Method Article
Automated Preparation of [68Ga]Ga-3BP-3940 on a Synthesis Module for PET Imaging of the Tumor Microenvironment
In This Article
Summary
This research describes the automated process for [68Ga]Ga-3BP-3940 production with the GAIA V2 synthesizer, for PET imaging of fibroblast activation protein. The results of quality control tests performed on three test batches are also presented.
Abstract
A fast, efficient method has been developed on the GAIA synthesis module for automated gallium-68 radiolabeling of 3BP-3940, a molecular imaging probe targeting the fibroblast activation protein for positron emission tomography imaging of the tumor microenvironment. The reaction conditions involved acetate buffer (final concentration: 0.1 M), methionine as an anti-radiolysis agent (final concentration: 5.4 mg/mL), and 30 µg of 3BP-3940, with heating for 8 min at 98 °C. A final purification step on a C18 cartridge was necessary to obtain a radiolabeled product of high purity. In contrast, the generator-produced 68Ga was used directly without a concentration step on a cation exchange cartridge. The production of three validation batches confirmed the method's reliability, allowing the synthesis of [68Ga]Ga-3BP-3940 in 22.3 ± 0.6 min with high radiochemical purity (RCP), as determined by both radio-HPLC (99.1% ± 0.1%) and radio-TLC (99.2% ± 0.1%). The average radiochemical yield, based on RCP values measured by radio-HPLC, was 74.4% ± 3.3%. The stability of the radiolabeled product was demonstrated for up to 4 h after preparation. This protocol provides a reliable, rapid, and efficient methodology for the preparation of [68Ga]Ga-3BP-3940, which can easily be transposed to a clinical setting.
Introduction
In recent years, targeting the tumor microenvironment (TME) has attracted considerable interest in diagnostic and therapeutic applications1. The abundance of cell types, signaling molecules, and extracellular matrix (ECM) macromolecules within the TME offers a wide range of potential molecular targets2. Among the resident and infiltrating host cells, cancer-associated fibroblasts (CAFs) form a distinct subset of fibroblasts within the TME, phenotypically different from normal fibroblasts. CAFs play crucial roles in tumor progression, metastasis, immune evasion, and therapy resistance through unique cellular and molecular characteristics3. These mesenchymal cells exhibit an activated phenotype marked by the expression of fibroblast activation protein (FAP). Molecularly, CAFs secrete a complex array of cytokines, chemokines, growth factors (e.g., TGF-β, IL-6, and CXCL12), and ECM proteins (e.g., collagen, fibronectin), which remodel the ECM and foster a pro-tumorigenic environment4.
As a highly specific protein that is overexpressed and localized on the extracellular surface of the CAF membrane, FAP displays all the characteristics of a reliable molecular target, especially for nuclear medicine and radiopharmaceutical applications5. In this context, quinoline-based small molecule inhibitors of FAP (FAPI), functionalized with a DOTA group, were developed and quickly introduced into clinical use6,7,8. Specifically, FAPI-04 and FAPI-46 radiolabeled with gallium-68 (β+ emitter, t1/2 = 68 min) for positron emission tomography (PET) imaging have demonstrated significant value in fibrotic diseases, cardiology, and oncology8,9, particularly for cancers where [18F]fluorodeoxyglucose ([18F]FDG) has limited utility10. However, while their contributions to oncology and nonmalignant diseases imaging are undeniable, small molecule FAPIs exhibit certain limitations for targeted radionuclide therapy (TRT) applications, particularly due to their suboptimal intratumoral residence time, which can lead to unintended irradiation of healthy tissue11. To address this issue, several strategies have been explored, such as the design of multivalent ligands11,12 or the use of therapeutic radionuclides with short half-lives13,14,15. New molecular scaffolds with a high affinity for FAP and triggering a high proportion of cell internalization have also been developed.
One of these is the pseudopeptide derivative FAP-2286. It contains a 7-amino acid sequence, cyclized and linked to a DOTA chelator by a 1,3,5-benzenetrimethanethiol moiety16. An initial study in humans demonstrated that [68Ga]Ga-FAP-2286 exhibits a biodistribution profile similar to [68Ga]Ga-FAPI-46, with slightly higher physiological uptake in the liver, kidneys, and heart17. In this study, 64 patients, primarily with cancers of the neck, liver, stomach, pancreas, ovaries, and esophagus, underwent PET imaging with [68Ga]Ga-FAP-2286 for cancer staging or detection of recurrence: uptake of [68Ga]Ga-FAP-2286 was notably higher than [18F]FDG in primary tumors, lymph node metastases, and distant metastases, enhancing image contrast and lesion detectability. All primary tumors were visible with [68Ga]Ga-FAP-2286 PET/CT, whereas [18F]FDG PET/CT missed almost 20% of the lesions. For involved lymph nodes, detection rates were higher with [68Ga]Ga-FAP-2286, as well as for bone and visceral metastases. Another study in a smaller group of 21 patients with a variety of cancer diseases also demonstrated the excellent sensitivity of this imaging agent, reflecting the diagnostic efficiency of [68Ga]Ga-FAP-228618. More specific studies have focused on a single type of cancer, such as urothelial or lung cancer, highlighting once again the high potential of [68Ga]Ga-FAP-2286 for clinical molecular imaging4,5. Regarding therapy, a preliminary study investigated the use of FAP-2286 radiolabeled with lutetium-177 (β- emitter, t1/2 = 6.7 d) in 11 patients with diverse progressive, metastatic cancers19. Most patients received two treatment cycles spaced 8 weeks apart, and the average administered dose per cycle was 5.8 ± 2.0 GBq of [177Lu]Lu-FAP-2286. The drug demonstrated prolonged intratumoral retention, with an effective half-life of approximately 44 h in bone metastases. Given the acceptable side effects, these findings paved the way for larger-scale clinical trials: the safety and efficacy of [177Lu]Lu-FAP-2286 are currently being assessed in the phase 1/2 LuMIERE clinical trial, sponsored by Novartis (NCT04939610)7,8. Further smaller-scale research protocols are documented in the literature9,20, and multiple case reports have been published21,22,23,24,25,26, demonstrating the efficacy and excellent tolerability of this TRT.
Minimal structure modifications made on FAP-2286 led to the optimized analog 3BP-3940 (Figure 1)27. Although scientific literature on this vector molecule remains limited, early studies have been conducted for both imaging and therapeutic applications. A preliminary report describes the use of [68Ga]Ga-3BP-3940 in 18 patients with various end-stage metastatic carcinomas and concludes that this radiopharmaceutical is a suitable PET imaging agent, emphasizing its excellent tumor-to-background ratio and very low kidney uptake28. In another work, a single pancreatic cancer patient with liver metastases received 150 MBq of [68Ga]Ga-3BP-3940 for PET imaging, which demonstrated intense uptake in the primary tumor and metastatic lesions29. The same patient subsequently received a single dose of 9.7 GBq of [177Lu]Lu-3BP-3940 for TRT. The treatment was well tolerated, with no significant changes in vital signs or biological parameters. A different study presented the initial human results of a theranostic approach using 3BP-3940: patients were selected with [68Ga]Ga-3BP-3940 PET imaging and then received 3BP-3940 labeled with different isotopes (177Lu, 90Y, or 225Ac), administered alone or in tandem combinations (e.g., 177Lu + 225Ac) in 1-5 treatment cycles30. Outcomes included one complete remission, four partial remissions, three stable diseases, and 12 disease progressions. The cohort's (n = 28) median overall survival was 9 months from the start of TRT.
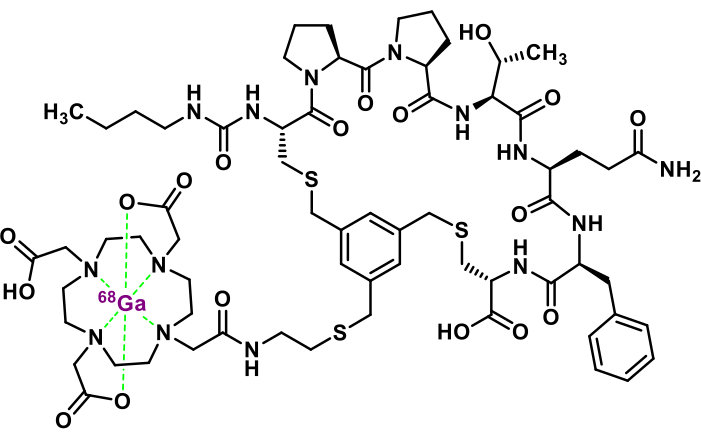
Figure 1: Chemical structure of [68Ga]Ga-3BP-3940. Please click here to view a larger version of this figure.
The 68Ga radiolabeling process for experimental radiopharmaceuticals such as FAP-2286 and 3BP-3940 generally involves a synthesis module to automate the preparation step. Notably, method automation ensures process robustness and GMP compliance and minimizes operator radiation exposure in comparison with manual preparation methods31,32,33. In many cases, such a protocol is expected by regulatory authorities as a part of an investigational medicinal product dossier (IMPD) before authorizing a center to manufacture the corresponding experimental radiopharmaceutical34. To date, very little detailed information on the automated 68Ga radiolabeling of anti-FAP pseudopeptides is available in the literature29,35,36,37,38. Moreover, the data reported generally applies only to a given model of synthesizer. The type of 68Ga generator used can also bring certain specificities, as the different commercially available solutions are characterized by specific volumes of 68Ga3+ eluate in HCl (usually 0.1 M), which can have a direct impact on automated radiolabeling conditions.
In this context, we present a detailed protocol for the rapid and efficient automated radiolabeling of the pseudopeptide 3BP-3940 with 68Ga, using the GAIA V2 synthesis module. This synthesizer relies on the use of a tubing set comprising three ramps of five manifolds each, connected to a peristaltic pump to control fluid flow. It also features a vial oven for reaction medium heating, several radioactivity probes, and a pressure sensor to monitor these parameters within the system. Although not as widespread as some other models, this automaton is used routinely in our center and is installed in a growing number of facilities31,39,40,41,42,43,44 . A GALLIAD 68Ge/68Ga generator was used in this work without prepurification of the 68Ga eluate. This method is designed to offer a robust, fast, and convenient solution for the production of [68Ga]Ga-3BP-3940, also optimizing radiation protection for operators during radiolabeling. This is also the first preparation protocol for this radiopharmaceutical to be reported on this specific synthesizer model, and in such detail.
Protocol
NOTE: This protocol involves working with radioisotopes. Anyone conducting this procedure must be properly trained in handling unsealed radioactive materials and must have approval from their institution's radiation safety officer. The automated synthesizer should be placed in a designated shielded hot cell. Any manual procedures involving radioactive materials should also be carried out in a shielded hot cell or behind appropriate radiation shielding.
1. Preparation of reagents
NOTE: The reagents required for the automated production of [68Ga]Ga-3BP-3940 (see Table of Materials) were prepared in a radiopharmaceuticals preparation unit (GMP grade C clean room). Reagents can be prepared in any order and up to 2 h prior to synthesis.
- Preparation of buffer solution (sodium acetate 0.8 M)
- Acquire the following raw material: sodium acetate trihydrate EMPROVE API Ph Eur, BP, JP, USP, FCC, E262.
- In a sterile container (e.g., microcentrifuge tube, 5 mL) correctly identified, weigh an exact mass of sodium acetate trihydrate close to 544.32 mg using a precision balance.
- Using a calibrated micropipette and sterile cones, solubilize sodium acetate in a volume of water for injection (WFI) close to 5 mL so that WFI is as described below.
WFI volume = (weighed buffer mass x 5)/544.32 - Vortex the solution and run it through an ultrasonic bath (40 kHz, ~1 min) to facilitate solubilization.
- Using a 10 mL syringe equipped with a 21G needle, withdraw the buffer solution. Install a 0.22 µm filter between the syringe and a new needle, then filter the buffer solution directly into a properly identified sterile sealed vial (e.g., TC-ELU 5) after disinfection of its septum.
- Preparation of anti-radiolysis compound solution (methionine 10 mg/mL)
- Acquire the following raw material: L-Methionine (Ph. Eur., USP) pure, pharma grade.
- In a sterile container (e.g., microcentrifuge tube 5 mL) correctly identified, weigh an exact mass of L-methionine close to 50 mg using a precision balance.
- Using a calibrated micropipette and sterile cones, solubilize L-methionine in a volume of WFI close to 5 mL so that WFI is as described below.
WFI volume = (weighed buffer mass x 5)/50 - Vortex the solution and run it through an ultrasonic bath (40 kHz, ~1 min) to facilitate solubilization.
- Using a 10 mL syringe equipped with a 21G needle, withdraw the methionine solution. Install a 0.22 µm filter between the syringe and new needle, then filter the solution directly into a properly identified sterile sealed vial (e.g., TC-ELU 5, Curium) after disinfection of its septum.
2. Preparation of equipment for quality controls
- pH control of the final product
- Behind an appropriate protective shield, position a strip of pH paper for subsequent checking of the final product.
- Radiochemical purity control by radio-TLC
- Prepare two TLC migration tanks containing appropriate mobile phases. Mobile phase A: citrate buffer 0.1 M pH 4 in water, with expected Rf = 0-0.2 for [68Ga]Ga-3BP-3940 and Rf = 0.8-1 for free 68Ga3+. Mobile phase B: ammonium acetate buffer 1 M in a 1:1 mixture of water and methanol (v/v), with expected Rf = 0-0.2 for [68Ga]gallium colloids and Rf = 0.8-1 for [68Ga]Ga-3BP-3940.
- Behind an appropriate protective shield, position the two TLC migration tanks next to two iTLC-SG plates for subsequent control of the RCP of the final product.
- Switch on the radiochromatograph and open the associated acquisition software on the operating computer. Pre-enter the identification information for the first analysis to be performed.
- Radiochemical purity control by radio-HPLC
- Check that the column model required for the analysis (C18 ACE Equivalence) is installed on the system.
- Prepare fresh mobile phase for HPLC, i.e., water for HPLC + 0.1% TFA (solvent A) and acetonitrile + 0.1% TFA (solvent B). Connect the solvent A and solvent B bottles to the corresponding lines on the radio-HPLC.
- Turn on the operating computer and connect to the control software.
- If necessary, open the purge valve on the pump module and purge the lines that will be used for analysis (i.e., line A and line B). Close the purge valve after this operation.
- Allow the system to equilibrate with a proportion of solvent equal to the start of the gradient (i.e., 95% A/5% B) with a 0.6 mL/min flow for at least 20 min. For the [68Ga]Ga-3BP-3940 HPLC analysis sequence, use the flow rate maintained at 0.6 mL/min, and program the mobile phase gradient from 0.1% TFA in water (A) to 0.1% TFA in acetonitrile (B) as follows: 0 - 1 min 95/5 A/B; 1 - 8 min linear gradient from 95/5 A/B to 60/40 A/B; 8 - 9 min 60/40 A/B; 9 - 10 min linear gradient from 60/40 A/B to 95/5 A/B; 10 - 12 min 95/5 A/B.
- Select the analytical method with the mobile phase gradient described in step 2.3.5., then pre-enter the identification information for the first analysis to be performed.
- In a shielded container, prepare an HPLC vial with a glass insert to receive the sample to be analyzed and position it behind an appropriate protective shield.
3. Preparation of the synthesis module
- Turn on the power and light of the shielded cell. Turn on the laptop controlling the synthesizer and log on to the module software.
- If required, remove the old kit from the synthesizer and dispose of waste in the appropriate containers.
- Perform cleaning of the inside of the shielded cabinet housing the synthesis module and of the synthesizer itself in accordance with applicable hygiene standards.
- On the synthesizer software, press User, then select a username and enter the associated password in the pop-up window. Press Method and select the automated protocol previously configured for the [68Ga]Ga-3BP-3940 synthesis.
- Press Preparation and input the synthesis title, vector batch number, reagent kit batch number and any comments in the appropriate text box. A checklist with the various kit preparation steps can also be set up and followed at this stage (Supplementary Figure 1).
4. Preparation of the synthesis cassette and cassette installation
- Clean the radiopharmacy lab workbench with a wipe and the appropriate detergent disinfectant and place a sterile drape over the workbench.
- Obtain a sterile [68Ga]Ga labeling cassette (reference RT-01-H) and a reagents kit (Supplementary Figure 2). The 3 ramps of the tubing set are identified as A, B, and C from left to right on the module; the manifolds of each ramp are numbered from 1 to 5 from top to bottom (ramps A and C) or from left to right (ramp B).
- Assemble the cassette following the steps below.
- Unwrap the [68Ga]Ga cassette envelope, check for any damage, and tighten each Luer connection on the cassette. Remove all the spike caps.
- Using a 5 mL Luer Lock syringe and 21G needle, draw up 5 mL of ethanol absolute from the reagent kit and pass it very slowly over the C18 cartridge, then draw up 5 mL of WFI (not supplied in the kit) and pass it very slowly over the same C18 cartridge to precondition it.
- Position ramp A of the tubing set on the synthesis module and turn the two latches to hold the ramp in place. Connect the free end of the vertical A1 tubing to a 19G needle and insert it into the waste vial.
- Add a venting needle to the waste vial and position it backward, optimally behind the shielded container that will receive the evacuation vial. Place a 0.22 µm filter in position A4.
- Connect the horizontal A1 tubing to the pressure sensor at the bottom left of the module front panel.
- Using a male/male adaptor, connect in horizontal A5 position a 30 cm extension ended by a 0.22 µ terminal filter and an 80 mm 20G needle.
- Insert the above-mentioned 20G needle into a sealed, sterile evacuation vial (e.g., TC-ELU 5), add an aeration needle and position the evacuation vial in its shielded container.
- Place the tubing line connecting vertical A1 to vertical C1 behind the retaining hooks above ramp B.
- Connect horizontal manifolds A2 and B1 with a short extension line (either not included in the kit, or the one initially connected in position C5 horizontal), using the adapter already mounted in position A2.
- Position ramp B on the synthesis module and turn the two latches to hold the ramp in place.
- Connect the pre-conditioned C18 cartridge to the horizontal C2 position, keeping the adapter connecting the horizontal B5 valve to C2 on its left.
- Position ramp C on the synthesis module and turn the two latches to hold the ramp in place.
- Using a male/male adapter, connect a 50 cm extension line from horizontal C5 to the GALLIAD 68Ga generator.
- Place the stained-glass reaction vial of the tubing set in the oven of the module. Carefully place the tubing from vertical A5 to vertical C5 in the peristaltic pump, close the pump, checking that the tubing is correctly positioned, and pass the tubing through the activity sensor on the left-hand side of the pump.
- Perform an intermediate check to ensure that the cassette and tubing are assembled on the radiosynthesizer as shown in Figure 2, without reagents yet.
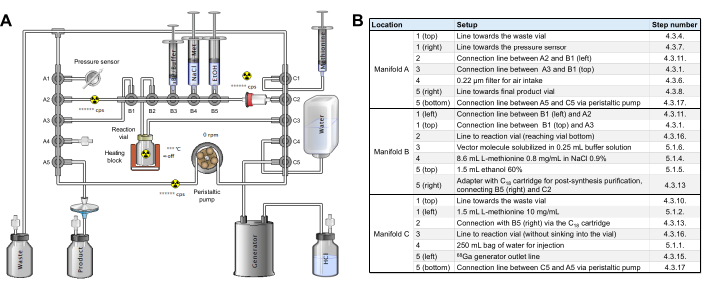
Figure 2: Synthesis module configuration. (A) Setup for automated synthesis of [68Ga]Ga-3BP-3940 on the synthesis module. (B) Details on the reagent positions for automated production of [68Ga]Ga-3BP-3940 using a GAIA synthesis module. Please click here to view a larger version of this figure.
5. Reagents installation
- Once the ramps have been installed on the module, install the reagents as described below.
- Connect the WFI 250 mL bag from the reagent kit to the C4 tubing using the Spike adaptor, and hang the bag on the dedicated hook on the right-hand side of the module.
- Using a 3 mL three-piece Luer Lock syringe equipped with a 20G needle, withdraw 1.5 mL of the previously prepared L-methionine 10 mg/mL solution, connect the syringe to the horizontal C1 tubing and hang the syringe in the dedicated slot on the right-hand side of the module. Leave approximately 2 mL of air between the liquid surface and the syringe plunger seal to ensure complete liquid transfer.
- Using a 1 mL syringe with a 20G needle, withdraw 750 µL of the previously prepared 10 mg/mL L-methionine solution and inject into the 0.9% NaCl vial (blue crimp) from the reagent kit, after disinfection of the septum.
- Using a 10 mL three-piece Luer Lock syringe with a 20G needle, withdraw the contents of the 0.9% NaCl + L-methionine vial (blue crimp), adjust to 8.6 mL, remove the Spike in position B4, then connect the syringe in B4 instead. Leave approximately 2 mL of air between the liquid surface and the syringe plunger seal to ensure complete liquid transfer.
- Using a 3 mL three-piece Luer Lock syringe with a 20G needle, withdraw the contents of the 60% ethanol vial (orange crimp) after disinfection of the septum. Check that the volume is at least equal to 1.5 mL, remove the Spike in position B5, then connect the syringe to B5 instead. Leave approximately 2 mL of air between the liquid surface and the syringe plunger seal to ensure complete liquid transfer.
- Using a low dead volume 1 mL syringe with a 20G needle, withdraw 0.25 mL of the 0.8 M sodium acetate buffer solution previously prepared and inject into the vial containing 30 µg of 3BP-3940 to solubilize it by successive injections/re-aspirations cycles. Withdraw the 0.25 mL solution into the same syringe, disconnect the needle, and place the buffer + vector syringe in B3. Leave approximately 0.25 mL of air between the liquid surface and the syringe plunger to ensure complete liquid transfer.
- Perform a final check before the start of the synthesis as described below.
- Verify that all the reagents are connected to the cassette or tubing, as shown in Figure 3.
- Ensure that each of the kit's connections is sufficiently tight. Make sure no tubing is pinched or angled.
- The system is ready. Close the front panel and switch on the ventilation of the shielded cell.
Figure 3: Kit setup. Final installation of the tubing set and reagents on the synthesizer for the radiolabeling of 3BP-3940 with 68Ga. Please click here to view a larger version of this figure.
6. Automated radiolabeling sequence for [68Ga]Ga-3BP-3940 production
- Click Run Synthesis when all reagents are placed on the ramps and all information is correctly recorded in the software.
- A typical profile of radioactivity distribution in the tubing set throughout the synthesis sequence is shown in Figure 4. Follow the successive steps in the automated method for preparing [68Ga]Ga-3BP-3940 as described below.
- Kit integrity tests: after a brief purge of the tubing towards the waste vial (essentially to remove any remaining liquid in the C18 cartridge), the system increases its internal pressure by opening A4 and pumping filtered air via the peristaltic pump. As soon as the pressure sensor connected to A1 detects a tubing pressure > 1500 mbar, the system closes, and the peristaltic pump stops. If the pressure drop does not exceed 400 mbar over 15 s, assess the integrity test of the kit as successful and begin synthesis. If the test fails, suspect a system leak and abort the synthesis sequence.
- Addition of vector to reaction vial: Ensure that the 3BP-3940 solubilized in the buffer solution is drawn into the reaction vial in around 30 s, through manifold B2.
- Addition of L-methionine to reaction vial: Ensure that the methionine solution contained in the syringe at C1 is transferred to the reaction vial via manifold B2, down ramp C and then up ramp A to branch off at ramp B by manifold A2.
- Activation of C18 cartridge with WFI: Ensure that the WFI contained in the bag at C4 is passed over the C18 cartridge, also rinsing ramps A, B, and C on the paths followed by the vector and methionine solutions. The same routes are then flushed with filtered air to ensure that no water remains in the tubing for the subsequent steps.
- Generator elution in reaction vial (manual intervention): To elute the GALLIAD generator, turn the green knob on top of the generator to 90° to the loading position before waiting for 10-20 s: this time allows the generator to prepare a fixed eluate volume of 1.1 mL. Return the green knob to its initial position, and click the prompter on the Control software. The peristaltic pump then draws the 68Ga eluate into the reaction vial over a 3 min period. Over this time interval, the temperature of the reaction vial is gradually raised to 60 °C over 1 min, then to 90 °C over 2 min, to reach the set temperature for the radiolabeling step more quickly.
- Radiolabeling: Check that at the start of the radiolabeling step, a setpoint temperature of 120 °C is applied for 30 s to enable rapid, efficient heating. Check that the setpoint temperature is then adjusted to 98 °C for 2 min. Due to the heating and pressure rise in the reaction vial, some of the reaction medium tends to flow back up into the tubing connected to B2. To limit this, a 10-second line purge is set after 2 min heating; then radiolabeling continues for around 5.5 min.
- Trapping on C18 cartridge: Ensure that filtered air is pumped into the reaction vessel by C3. The pressure exerted on the surface of the liquid allows the reaction medium to flow upwards through the tubing connected at B2 and then to the SPE cartridge. The reaction vial is then rinsed with WFI from the bag at C4, and this rinsing liquid is transferred to the SPE cartridge in the same way. The cartridge is eventually rinsed with fresh WFI and flushed with filtered air.
- Elution of C18 cartridge and formulation (addition of NaCl 0.9% + L-methionine): The SPE cartridge initially retains the radiolabeled product and any gallium colloids and allows free 68Ga3+ to pass through. To elute the cartridge and recover [68Ga]Ga-3BP-3940 in the terminal vial, ensure successive fractions of ethanol 60% and methionine ~0.9 mg/mL in NaCl 0.9% (3x 0.5 mL each) are drawn over the cartridge for elution. The entire contents of the syringe at B4 are then passed through the C18 cartridge in a formulation step to achieve a final ethanol concentration of <10% in the terminal vial.
- Filter integrity test (manual intervention): After synthesis and removal of the terminal vial, follow the prompt that asks to connect the terminal filter to the waste vial and remove the venting filter from the waste vial in order to check the integrity of the terminal filter using a bubble point test. During the bubble point test, the filter is initially wetted by WFI for 30 s. After purging the lines, pressure in the system is slowly increased up to >2500 mbar over <2 min, during which the synthesizer checks that there is no pressure increase on the outlet of the filter. Then, the system slowly increases the pressure to check the bubble point. The synthesizer will finally record the pressure when pressure increases on the filter outlet to determine the bubble point pressure.
- At the end of the synthesis, the automaton software generates a synthesis report describing the course of the radiolabeling and tracking the temperatures in the reaction vial, the pressures in the system, and the quantities of radioactivity involved (Figure 4). As a complement, manually calculate the decay-corrected radiochemical yield (RCY) according to the formula:
RCY =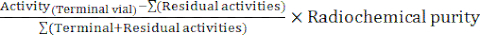
with residual activities describing the activities remaining in the reaction vial, on the C18 cartridge, and in the waste vial at the end of the synthesis.

Figure 4: Typical distribution profile of radioactivity within the module. (A) reaction vial; (B) C18 cartridge during synthesis of [68Ga]Ga-3BP-3940. The flow of 68Ga eluate into the reaction vial occurs at 6 min. The activity remains in the reaction vial throughout the radiolabeling reaction. After 16 min, the activity is transferred to the SPE cartridge. The cartridge is eluted after 19.5 min, after which a residual activity of around 150 MBq remains on the stationary phase. Please click here to view a larger version of this figure.
7. Dispensing and quality controls of [68Ga]Ga-3BP-3940
- Transfer the terminal vial to an appropriate shielded cell for radioactivity measurement and patient dose preparation.
- Measure the activity of the terminal vial with a properly calibrated dose calibrator and record the preparation on the computer.
- Identify the vial correctly and place it in an appropriate shielded container. Using appropriate aseptic techniques and radiation protection techniques, withdraw a ~0.5 mL sample from the terminal vial for quality controls.
- Assess the appearance of the preparation by visual inspection. Assess the pH of the preparation by depositing a drop of product solution on the pH paper strip.
- Measure the radiochemical purity by radio-TLC by depositing a drop of product solution on each of the two previously prepared iTLC-SG plates. Then, allow the plates to migrate into the corresponding mobile phases and read by the radiochromatograph. Integrate the resulting radiochromatogram by measuring the area under the curve of the product signal and the impurity signal, then calculate the radiochemical purity according to the formula:
RCP (%) = 100 - %AUCimpurity 1 - %AUCimpurity 2 - Measure the radiochemical purity by radio-HPLC by injecting ~50 µL of the preparation in the HPLC vial prepared beforehand (see step 2.3.7). Position the vial in the HPLC autosampler at the required position and start the analysis sequence. Once the sample has been injected into the system, remove the vial from the autosampler and replace it in its shielded container to minimize irradiation. At the end of the analysis, integrate the resulting radiochromatogram by measuring the area under the curve of the product signal and the impurities signals, then calculate the radiochemical purity according to the formula:
RCP (%) = 100 - Σ(%AUCimpurities) - Check the half-life (gamma counter) of the radioisotope contained in the preparation by adding ~5 µL of preparation to a gamma counter tube containing 1 mL of WFI. Count the tube with the gamma counter 10x consecutively (counts of 1 min each). Calculate the half-life based on the radioactive decay observed over the 10 measurements.
- Perform the radionuclidic identity assay on the same sample as the half-life assay by conducting gamma spectrometry analysis using the gamma counter, seeking the 511 keV and 1077 keV peaks from annihilation photons.
- Assess the radionuclidic purity after a 48 h decay period of the previous sample. Perform a 120 min measurement in the gamma counter, allowing the detection of any residual 68Ga activity formed in situ from 68Ge breakthrough and other long half-life radionuclide impurities.
8. Stability of the [68Ga]Ga-3BP-3940 preparation
- Assess three test batches for stability over time. For this purpose, withdraw approximately 200 µL of preparation from the terminal vial every hour immediately after the EoS and up to 4 h post-synthesis.
- Perform a radio-HPLC analysis at each time point to measure RCP by HPLC according to the procedure described above. Perform Radio-TLC analyses at each timepoint to measure RCP by TLC according to the procedure previously described.
Results
The synthesis process developed on the GAIA module allows fast 68Ga radiolabeling of 3BP-3940 in 21-22 min. This protocol was designed to work with pharmaceutical grade 68Ge/68Ga generator GALLIAD, which produces 1.1 mL of 68Ga eluate in 0.1 M HCl. The volume and molarity of the reaction buffer were finely tuned according to this amount of acid to obtain a reaction pH between 3.5 and 4, necessary for optimal radiolabeling45. Thus, sodium acetate with a final molarity of 0.1 M was used. Methionine 10 mg/mL was added to the reaction medium as an anti-radiolysis agent to limit the degradation of the vector molecule during the heating step. Preliminary tests without terminal purification compared this antioxidant to gentisic acid 16 mg/mL and ascorbic acid 12 mg/mL and showed better final RCP with methionine than with either of these two other compounds (Figure 5).
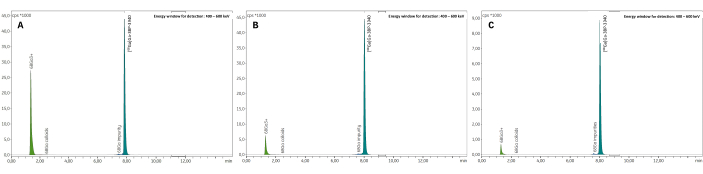
Figure 5: HPLC radiochromatograms of crude reaction media from preliminary experiments comparing anti-radiolysis compounds. (A) Gentisic acid 16 mg/mL (RCP = 75.5%); (B) Ascorbic acid 12 mg/mL (RCP = 86.4%); (C) Methionine 10 mg/mL (RCP = 94.7%). Please click here to view a larger version of this figure.
To validate this automated radiolabeling method, three test batches were produced, resulting in preparations with an average final activity of 737 ± 2.8 MBq (generator at +2 weeks after calibration). Of note, activity losses in the single-use kit system during the synthesis were low, with a mean of 24.9% ± 3.4% over the three test productions (Figure 6), demonstrating the efficiency of the process.
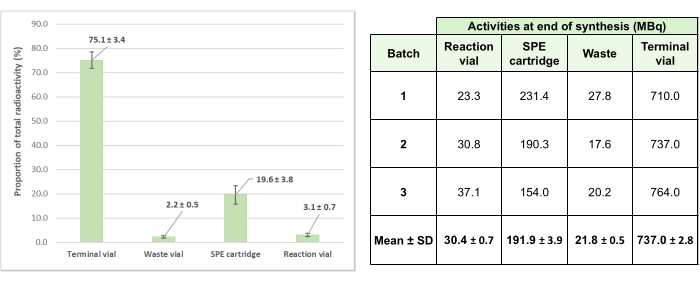
Figure 6: Distribution of average residual activities for the three validation batches of [68Ga]Ga-3BP-3940. The average proportion of total activity involved in the 3 test radiolabeling operations and found in the various elements of the system is detailed as a diagram, and the corresponding absolute activity values for each test synthesis are provided as a table. Please click here to view a larger version of this figure.
The three validation batches passed all quality controls, including radionuclide identification (Supplementary Table 1), calculated half-life (Supplementary Table 2), and radionuclide purity (Supplementary Table 3). In particular, excellent RCP values were obtained in both radio-TLC (mean RCP = 99.19 ± 0.07%; %CV = 0.07) (Supplementary Table 4) and radio-HPLC (mean RCP = 99.19 ± 0.07%; %CV = 0.07; Figure 7, Supplementary Table 5). The reproducibility of RCP values is excellent and may be further confirmed by the results of future syntheses. Also, the RCP values of the three test batches showed great stability over time, both in radio-TLC and radio-HPLC, with values consistently >98% up to 4 h after EoS (Figure 8, Supplementary Table 6). The comprehensive results of the analyses performed on these samples are compiled in Table 1. Overall, the automated 68Ga radiolabeling method of 3BP-3940 was validated with a mean of 74.4% ± 3.3% decay-corrected RCY (calculated from RCP in HPLC). With the use of a pharmaceutical-grade vector, this method could be transposed unchanged to clinical use.
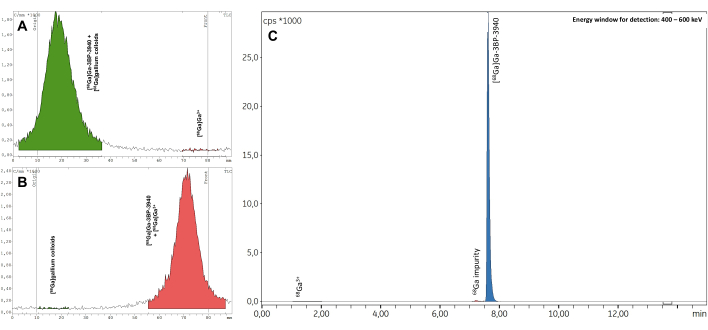
Figure 7: Representative radio-TLC. The results in (A) citrate buffer 0.1 M pH 4 and (B) ammonium acetate 1 M in 1:1 water/methanol. (C) Representative radio-HPLC spectra obtained when measuring the RCP of [68Ga]Ga-3BP-3940. Please click here to view a larger version of this figure.
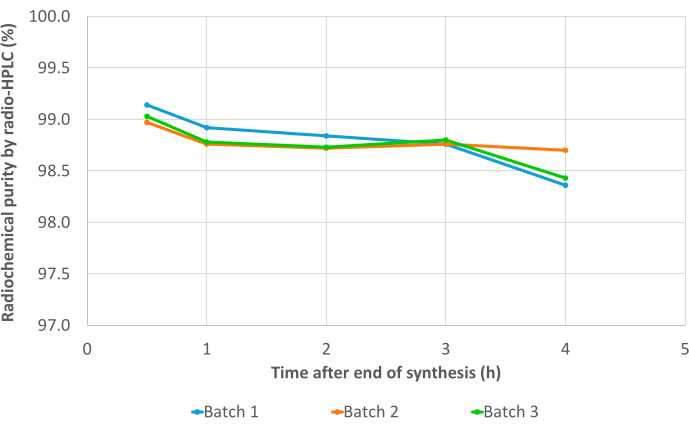
Figure 8: Stability results. Radiochemical purity of the three validation batches of [68Ga]Ga-3BP3940 over time, from EoS to 4 h post-synthesis, determined by radio-HPLC. Please click here to view a larger version of this figure.
| Test | Batch 1 | Batch 2 | Batch 3 | |
| Appearance | Clear, colorless solution | Clear, colorless solution | Clear, colorless solution | |
| Identification | ||||
| Energy of gamma photons (MeV) | 0.511 and 1.077 | 0.511 and 1.077 | 0.511 and 1.077 | |
| Half-life (min) | 69.94 ± 1.03 | 69.34 ± 0.66 | 68.66 ± 0.28 | |
| pH | 6 | 6 | 6 | |
| Radionuclidic purity | ||||
| (68Ga) Gallium (%) | 99.9999861 | 99.9999861 | 99.9985764 | |
| (68Ge) Germanium and other γ-emitting impurities (%) | 1.39 × 10-5 | 5.47 × 10-6 | 1.42 × 10-3 | |
| Radiochemical purity | ||||
| [68Ga]Ga-3BP-3940 (HPLC) | 99.14 | 98.97 | 99.03 | |
| [68Ga]gallium impurities (HPLC) | 0.76 | 1.03 | 0.97 | |
| [68Ga]Ga-3BP-3940 (TLC) | 99.2 | 99.25 | 99.11 | |
| [68Ga]gallium impurities (TLC) | 0.8 | 0.75 | 0.89 | |
| Filter integrity test (mbar) | 4046 | 4082 | 3901 | |
| Volume activity at EoS (MBq/mL)* | 69.27 | 71.9 | 74.54 | |
| Specific activity at EoS (MBq/µg) | 23.46 | 24.31 | 25.22 | |
| Molar activity at EoS (GBq/µmol) | 34.53 | 35.78 | 37.12 | |
| Radiochemical yield (Based on RCP determined by HPLC) | 70.93 | 74.75 | 77.58 | |
| Stability over 4 h (HPLC) | ≥98.36% | ≥98.70% | ≥98.43% | |
| *Calculated with total theoretical volume of 10.25 mL | ||||
Table 1: Mains quality control results for three [68Ga]Ga-3BP-3940 test batches.
Supplementary Figure 1: Supplies for the synthesis module setup. Please click here to download this File.
Supplementary Figure 2: Checklist for installing the kit on the synthesis module. Please click here to download this File.
Supplementary Figure 3: Alternative automated synthesis protocol with SCX cartridge. Please click here to download this File.
Supplementary Table 1: Radionuclide identification for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Supplementary Table 2: Calculated half-life for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Supplementary Table 3: Radionuclide purity for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Supplementary Table 4: Radiochemical purity determined by radio-TLC for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Supplementary Table 5: Radiochemical purity determined by radio-HPLC for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Supplementary Table 6: Radiochemical stability over 4 h determined by radio-TLC for the 3 test batches of [68Ga]Ga-3BP-3940. Please click here to download this File.
Discussion
This work presents a GMP-compliant automated preparation protocol for the synthesis of [68Ga]Ga-3BP-3940 using a GAIA module and a GALLIAD generator. This method was adapted from protocols used in our center for gallium-68 radiolabeling of vectors such as PSMA ligands44 and other FAP inhibitors43,46 for clinical PET imaging, with slight modifications.
The production process was designed to be simple and straightforward and can be divided into three major phases: (i) transfer of all reagents into the reaction vial, (ii) radiolabeling of 3BP-3940 with 68Ga, and (iii) product purification and formulation. Importantly, one of the great strengths of this automated method is its short duration, with the three test batches produced in 22.3 ± 0.6 min (filter integrity test time not included). In comparison, Hörmann et al., who reported on the automated synthesis of [68Ga]Ga-FAP-2286 on a GRP-3V module, underlined a synthesis time of 35 min38.
To ensure a radiolabeled product with high specific activity, only 30 µg of 3BP-3940 was used in the reaction, resulting in a mean specific activity of 24.3 ± 0.9 MBq/µg at EoS for the three test batches. Comparatively, average specific activities reported in the literature range from 6.9 MBq/µg38 to 19.7 MBq/µg29. The only exception is the [68Ga]Ga-FAP-2286 preparation protocol described by Pang et al., which uses 25 µg of vector, resulting in an estimated specific activity of 40.7 MBq/µg (uncorrected for decay during the synthesis time)17.
Several automated protocols in the literature include an initial step of enrichment and purification of the gallium-68 eluate on a strong cation exchange (SCX) cartridge in order to control the volume of 68Ga in an acidic solution added to the reaction medium47. To shorten the preparation time and given the pharmaceutical grade of the GALLIAD 68Ga generator, we decided to omit this step; consequently, the protocol described here is compatible with radiolabeling involving ~1.1 mL of 68Ga eluate in 0.1 M HCl.
The type and molarity of the reaction buffer are crucial parameters in optimizing 68Ga radiolabeling reactions48. The challenge is to maintain a pH of approximately 3.6 to facilitate the chelation of the radiometal by the DOTA chelator while minimizing the formation of insoluble gallium hydroxides49. Sodium acetate, at a final concentration of 0.07 M in the reaction medium, was retained based on its excellent performance in radiolabeling other vectors in our practice46. Consistently, it demonstrated high efficiency in radiolabeling [68Ga]Ga-3BP-3940 in this study. Moreover, this buffer is the most commonly reported in the literature for 68Ga radiolabeling of FAP-targeting pseudopeptides, though typically used at higher molarities (0.25 M to 1 M)17,19,29,35,36. A single article mentions the use of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) as a reaction buffer38. Its weak metal complexing properties make HEPES a very suitable compound for controlling the pH of 68Ga radiolabeling reactions50. However, regulatory constraints on this compound require additional tests to determine its residual quantities in the final radiopharmaceutical preparation, causing further delays between synthesis completion and product use51,52.
Reported temperature settings for the preparation of 68Ga-labeled FAP-inhibiting pseudopeptides vary widely in the literature. In preliminary works and in the corresponding scientific article29,36, the team of Richard Baum observed increased by-product formation when radiolabeling reactions were conducted at temperatures exceeding 90 °C. In this context, lowering the heating temperature appeared as an effective strategy for minimizing the formation of degradation products. Of note, no antioxidant compounds were added to the reaction mixture under these conditions. Nevertheless, the most commonly reported conditions in the literature therefore include heating at 85 °C for 15 min. Even lower heating temperatures can be found, with the 68Ga radiolabeling conditions of a close analog of 3BP-3940, additionally functionalized with a fluorescent group, involving 80 °C heating for 15 min53. Conversely, two protocols for the automated preparation of [68Ga]Ga-FAP-2286 mentioned a heating temperature of 120 °C for 10 min54 and 125 °C for 6 min38, respectively, but resulting in the appearance of by-products for the second. We therefore opted for an intermediate temperature, heating at 98 °C for 8 min, similar to some published processes19,35. Based on the RCP of the three test batches, heating under these conditions in the presence of an antioxidant does not appear to result in the significant formation of radiolysis products during labeling.
Even though it is not systematically used, the addition of an antioxidant compound to the reaction medium can enhance the radiolabeling outcome by achieving higher RCP43. In the final radiopharmaceutical formulation, an antioxidant compound can limit radiolysis phenomena and improve the stability of the radiocomplex over time55,56,57. In the case of 68Ga-labeled, FAP-targeting DOTA-pseudopeptides, some studies suggested insufficient efficacy of ascorbic acid (10 mg or 50 mg in a reaction volume of ~2.6 mL) in preventing the formation of radiolysis by-products38. Interestingly, forced degradation tests in the presence of hydrogen peroxide showed an increase in the proportion of these impurities, suggesting oxidation products, probably formed through the transformation of one or more sulfide groups of the pseudopeptide into sulfoxides and/or sulfones58. This underlines the importance of antioxidants in the reaction medium for the radiolabeling of 3BP-3940 and related molecules. In the present work, preliminary assays (see Figure 5) have identified methionine as an ideal compound for this specific purpose. Continuing with the above hypothesis, it is possible that under oxidizing conditions, sulfone and/or sulfoxide derivatives are preferentially formed with methionine, thereby efficiently preserving the integrity of the sulfide functions in 3BP-3940. In addition to its use in the automated 68Ga radiolabeling protocols of various other vectors labeled with 68Ga59,60 or 177Lu56,61,62,63, methionine is justifiably reported to be used in the preparation of [68Ga]Ga-3BP-394035 and [68Ga]Ga-FAP-228619, in combination with ascorbic acid. Notably, in this protocol, the volume and concentration of each reagent (buffer and antioxidant) were selected to achieve the optimal reaction pH without requiring prior adjustment with concentrated HCl, as described in some methods47,48,64. Again, accurate control of the reaction pH is a key parameter for successful gallium-68 radiolabeling.
The final purification step by solid-phase extraction is necessary to remove traces of free 68Ga3+ that have not been complexed with 3BP-3940. As the C18 cartridge used in the assays showed good results, with an excellent recovery rate (80.3% ± 3.3% of the activity bound to the cartridge recovered after elution), other models were not tested. Interestingly, water-wettable hydrophilic-lipophilic balanced (HLB) reverse-phase cartridges are also reported for the terminal purification of [68Ga]Ga-3BP-394029,36.
As it stands, the primary limitation of this protocol is its compatibility with only a single model of 68Ga generator. However, solutions can be considered for adapting the method to other generators, such as increasing the buffer volume proportionally to the volume of 68Ga eluate. For instance, using 1.25 mL instead of 0.25 mL of 0.8 M sodium acetate buffer could be suitable for a 5 mL 68Ga eluate. Another approach would involve incorporating an SCX cartridge into the setup (typically between manifolds A2 and B1) to concentrate the 68Ga3+ ions from the generator eluate. Then, gallium could be recovered in a fixed volume by eluting the SCX cartridge with a saturated solution of NaCl (5 M) at pH 1. This modification also enables multi-generator syntheses, as demonstrated by Mueller et al., who employed up to four generators for the preparation of [68Ga]Ga-FAP-228619,35. Thus, an alternative protocol incorporating an SCX cartridge is proposed in Supplementary Figure 3. Such an approach would allow a significant upscaling of the method, and could greatly increase the number of patients who could benefit from a given preparation. However, in the method, it is essential to note that buffer volumes should once again be carefully adjusted to allow, under these alternative conditions, a reaction pH of around 3.6, ideal for 68Ga radiolabeling65. The anti-radiolysis agent and buffer should also be prepared directly in the same syringe at B3 to make the C1 position free for the SCX cartridge eluent.
Lastly, as the automated process presented here is GMP-compliant, the use of GMP-grade 3BP-3940 in association with the development and validation of additional quality controls (i.e., preparation sterility, bacterial endotoxins levels, radiochemical identity by radio-HPLC and residual solvent content quantification) would ensure a straightforward transition to clinical applications. This implementation would obviously have to meet all current regulatory requirements to be validated, in particular, the constitution of an IMPD.
Disclosures
The authors have no commercial partnerships or funding sources that would result in a real or perceived conflict of interest relating to this work to disclose.
Acknowledgements
The authors thank Yasmine Soualy, Stéphane Renaud and Élodie Gaven for their help in preparing the radiolabeling reactions presented in this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 µ filters | VWR | 514-0515 | For filtration of buffer and antioxidant solutions and final radiolabeling product |
| Acetonitrile for HPLC | Sigma Aldrich | 34851-2.5L | For HPLC control of radiochemical purity |
| Ammonium acetate | Sigma Aldrich | 238074 | For the preparation of one of the mobile phases for TLC control |
| C18 column for HPLC | VWR | EQV-3C18-1503 | For HPLC control of radiochemical purity |
| Calibrated dose calibrator (CRC25) | Capintec | - | For measuring the radioactivity of the final product and the various components of the module post-synthesis |
| Citrate buffer solution, pH 4 | Thermofisher | 258585000 | Mobile phase for TLC controls |
| Eppendorf tube 5 mL Biopur | Sigma Aldrich | EP0030119479 | For the preparation of buffer and antioxidant solutions |
| Extension line (30 cm) | Vygon | 1159.03 | For the connection of the generator to the tubing set |
| Gallium-68 generator | IRE Elit | - | For in situ generation of [68Ga]gallium chloride |
| Gamma counter (Hidex AMG) | Hidex | - | For half-life and radiochemical purity assessment |
| HPLC station | Shimadzu | - | For HPLC control of radiochemical purity |
| iTLC-SG plates | Agilent | SGI0001 | For TLC control of radiochemical purity |
| L-methionine | AppliChem | A1340 | For antioxidant solution preparation |
| Male/male adapter | Vygon | 893.00 | For the connection of the generator to the tubing set |
| Methanol | Sigma Aldrich | 320390-1L | For the preparation of one of the mobile phases for TLC control |
| Needles (21G, Sterican) | B Braun | 4657543B | For solution transfers prior to radiolabeling |
| pH paper | VWR | 85409.600 | To test the pH of the radiolabelling product |
| Pipette 1000 µL (Gilson PIPETMAN) | Fisher Scientific | 12346132-1000 | For precise liquid measurement and transfer |
| Pipette 200 µL (Gilson PIPETMAN) | Fisher Scientific | 12326132-200 | For precise liquid measurement and transfer |
| Pipette Tips, 100-1000 μL | Charles River | D1000IW | For precise liquid measurement and transfer |
| Pipette Tips, 2-200 μL | Charles River | D200IW | For precise liquid measurement and transfer |
| Radiochromatograph | Elysia-Raytest | - | For TLC control of radiochemical purity |
| Radiosensor for HPLC | Elysia-Raytest | - | For HPLC control of radiochemical purity |
| Reagents kit | ABX | RT-101 | Provides ethanol 60%, NaCl 0.9%, WFI bag, C18 cartridge, 0.2 µ terminal filter, aeration needles, terminal needle and waste vial |
| Shielded container | LemerPax | For radiation attenuation of the radiolabeling product | |
| Single-use plastic spatula | Corning | 3005 | For the preparation of reagents |
| Sodium acetate trihydrate EMPROVE | Sigma Aldrich | 1.28204 | For reaction buffer preparation |
| Sterile sealed vials (glass type 1) | Curium | TC-ELU-5 | For final conditioning of buffer, antioxidant and radiolabeling solutions |
| Sterile tubing set | ABX | RT-01-H | For automated synthesis of [68Ga]Ga-3BP-3940 |
| Sterile water for irrigation | B Braun | 0082479E | For the preparation of one of the mobile phases for TLC control |
| Synthesis module (GAIA) | Elysia-Raytest | - | For automated synthesis of [68Ga]Ga-3BP-3940 |
| Syringe (1 mL, low dead-volume) | B Braun | 9166017V | For peptide in buffer conditionning and addition of methionine in NaCl 0.9% |
| Syringes (10 mL) | Becton Dickinson | 309649 | For methionine in NaCl 0.9% and conditionning |
| Syringes (3 mL) | Becton Dickinson | 309658 | For methionine and ethanol 60% conditionning |
| TLC migration tanks | Fisher Scientific | 50-212-281 | For TLC control of radiochemical purity |
| Trifluoroacetic acid (suitable for HPLC) | Sigma Aldrich | 302031-100ML | For HPLC control of radiochemical purity |
| Tubes for gamma counter | - | - | For half-life and radiochemical purity assays preparation |
| Ultrasonic bath | Selecta | 3000683 | For sonication of prepared solutions |
| Vector molecule (3BP-3940) | MedChemExpress | HY-P10131 | Vector molecule to be radiolabeled |
| Vial for HPLC with glass insert | Sigma Aldrich | 29385-U and SU860066 | For HPLC control of radiochemical purity |
| Vortex mixer | VWR | 444-5900P | For stirring the prepared solutions |
| Water for HPLC | Sigma Aldrich | 34877-2.5L-M | For HPLC control of radiochemical purity |
| Water for injection, 10 mL flasks | Aguettan | 34009 370 641 0 1 | For solutions preparation |
References
- Xiao, Y., Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 221, 107753 (2021).
- Zhang, L. et al. Targets of tumor microenvironment for potential drug development. MedComm Oncol. 3 (1), e68 (2024).
- Fouillet, J., Torchio, J., Rubira, L., Fersing, C. Unveiling the Tumor Microenvironment Through Fibroblast Activation Protein Targeting in Diagnostic Nuclear Medicine: A Didactic Review on Biological Rationales and Key Imaging Agents. Biology. 13 (12), 967 (2024).
- Chen, Y., McAndrews, K. M., Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 18 (12), 792-804 (2021).
- Lindner, T. et al. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm Chem. 4 (1), 16 (2019).
- Jansen, K. et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. 57 (7), 3053-3074 (2014).
- De Decker, A. et al. Novel Small Molecule-Derived, Highly Selective Substrates for Fibroblast Activation Protein (FAP). ACS Med Chem Lett. 10 (8), 1173-1179 (2019).
- Van Rymenant, Y. et al. In Vitro and In Situ Activity-Based Labeling of Fibroblast Activation Protein with UAMC1110-Derived Probes. Front Chem. 9, 640566 (2021).
- Lindner, T. et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 59 (9), 1415-1422 (2018).
- Guglielmo, P. et al. Head-to-Head Comparison of FDG and Radiolabeled FAPI PET: A Systematic Review of the Literature. Life. 13 (9), 1821 (2023).
- Ora, M. et al. Fibroblast Activation Protein Inhibitor-Based Radionuclide Therapies: Current Status and Future Directions. J Nucl Med. 64 (7), 1001-1008 (2023).
- Zhao, L. et al. Synthesis, Preclinical Evaluation, and a Pilot Clinical PET Imaging Study of 68Ga-Labeled FAPI Dimer. J Nucl Med. 63 (6), 862-868 (2022).
- Ferdinandus, J. et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: a case series of nine patients. J Nucl Med. 63 (5), 727-734 (2021).
- Rathke, H. et al. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin Nucl Med. 46 (10), 842-844 (2021).
- Fendler, W. P. et al. Safety and Efficacy of 90Y-FAPI-46 Radioligand Therapy in Patients with Advanced Sarcoma and Other Cancer Entities. Clin Cancer Res. 28 (19), 4346-4353 (2022).
- Zboralski, D. et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 49 (11), 3651-3667 (2022).
- Pang, Y. et al. PET Imaging of Fibroblast Activation Protein in Various Types of Cancer Using 68Ga-FAP-2286: Comparison with 18F-FDG and 68Ga-FAPI-46 in a Single-Center, Prospective Study. J Nucl Med. 64 (3), 386-394 (2023).
- Banihashemian, S. S. et al. [68Ga]Ga-FAP-2286, a novel promising theragnostic approach for PET/CT imaging in patients with various type of metastatic cancers. Eur J Nucl Med Mol Imaging. 51 (7), 1981-1988 (2024).
- Baum, R. P. et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using 177Lu-FAP-2286: First-in-Humans Results. J Nucl Med. 63 (3), 415-423 (2022).
- Loktev, A. et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med. 60 (10), 1421-1429 (2019).
- Banihashemian, S. S. et al. The complete metabolic/molecular response to chemotherapy combined with [177Lu]Lu-FAP-2286 in metastatic breast cancer. Eur J Nucl Med Mol Imaging. 51 (13), 4185-4187 (2024).
- Rao, Z., Zhang, Y., Liu, L., Wang, M., Zhang, C. [177Lu]Lu-FAP-2286 therapy in a case of right lung squamous cell carcinoma with systemic metastases. Eur J Nucl Med Mol Imaging. 50 (4), 1266-1267 (2023).
- Yang, H., Liu, H., Zhang, Y., Zhang, Y., Chen, Y. Metastatic Lung Adenocarcinoma Received Combined 177Lu-FAP-2286 Radiation Therapy and Targeted Therapy. Clin Nucl Med. 49 (6), 569-571 (2024).
- Wan, Z., Wang, W., Chen, Y., Zheng, W., Huang, Z. 177Lu-FAP-2286 Therapy in a Patient With Metastatic Rhabdoid Meningioma. Clin Nucl Med. 49 (9), 879-881 (2024).
- Yang, H., Liu, H., Li, H., Zhang, Y., Chen, Y. 177Lu-FAP-2286 Therapy in a Metastatic Bone Malignant Solitary Fibrous Tumor. Clin Nucl Med. 49 (5), 472-474 (2024).
- Li, L., Yang, J., Peng, D., Zhang, Y., Chen, Y. 177 Lu-FAP-2286 Therapy in a Case of Recurrent Bladder Cancer With Multiple Metastatic Lesions. Clin Nucl Med. 48 (11), 1012-1014 (2023).
- Osterkamp, F. et al. Compounds comprising a fibroblast activation protein ligand and use thereof. https://patents.google.com/patent/US20230212549A1/en (2023).
- Jakobsson, V. et al. First-in-human study of a novel radiolabeled fibroblast activating protein (FAP)-targeted peptide 68Ga-FAP-3BP-3940 for PET/CT imaging in patients with solid tumors. J Nucl Med. 64 (supplement 1), P1615-P1615 (2023).
- Greifenstein, L. et al. 3BP-3940, a highly potent FAP-targeting peptide for theranostics - production, validation and first in human experience with Ga-68 and Lu-177. iScience. 26 (12), 108541 (2023).
- Baum, R. P. et al. FAP-Targeted Radiopeptide Therapy using 177Lu-, 225Ac- and 90Y-labeled 3BP-3940 in Diverse Advanced Solid Tumors: First-in-Humans Results (Abstract P1612). J Nucl Med. 64 (supplement 1), P1612-P1612 (2023).
- Meisenheimer, M., Kürpig, S., Essler, M., Eppard, E. Manual vs automated 68Ga-radiolabelling-A comparison of optimized processes. J Labelled Comp Radiopharma. 63 (4), 162-173 (2020).
- Kleynhans, J. et al. Production of [68Ga]Ga-PSMA: Comparing a manual kit-based method with a module-based automated synthesis approach. J Labelled Comp Radiopharma. 63 (13), 553-563 (2020).
- le Roux, J., Rubow, S., Ebenhan, T. A comparison of labelling characteristics of manual and automated synthesis methods for gallium-68 labelled ubiquicidin. Appl Radiation Isotopes. 168, 109452 (2021).
- Todde, S. et al. EANM guideline for the preparation of an Investigational Medicinal Product Dossier (IMPD). Eur J Nucl Med Mol Imaging. 41 (11), 2175-2185 (2014).
- Mueller, D. et al. Radiolabeling and Stability of FAP- Seeking Radiopharmaceuticals for Radio-Molecular Imaging and Therapy. J Nucl Med. 61 (supplement 1), 1129-1129 (2020).
- Greifenstein, L. et al. Radiolabeling of 3BP-3940 with 68Ga, 90Y, 177Lu and 225Ac for imaging and Peptide Targeted Radiotherapy (PTRT) (Abstract 2532). J Nucl Med. 63 (supplement 2), 2532-2532 (2022).
- Greifenstein, L. et al. Radiolabeling of 3BP-3940 with 68Ga, 90Y, 177Lu and 225Ac for imaging and Peptide Targeted Radiotherapy (PTRT) (Abstract P61). Nuklearmedizin. 62, P61 (2023).
- Hörmann, A. A. et al. [68Ga]Ga-FAP-2286-Synthesis, Quality Control and Comparison with [18F]FDG PET/CT in a Patient with Suspected Cholangiocellular Carcinoma. Pharmaceuticals. 17 (9), 1141 (2024).
- Lütje, S. et al. Optimization of Acquisition time of 68Ga-PSMA-Ligand PET/MRI in Patients with Local and Metastatic Prostate Cancer. PLoS One. 11 (10), e0164392 (2016).
- Haendeler, M. et al. Biodistribution and Radiation Dosimetric Analysis of [68Ga]Ga-RM2: A Potent GRPR Antagonist in Prostate Carcinoma Patients. Radiation. 1 (1), 33-44 (2020).
- Daniel, T., Balouzet Ravinet, C., Clerc, J., Batista, R., Mouraeff, Y. Automated synthesis and quality control of [68Ga]Ga-PentixaFor using the Gaia/Luna Elysia-Raytest module for CXCR4 PET imaging. EJNMMI Radiopharm Chem. 8 (1), 4 (2023).
- Rusu, T. et al. Fully automated radiolabeling of [68Ga]Ga-EMP100 targeting c-MET for PET-CT clinical imaging. EJNMMI Radiopharm Chem. 8 (1), 30 (2023).
- Rubira, L. et al. [68Ga]Ga-FAPI-46 synthesis on a GAIA® module system: Thorough study of the automated radiolabeling reaction conditions. Appl Radiation Isotopes. 206, 111211 (2024).
- Fouillet, J. et al. "One Method to Label Them All": A Single Fully Automated Protocolfor GMP-Compliant 68Ga Radiolabeling of PSMA-11, Transposable toPSMA-I&T and PSMA-617. Curr Radiopharm. 17 (3), 285-301 (2024).
- Nelson, B. J. B., Andersson, J. D., Wuest, F., Spreckelmeyer, S. Good practices for 68Ga radiopharmaceutical production. EJNMMI Radiopharm Chem. 7 (1), 27 (2022).
- Rubira, L., Torchio, J., Fouillet, J., Vanney, J., Fersing, C. GMP-Compliant Automated Radiolabeling and Quality Controls of [68Ga]Ga-FAPI-46 for Fibroblast Activation Protein-Targeted PET Imaging in Clinical Settings. Chem Pharma Bullet. 72 (11), 1014-1023 (2024).
- Mueller, D. et al. Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat Protoc. 11 (6), 1057-1066 (2016).
- Bauwens, M., Chekol, R., Vanbilloen, H., Bormans, G., Verbruggen, A. Optimal buffer choice of the radiosynthesis of 68Ga-Dotatoc for clinical application. Nucl Med Comm. 31 (8), 753-758 (2010).
- Kulprathipanja, S., Hnatowich, D. J. A method for determining the pH stability range of gallium radiopharmaceuticals. Int J Appl Radiation Isotopes. 28 (1-2), 229-233 (1977).
- Martins, A. F. et al. Spectroscopic, radiochemical, and theoretical studies of the Ga3+-N-2-hydroxyethyl piperazine-N'-2-ethanesulfonic acid (HEPES buffer) system: evidence for the formation of Ga3+ - HEPES complexes in (68) Ga labeling reactions. Contrast Media Mol Imaging. 8 (3), 265-273 (2013).
- European Directorate for the Quality of Medicines & Healthcare (EDQM) Gallium (68Ga) PSMA-11 injection. Eur Pharma 11.0. 3044, 1276-1277 (2021).
- European Directorate for the Quality of Medicines & Healthcare (EDQM) Gallium (68Ga) edotreotide injection. Eur Pharma 11.0. 2482, 1274-1276 (2022).
- Li, D. et al. Development of a fibroblast activation protein-targeted PET/NIR dual-modality probe and its application in head and neck cancer. Front Bioeng Biotechnol. 11, 1291824 (2023).
- Koshkin, V. S. et al. Initial Experience with 68Ga-FAP-2286 PET Imaging in Patients with Urothelial Cancer. J Nucl Med. 65 (2), 199-205 (2024).
- De Blois, E., Sze Chan, H., Konijnenberg, M., De Zanger, R., A.P. Breeman, W. Effectiveness of Quenchers to Reduce Radiolysis of 111In- or 177Lu-Labelled Methionine-Containing Regulatory Peptides. Maintaining Radiochemical Purity as Measured by HPLC. Curr Topics Med Chem. 12 (23), 2677-2685 (2013).
- Larenkov, A., Mitrofanov, I., Pavlenko, E., Rakhimov, M. Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules. 28 (4), 1884 (2023).
- Baudhuin, H. et al. 68Ga-Labeling: Laying the Foundation for an Anti-Radiolytic Formulation for NOTA-sdAb PET Tracers. Pharmaceuticals. 14 (5), 448 (2021).
- Kaczorowska, K., Kolarska, Z., Mitka, K., Kowalski, P. Oxidation of sulfides to sulfoxides. Part 2: Oxidation by hydrogen peroxide. Tetrahedron. 61 (35), 8315-8327 (2005).
- Breeman, W. A. P. et al. Optimised labeling, preclinical and initial clinical aspects of CCK-2 receptor-targeting with 3 radiolabeled peptides. Nucl Med Biol. 35 (8), 839-849 (2008).
- Haskali, M. B. Automated preparation of clinical grade [68Ga]Ga-DOTA-CP04, a cholecystokinin-2 receptor agonist, using iPHASE MultiSyn synthesis platform. EJNMMI Radiopharm Chem. 4, 23 (2019).
- Chen, J. et al. Synthesis, stabilization and formulation of [177Lu]Lu-AMBA, a systemic radiotherapeutic agent for Gastrin Releasing Peptide receptor positive tumors. Appl Radiation Isotopes. 66 (4), 497-505 (2008).
- Chatalic, K. L. S. et al. In Vivo Stabilization of a Gastrin-Releasing Peptide Receptor Antagonist Enhances PET Imaging and Radionuclide Therapy of Prostate Cancer in Preclinical Studies. Theranostics. 6 (1), 104-117 (2016).
- De Zanger, R. M. S., Chan, H. S., Breeman, W. A. P., De Blois, E. Maintaining radiochemical purity of [177Lu]Lu-DOTA-PSMA-617 for PRRT by reducing radiolysis. J Radioanal Nucl Chem. 321 (1), 285-291 (2019).
- Hörmann, A. A. et al. Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting. Molecules. 27 (6), 2034 (2022).
- Breeman, W. A. P., Jong, M., Visser, T. J., Erion, J. L., Krenning, E. P. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging. 30 (6), 917-920 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved