopathological analysis of uterine allografts including proposed scoring of ischemia reperfusion injury and t-cell-mediated rejection-dallas uterus transplant study: A pilot study. Transplantation. 106 (1), e10-e20 (2022).
- Díaz-García, L., et al. Pregnancy in transplanted mouse uterus after long-term cold ischaemic preservation. Hum Reprod. 18 (10), 2142-2150 (2003).
- Tricard, J., et al. Uterus tolerance to extended cold ischemic storage after auto-transplantation in ewes. Eur J Obstet Gynecol Reprod Biol. 214, 162-167 (2017).
- Dion, L., et al. Hypothermic machine perfusion for uterus transplantation. Fertil Steril. 120 (6), 1259-1261 (2023).
- Brännström, M., et al. Experimental uterus transplantation. Hum Reprod Update. 16 (3), 329-340 (2010).
- Ozkan, O., Ozkan, O., Dogan, N. U. The Ozkan technique in current use in uterus transplantation: from the first ever successful attempt to clinical reality. J Clin Med. 12 (8), 2812 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved