Method Article
Avoiding Ischemia Reperfusion Injury in Liver Transplantation
* These authors contributed equally
In This Article
Summary
Presented here is a protocol to provide a step-by-step ischemia-free liver transplantation protocol under ex situ normothermic machine perfusion (37 °C) of human livers from donors to recipients.
Abstract
Currently, ex situ machine perfusion is a burgeoning technique that provides a better preservation method for donor organs than conventional static cold preservation (0–4 °C). A continuous blood supply to organs using machine perfusion from procurement and preservation to implantation facilitates complete prevention of ischemia reperfusion injury and permits ex situ functional assessment of donor livers before transplantation. In this manuscript, we provide a step-by-step ischemia-free liver transplantation protocol in which an ex situ normothermic machine perfusion apparatus is used for pulsatile perfusion through the hepatic artery and continuous perfusion of the portal vein from human donor livers to recipients. In the perfusion period, biochemical analysis of the perfusate is conducted to assess the metabolic activity of the liver, and a liver biopsy is also performed to evaluate the degree of injury. Ischemia-free liver transplantation is a promising method to avoid ischemia-reperfusion injury and may potentially increase the donor pool for transplantation.
Introduction
Ischemia reperfusion injury (IRI) is a well-known and widespread complication in organ transplantation. Obvious nonimmunological events lead to poor graft outcomes and delayed graft function, which are related to the high proportions of organ failure, re-transplantation, and recipient death1. Conventional cold storage (CCS) of organs was previously identified as a classic method to slow down metabolism but it does not have an influence on preventing progressive dysfunction and damage to cellular integrity. Furthermore, leukocyte accumulation is induced by reactive oxygen metabolites in the reperfusion phase. All of these biological processes become even more relevant when we use extended criteria donor (ECD) grafts such as fatty livers and those from donors older than 65 years. These ECD grafts are more vulnerable to damage and some other detrimental impacts, especially those from CCS2. The technology of normothermic ex situ liver machine perfusion to preserve donor organs has achieved great progress over the past few decades and is entirely feasible in clinical practice3. The safety and viability of warm perfusion techniques in donor organs have been evaluated in preclinical studies, and some study groups have designed new type of perfusate and rewarming tactics in animal models. Some clinical trials of warm perfusion to preserve donor livers have been launched in East Asia, Europe and North America4,5.
Normothermic machine perfusion (NMP) facilitates a metabolically active scenario in which organs can achieve homeostasis with continuously provisioned oxygen and nutrients. The metabolism of grafts is activated, and we can judge during perfusion whether the donor organs are suitable for transplantation to recipients according to the biochemical index of the perfusate or biopsy of the perfused organs. Available parameters during the preservation period also offer a means for surgeons to treat grafts or restore ECD grafts6,7.
Red blood cells are the most frequently used oxygen carrier. Some other essential ingredients, including antibiotics, antithrombotic agents, and nutrients are also included in the perfusate8. In the current practice, after a liver has been retrieved, it is preserved and back-table prepared in a 0-4︒C solution. Then, the cold liver is perfused in the already prepared NMP apparatus for several hours for assessment and restoration. However, the liver suffers double attacks of IRI at the start of NMP and after implantation, although the liver is protected and repaired to some extent during the NMP process9,10. Therefore, we attempted to reevaluate the process and reflect on avoidance of the two IRI attacks. We hypothesized that IRI was avoidable if a continuous blood supply was provided to the liver. To verify this hypothesis, we changed the conventional double conversion protocol into an uninterrupted hepatic artery (HA) and portal vein (PV) supply using a Liver-Assist device. This novel transplant procedure was named ischemia-free liver transplantation (IFLT). The first case of IFLT has previously been published and has attracted considerable attention from organ transplantation experts11.
Two rotary pumps providing pulsatile hepatic arterial flow and a continuous PV supply were used in the perfusion device in which the flow was controlled by relevant pressure. The system is controlled by pressure and allows the flow through the liver to be automatically adjusted according to the resistance in the liver. Oxygenation and CO2 elimination of the perfusate are regulated by two hollow fiber membrane oxygenators. We can set different temperatures according to different types of machine perfusion (ranging from 10 °C to 37 °C). We can monitor and record the real-time pressure, temperature, flow and resistance index in the instrument panel during the perfusion process. Liver assist is not a transportable device. Therefore, the donors used for IFLT should be transferred to the transplant center.
This article aimed to offer a step-by-step IFLT protocol in which an ex situ NMP apparatus is used to provide pulsatile perfusion to the HA and maintain continuous perfusion of the PV from human donor liver procurement to implantation.
Protocol
This protocol was reviewed and approved by the ethics committee of The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. Informed consent was obtained from all the participants. All the procedures in studies involving human participants were performed in accordance with the 1964 Helsinki Declaration and its later amendments or revisions.
1. Preparation of the perfusion solution and device
NOTE: The total volume of the perfusion solution prepared for NMP according to this protocol is approximately 3,000 mL, as reported previously1, and the final hemoglobin concentration is 6–10 g/L. The components of the perfusion solution are listed in Table 1.
- Add the components of the perfusate to the organ reservoir of artificial hepatic assist device (Table of Materials) through the connector at the top of the oxygenator and remove all bubbles from the pipeline.
- Turn on the venous pump according to the manufacturer’s instructions, which is displayed on the screen. Turn on the arterial pump in a similar manner. Null the pressure according to the instructions on the device screen. Click on the Pressure button to set the HA pressure within the range of 50–60 mmHg and PV pressure within the range of 5–10 mmHg during the whole process of normal temperature mechanical perfusion.
- Start oxygenation using a mixture of O2 and air (30% O2) at a combined flow rate of 400 mL/min. Warm the perfusion solution to 37 °C.
- Obtain a sample of perfusate solution from the T-branch pipe of arterial perfusion line for microbial culture (8 mL), blood gas analysis (0.5 mL) and liver function test (3 mL) after the device has been primed (pO2, pCO2, pH and electrolyte within normal range, and temperature near 37 °C), and monitor the biochemical parameters accordingly.
NOTE: The perfusate should be prepared fresh before use in a laminar flow operating room. Bicarbonate or insulin is added, if necessary.
2. Ischemia-free procurement of donor liver
- Conduct the abdominal cruciate incision as follows: vertical, from the sternal notch to the symphysis pubis, and transverse, laterally to both flanks at the level of umbilicus. When procurement of the lung or heart is needed, a sternotomy can be utilized. Use a large C-shaped retractor to provide exposure.
- Perform a detailed inspection of the abdominal viscera. Take a liver biopsy specimen for histological observation and clinical research. Mobilize the liver with a precision technique.
- Place a cannula in the common bile duct for bile drainage and ligate the cystic duct. Cut a full-circumference tissue sample (width: 3–5 mm) from the end of the common bile duct for histological observation and clinical research.
- Dissect the celiac artery (CA), gastroduodenal artery (GDA), splenic artery (SA), inferior vena cava (IVC), and PV. Insert an 8 Fr/12 Fr arterial cannula into the GDA or SA. Ensure that there is no interruption of the arterial supply for the liver from the CA.
- Harvest a 3 cm-long right external iliac vein and anastomose the vessel to the PV in end-to-side fashion with partial blockage of the PV for making an interposition vein.
- Place a 32–34 Fr caval cannula in the infrahepatic inferior vena cava (IHIVC) for outflow to the organ reservoir of the device. Connect a straight 24 Fr cannula to the PV perfusion line of the device and then, via the interposition vein, completely insert into the PV. Block the suprahepatic inferior vena cava (SHIVC) thereby blocking the venous drainage to the right atrium. Connect the arterial cannula to the HA perfusion line of the Liver Assist device. Then, start NMP, and establish the circuit in situ.
- Harvest the liver and transfer to the organ reservoir under continuous NMP. Immediately after the liver is removed from the abdominal cavity, cold-flush the kidneys via the preplaced cannula within the abdominal aorta and procure the kidneys in the conventional manner.
NOTE: In the process of procurement, fully isolate the common hepatic artery (CHA), ligate the left gastric artery (LGA), and isolate the CA to the abdominal aorta. In the case of the accessory HA, bypass the artery in situ before NMP starts.
3. Ischemia-free preservation of the donor liver
- Transfer the liver to the perfusion device. Remove the caval cannula immediately when the liver is moved to the organ reservoir. Continuous ex situ NMP the liver graft until allograft revascularization.
- Set the PV perfusion pressure at 6–10 mmHg with a targeted flow rate higher than 500 mL/min. Set the HA pressure at 50–60 mmHg with a targeted flow rate higher than 150 mL/min. During the NMP, ensure that the perfusion parameters are stable, and monitor the pressure and flow rate within an appropriate range.
- Remove redundant tissues from the liver and blood vessels. Transiently block the overall IVC to examine SHIVC and IHIVC for leaks. Cover any dry surfaces with wet sterile gauze to prevent dehydration.
- Collect the bile tubing into a 15 mL collection container. Place the opening of the bile drain below the liver to allow bile to run out freely. Record the amount of bile production, and monitor the biochemical parameters every 30 min.
- Obtain a perfusion sample (0.5 mL) for blood gas analysis every 10–20 min, liver function tests (3 mL) every 30 min and monitor the biochemical parameters accordingly.
- Assess the viability of the liver by blood gas analysis and liver function tests of the perfusate, as well as bile biochemical parameters as previously reported2.
NOTE: For patient safety, confirm the graft viability during NMP before initiation of the recipient surgical procedures. Add 1 mL of papaverine to reduce vascular resistance, if necessary.
4. Ischemia-free implantation of the donor liver
- Resect the recipient’s diseased liver using a conventional technique. Recannulate the donor IHIVC a 32–34 Fr caval cannula, remove the diseased liver and block the SHIVC with a clamp. Then, move the donor liver from the reservoir to the recipient’s abdominal cavity so that an NMP circuit in situ can be re-established.
- Suture the donor SHIVC to the recipient counterparts using 3–0 non absorbable polypropylene sutures with a bi-caval or piggy-back technique.
- Suture the donor PV and HA to the recipient’s counterparts in an end-to-end fashion using 5–0 and 7–0 non absorbable polypropylene sutures, respectively. Perform these anastomoses under continuous NMP of the allograft as both HA and PV contains branches both native and artificial in nature.
- Collect the liver biopsy specimen before reperfusion. Afterward, release the clamps on the PV and HA in order to re-establish the native dual blood supply for the liver. At the same time, cease the NMP after removal of the HA and PV cannula. Then, flush out approximately 200 mL perfusate within the liver form IHIVC cannula. Block IHIVC cannula and release of the clamp on the SHIVC. The anhepatic phase is over. Obtain a perfusate sample (8 mL) for microbial culture again.
- Remove of the cannulas in the SA or GDA, and interposition vein. Ligate the donor SA or GDA and interposition vein. Withdraw the cannula in the IHIVC and anastomose the donor’s IHIVC to the recipient IHIVC (bi-caval) or ligate it (piggy-back) according to the surgical procedure. Collect the common bile duct specimen again after withdrawal of the bile drainage cannula. Anastomose the donor’s common bile duct to the recipient’s common bile duct with end-to-end fashion after withdrawal of the draining tube.
- Collect liver biopsy specimen again after meticulous hemostasis. Close the abdominal wall in the routine procedure.
NOTE: During the implantation process, monitor the portal and arterial cannula closely to avoid twisting or bending, and scrutinize the flow rate parameters in real time to ensure the blood supply of the HA and PV. Increase the perfusion pressure slightly when necessary to ensure that the flow rate is sufficient to the liver. During the anastomosis of the SHIVC, PV, or IHIVC, shorten the venous stump as much as possible to avoid postsurgical obstruction of the venous flow.
Results
In April 2018, a 66-year-old male donor with brain death was not considered by local transplant centers because of the high risk of graft loss in such donors. The reasons for discarding the liver, at the time of procurement were older age and macroscopic appearance of moderate firmness, round liver edges and suboptimal liver graft perfusion along with major donor comorbidities, which included hypertension, hypertensive heart disease, and the following associated factors: hypernatremia (sodium, 156 mmol/L) and hemodynamic instability with the need for amine administration (dopamine, 1.5 µg/kg/min, noradrenaline, 0.12 µg/kg/min). Normothermic perfusion of the human donor liver grafts was performed for 5 h as described in the presented protocol. Macroscopic homogeneity of liver perfusion was evaluated to assess the quality of the liver graft. (Figure 1A–D). The hemodynamics of the liver was also studied by monitoring the changes in the arterial and portal flows. Stable hemodynamics of the liver grafts was observed during perfusion (Figure 2A). Blood gas analysis of the perfusate samples collected from arterial perfusion fluid was used to monitor the oxygenation status in the perfusion fluid. Oxygenation with a mixture of O2 and air (30% O2) at a flow rate of 400 mL/min resulted in a continuous O2 saturation of 100%. Figure 2B displays the oxygenation of the perfusion fluid and subsequent extraction of carbon dioxide in our experience. Notably, the perfusate maintained a physiological pH during the whole perfusion process. Lactate levels subsequently decreased rapidly and were normal at 2.5 h of NMP (Figure 2C). An increase in the quantities of total bilirubin represented an improvement in the quality of the bile produced during NMP (Figure 2D).
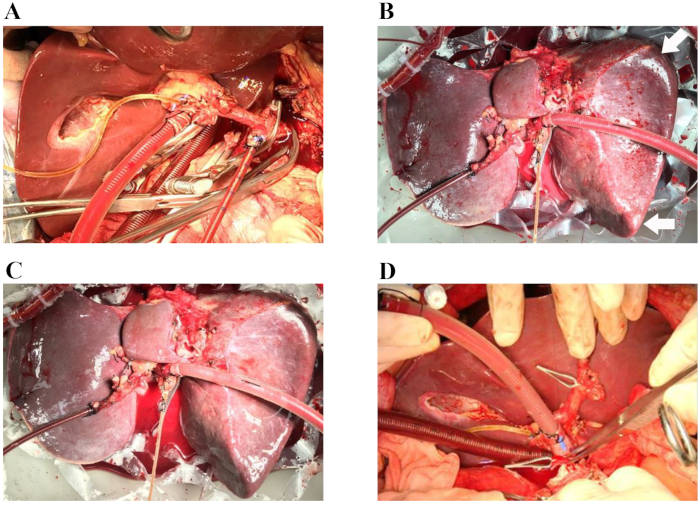
Figure 1: Representative procedures of ischemia-free liver transplantation. (A) The arterial cannula is inserted into the spleen artery, and the venous cannula is inserted into the portal vein patch. The bile duct is cannulated with a silicon biliary catheter. (B) Sixty minutes after the start of normothermic machine perfusion. Arrows: round liver edges. (C) Four hours after the start of normothermic machine perfusion. (D) The donor liver is implanted into the recipient (the suprahepatic vena cava anastomosis is completed). During the operation, the organ chamber is covered by a nontransparent cover to maintain a sterile moist environment for the liver (not shown in these images). Please click here to view a larger version of this figure.
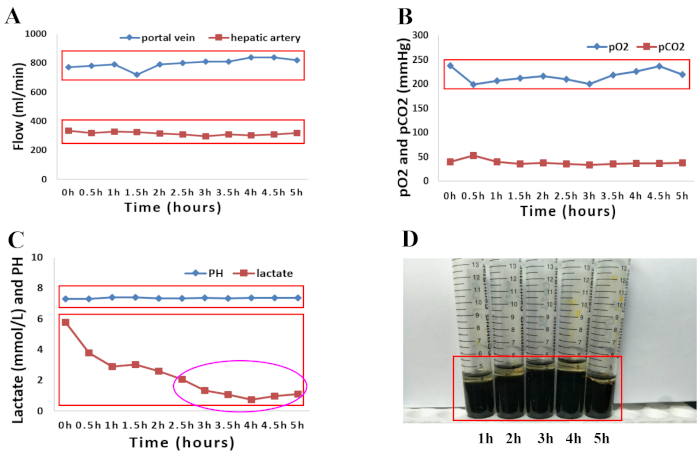
Figure 2: Graphical presentation of perfusion parameters of both the perfusion fluid and bile during 5 h of normothermic machine perfusion. (A) Changes in arterial and portal flow. (B) Evolution of oxygenation characteristics and pCO2 during 5 h of normothermic perfusion. (C) pH and lactate levels during 5 h of normothermic perfusion. (D) Increasing quantities of bilirubin in bile samples taken during machine perfusion. Please click here to view a larger version of this figure.
Discussion
This IFLT technique was established to completely avoid IRI. This article provides a step-by-step IFLT protocol from organ procurement, ex situ preservation to implantation.
Based on NMP, IFLT provides an uninterrupted supply of blood and oxygen to grafts from procurement and perseveration to implantation. Numerous studies have shown that NMP has significant advantages in reducing IRI, improving organ viability, and repairing graft damage compared to static cold preservation12. Through the innovation of surgical techniques and the advancement of NMP technology in various organs, the concept of ischemia-free organ transplantation (IFOT) is expected to extend to all solid organ transplants, significantly improving the early and long-term prognosis of organ transplantation and maximizing the use of marginal organs. IFOT technology is currently only used in organ transplantation derived from donation after brain death (DBD), but it is also applicable to transplantation of relative living organ donation (LDOD) by selecting reasonable vessel intubation and perfusion parameters. Donation after cardiac death (DCD) can be divided into two categories: manipulation of DCD (stopped after intentional recall of life support in patients with mechanical ventilation who do not meet brain death criteria, cDCD) and to a lesser extent uncontrolled DCD (unsuccessful resuscitation after cardiac arrest, uDCD)12. In uDCD-derived grafts in which organ warm ischemia injury has occurred, regional NMP should be established rapidly prior to organ harvesting. In this case, although the technique cannot completely avoid IRI, the damage to the organ can be maximally repaired. Notably, cDCD-derived grafts are widely used in most countries. With the support of regional NMP technology, IFOT can also be applied to organ transplants derived from such donations to avoid the subsequent occurrence of IRI. Since the IRI of a DCD organ is more severe than that of DBD and LDOD organs, this type of organ will likely benefit the most from IFOT. Therefore, IFOT is a promising method for organ transplants from almost all sources of donation, and its great application prospects warrant exploration.
There are several aspects to be aware of during this procedure. During the procurement process, the CHA is fully dissociated, the LGA is ligated, the celiac trunk is freed to the abdominal aorta, and the variant accessory HA needs to be reconstructed in the body.
During the preservation process using machine perfusion, the perfusion parameters are ensured to be stable, and the pressure and flow rate of the HA and PV are controlled in the physiological state range. The perfusion pressure can be slightly increased to ensure that the flow is sufficient to supply the liver during implantation.
For the process of donor liver implantation, attention should be paid to intubation of the PV and HA. The flow parameters should be monitored in real time to ensure the supply of arterial and portal blood flow. When the donor-to-recipient SHIVC and PV were anastomosed, redundant and twisted vessels should be avoided.
A continuous blood supply throughout the transplant process and the opportunity to add additional agents to the perfusion fluid during organ perfusion offer the potential to assess and improve organ quality prior to transplantation. Therefore, this method can considerably improve transplant outcomes and increase the number of available organs for transplantation.
Disclosures
The authors have no competing interests to declare.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81401324 and 81770410), Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002), Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), Special Support Program for Training High-Level Talent in Guangdong Province (2015TQ01R168), Pearl River Nova Program of Guangzhou (201506010014), and Scientific Program for Young Teachers of Sun Yat-sen University (16ykpy05), China.
Materials
| Name | Company | Catalog Number | Comments |
| 10% calcium gluconate | Hebei Tiancheng Pharmaceutical Co, Ltd | 1S181124101 | 30 mL |
| 25% magnesium sulphate | Hebei Tiancheng Pharmaceutical Co, Ltd | H20033861 | 3 mL |
| 5% sodium bicarbonate | Huiyinbi Group Jiangxi Dongya Pharmaceutical Co, Ltd | H36020283 | The amount depends on the pH |
| Cefoperazone sodium and sulbactam sodium | Pfizer | H20020597 | 1.5 g |
| Compound Amino Acid Injection | Guangdong Litai Pharmaceutical Co., Ltd | H20063797 | 250 mL |
| Crossed-matched leucocyte-depleted washed red cells | Guangzhou Blood Center | H20033739 | 1300 mL |
| Heparin | Chengdu Hepatunn Pharmaceutical Co., Ltd | H51021209 | 37500 U |
| Liver Assist | Organ Assist | OA.Li.Li.140 | Perfusion device |
| Liver Assist disposable package | Organ Assist | OA.Li.DP.540 | Disposable set and cannulas |
| Metronidazole | Shanghai Baxter Healthcare Co., Ltd. | H20003301 | 0.5 g |
| scalp acupuncture | Wuhan W.E.O.Science & Technology Development Co., Ltd | WEO-JX-32B-5.0 0.7*25mm | Bile duct cannula |
| Succinylated gelatinor | B. Braun Medical Suzhou Co., Ltd | H20113119 | 1400 mL |
References
- Hanidziar, D., Koulmanda, M. Towards cytoprotection in the peritransplant period. Seminars in Immunology. 23 (3), 209-213 (2011).
- Eltzschig, H. K., Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nature Medicine. 17 (11), 1391-1401 (2011).
- Ravikumar, R., Leuvenink, H., Friend, P. J. Normothermic liver preservation: a new paradigm. Transplant International. 28 (6), 690-699 (2015).
- Jayant, K., Reccia, I., Shapiro, A. M. J. Normothermic ex-vivo liver perfusion: where do we stand and where to reach. Expert Review of Gastroenterology & Hepatology. 12 (10), 1045-1058 (2018).
- Hessheimer, A. J., Riquelme, F., Fundora-Suarez, Y., Garcia Perez, R., Fondevila, C. Normothermic perfusion and outcomes after liver transplantation. Transplantation Reviews (Orlando, Fla). 33 (4), 200-208 (2019).
- Weissenbacher, A., Vrakas, G., Nasralla, D., Ceresa, C. D. L. The future of organ perfusion and re-conditioning. Transplant International. 32 (6), 586-597 (2019).
- von Horn, C., Minor, T. Modern concepts for the dynamic preservation of the liver and kidneys in the context of transplantation. Pathologe. 40 (3), 292-298 (2019).
- Czigany, Z., et al. Machine perfusion for liver transplantation in the era of marginal organs-New kids on the block. Liver International. 39 (2), 228-249 (2019).
- Wettstein, D., et al. Machine perfusion: new opportunities in abdominal organ transplantation. Orvosi Hetilap. 159 (46), 1882-1890 (2018).
- Lai, Q. R. N., et al. Role of perfusion machines in the setting of clinical liver transplantation: A qualitative systematic review. Clinical Transplantation. 32 (8), 11(2018).
- He, X., et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. American Journal of Transplantation. 18 (3), 737-744 (2017).
- Jassem, W., et al. Normothermic Machine Perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology. 70 (2), Baltimore, Md. 682-695 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved