Method Article
Optimizing Isolation and Purification of Murine Glomerular Mesangial Cells
In This Article
Summary
This study developed an optimized protocol for the isolation of murine mesangial cells (MCs) and their ex vivo cell culture. These cells can be passaged multiple times, frozen, revived, and cultured without compromising cell growth or protein expression.
Abstract
Mesangial cells (MCs) are stromal cells located in the middle space of the glomerulus, with pivotal functions in glomerular homeostasis. Methods for isolating, purifying, and culturing glomerular MCs have been developed and optimized since the 1980s for use in biomedical research, particularly in the field of nephrology. Mice are the most frequently used experimental animal models in research on renal diseases. In this study, we developed an optimized protocol for murine MCs isolation and ex vivo cell culture. These cells can be passaged multiple times, frozen, revived, and cultured without compromising cell growth or protein expression. This optimized approach significantly reduces the study duration for researchers and enables long-term cell preservation. The necessary equipment for the procedures is easily accessible in a basic biomedical laboratory, and the procedural steps are straightforward. The acquisition of target cells requires only 2-3 weeks, a reduction of at least 1 week compared to existing methods.
Introduction
The glomerulus is a network of capillaries that performs the essential task of filtering blood to form urine1. Mesangial cells (MCs) are embedded within the mesangial matrix, situated between the glomerular capillaries, and are uniquely positioned to influence glomerular dynamics through their diverse functions2. MCs play crucial roles in the glomerulus, including glomerular development, structural support for glomerular capillaries, phagocytosis, and the production of the glomerular basement membrane matrix3. The study of mesangial cells is pivotal for advancing our understanding of renal physiology and pathology.
The involvement of mesangial cells in pathological conditions is also noteworthy. In response to glomerular injury or disease, such as diabetic nephropathy or glomerulonephritis, mesangial cells can proliferate and secrete excess extracellular matrix components, leading to glomerulosclerosis and impaired kidney function4,5. Understanding the functions and regulatory mechanisms of mesangial cells is, therefore, essential for developing therapeutic strategies for kidney diseases.
Murine mesangial cells enable researchers to model and explore the molecular and cellular processes involved in conditions such as IgA nephropathy6, diabetic nephropathy7, and focal segmental glomerulosclerosis (FSGS)8,9. Given their roles in renal fibrosis and inflammation, murine glomerular MCs are frequently used in clinical studies to assess the efficacy of therapeutic compounds9,10. Additionally, murine mesangial cells are an important tool for studying the effects of various signaling pathways on renal function, including the RhoA/ROCK pathway11 and the Transforming Growth Factor-beta (TGF-β) pathway12. These studies help elucidate how these signaling molecules contribute to the progression of kidney disease. Whether used for disease modeling, therapeutic development, or signal transduction research, murine MCs continue to serve as a crucial resource for advancing our understanding of kidney health and disease.
Mackay et al. established a method to acquire ex vivo cell lines of glomerular epithelial, mesangial, and endothelial cells from transgenic mice in 198813. Wilson and Stewart developed a method for isolating and purifying primary MCs from patient kidney tissue, which involves three rounds of sieving and extensive washing with media14. Menè and Stoppacciaro also proposed a method for isolating primary MCs from patient or rat kidney tissue. This technique involves two rounds of sieving, two needle pushes, and collagenase digestion. Cells obtained from 4-8 rat kidneys are plated on a six-well plate, though the yield is relatively low15. These methods necessitate the dissection of the kidneys into small pieces before processing. Additionally, these approaches take approximately 3-4 weeks to yield purified MCs.
Mice are the most frequently employed experimental animal models in research on renal diseases. However, a systematic method for isolating murine MCs is still lacking. In this study, we developed an optimized protocol for murine MCs isolation and ex vivo cell culture. This method can be employed when using primary murine kidney MCs for experimental research. Compared to previous methods, this approach eliminates the need for tissue cutting and sieving prior to digestion. Instead, the entire mouse kidney is ground using a cell grinder and directly digested with collagenase. The digestion solution is then sieved twice, with all cells collected on the second sieve and resuspended. This method allows two mouse kidneys to produce enough cells to seed two to three 100 mm culture dishes within 10 days. Purified MCs are subsequently obtained through culture and purification using a specialized media containing D-valine. These cells can be passaged multiple times, frozen, revived, and cultured without compromising cell growth or protein expression. The equipment required for these procedures is easily available for basic biomedical laboratories, and the entire process takes just 2-3 weeks to obtain the target cells. This method is suited for studies involving murine MCs to investigate kidney-related diseases or mechanisms, as it is efficient and time-saving.
Protocol
Animal experiments complied with the ARRIVE guidelines, and all animal procedures were conducted in accordance with national legislation and the European Commission Directive (2010/63/EU). Mice were housed and maintained in pathogen-free conditions, in compliance with the requirements of the Animal Care and Use Committees of West China Hospital, Sichuan University. All experimental animal studies were approved by the Animal Care and Use Committees of West China Hospital, Sichuan University. Eight-week-old C57BL/6JGpt male mice were used for mesangial cell isolation in this assay. The details of the reagents and equipment used in this study are listed in the Table of Materials. Figure 1 illustrates the procedures for isolating and purifying murine glomerular mesangial cells.
1. Preparation of reagents for murine MCs' isolation
NOTE: Ensure all reagents and equipment are sterile.
- Type I collagenase (750 U/mL): Weigh powder and dissolve shortly before use in RPMI 1640.
- Mesangial cell culture media 1: Mix RPMI 1640 with 2 mM of glutamine, 17% FBS, antibiotics (100 units/mL Penicillin plus 10,000 µg/mL Streptomycin), and 0.1 U/mL insulin.
- Mesangial cell culture media 2: Combine RPMI 1640 (D-valine) with 2 mM of glutamine, 10% FBS, antibiotics (100 units/mL Penicillin plus 10,000 µg/mL Streptomycin), ITS-G (Insulin, Transferrin, Selenium solution 100x), and 0.1 U/mL insulin. Store mesangial cell culture media at 4 °C and use within 1 month after preparation.
- Cell cryopreservation media: Prepare a mixture of RPMI 1640 medium, serum, and DMSO in a 7:2:1 ratio.
2. Isolation of murine MCs
- Humanely sacrifice mice via CO2 asphyxiation followed by cervical dislocation (following institutionally approved protocols). Immerse the carcasses in a beaker containing 75% alcohol for sterilization, then transfer the animal to an aseptic work surface.
- Aseptically remove the kidneys of the mouse using scissors and tweezers, place them into a Petri dish, rinse with EBSS (Earle's Balanced Salt Solution), then carefully remove the connective tissue and kidney capsule using tweezers.
- Halve the kidney vertically, add 3-4 mL of collagenase I solution, and grind with a plastic cell pestle.
- Pipette all tissues and solution from the Petri dish into a 15 mL centrifuge tube. Rinse the Petri dish with 1-2 mL of collagenase I solution to ensure all tissue is transferred. Place the solution in the shaker at 37 °C, shaking at 120 rpm for 30 min. Then, add an equal volume of stop solution (culture media 1).
- Pipette the solution from the 15 mL centrifuge tube, pass it through a 100 µm cell strainer, and collect the filtrate in a 50 mL centrifuge tube.
- Pass the fluid from step 2.5 through a 40 µm cell strainer, retaining only the cells on the 40 µm cell strainer. Rinse the strainer repeatedly with culture media 1 to ensure the collection of as many cells as possible on the strainer. Transfer the collected cells from the strainer into a new 50 mL centrifuge tube.
- Centrifuge the suspension at 600 x g for 5 min (at room temperature), then resuspend the cells in a cell culture dish according to the cell density. Generally, resuspend the cells from two kidneys in two or three 100 mm culture dishes (10 mL culture media 1/100 mm dish). Place the dishes in a 37 °C cell incubator with 95% air/5% CO2.
- Examine the cell morphology and adhesion under a microscope (40×) the following day. If floating dead cells are present in the culture media, use a pipette to remove the media from the bottom of the culture dish, being careful not to disturb the attached cells.
- Add 1-2 mL PBS along the inner edge of the culture dish to rinse the cells. Use a pipette to remove all the PBS from the bottom of the culture dish, and finally add 10 mL culture media 1 along the inner edge of the culture dish.
3. Purification of murine MCs
- One day after separation, remove the non-adherent cells by gently washing and replacing the culture media with fresh culture media 1 as described in step 2.8. Observe the glomerulus cells under a phase contrast microscope (40×).
NOTE: The bright spherical shapes represent the glomerulus, while the surrounding cells exhibit cobblestone-like features, characteristic of epithelial cells (Figure 2A). - Observe the cell morphology after 1-3 days (Figure 2B-C). Stellate-shaped and spindle-shaped cells will appear around the cobblestone cells, as shown in Figure 2D.
- When the cells reach 80% confluence (usually within 7-10 days), wash the cells twice with sterile PBS, then trypsinize the cells with 0.25% trypsin solution (2 mL of 0.25% trypsin solution per 100 mm dish).
- The initial cell trypsinization process may take approximately 20 min. Upon adding trypsin, place the dish in a 37 °C incubator. Observe the process in real-time under a phase contrast microscope (40×) to evaluate the trypsinization of cells.
- Terminate the trypsinization process by adding an equal volume of media 2 when most cells exhibit a rounded morphology and detach from the dish surface.
- Centrifuge the cells at 600 × g for 5 min (at room temperature), then resuspend them in media 2. Generally, resuspend the cells from one 100 mm dish in two 100 mm culture dishes (10 mL culture media 2/100 mm dish).
- Change the culture media 2 the next day. Observe numerous suspended cells and cell fragments under the microscope (40×), as only MCs can survive and proliferate in D-valine's media16. Change the media every 1–2 days to remove dead cells. The adherent cells in the culture are MCs (Figure 3A-D). Determine the growth rate of isolated MCs (Figure 3E).
- Once the cells reach 80% confluence, trypsinize and passage them. MCs typically take approximately 5-10 min for trypsinization.
4. Long-term preservation of murine MCs
NOTE: As primary cells may differentiate and mutate with time and multiple passages, freeze and store MCs after cell purification for long-term preservation.
- Carefully remove the supernatant along the bottom edge of the dish using a pipette, wash the cells twice with sterile PBS, then trypsinize with 0.25% trypsin solution (2 mL of 0.25% trypsin solution per 100 mm dish). Follow the trypsinization steps as described in steps 3.3-3.8.
- After trypsinization, add an equal volume of media 2 to terminate the process, and transfer the entire liquid to a 15 mL centrifuge tube. Centrifuge the cells at 600 × g for 5 min (at room temperature), discard the supernatant, and resuspend the cells in 1 mL PBS to count the cells.
- Use an automatic cell counter to count the cells. Take 20 µL from the 1 mL of resuspended cell solution and add it to a dedicated cell counting plate to determine the total number of cells. Based on the cell count, resuspend them in cell cryopreservation media (1 mL cell cryopreservation media per 106 cells).
- Transfer the cell solution into cryopreservation tubes (1 mL cell cryopreservation media per 2 mL cryopreservation tube) and perform slow freezing to -80 °C before transferring to liquid nitrogen for preservation.
NOTE: Resuspend and seed the cells in cell culture dishes for amplification as needed. Cells should be used between passages 3 and 8 after being fully characterized17.
5. Identification of murine MCs
NOTE: After the cells are passaged twice, identify the murine MCs using the following techniques.
- Western blot of murine MCs
NOTE: The reagents needed for the procedure are provided in Supplementary Table 1.- Trypsinize the cells following the same steps as in 3.3-3.8 and step 4.1.
- After collecting the cells, lyse the cells to extract proteins and measure the protein concentration.
- Take an appropriate amount of sample (usually 20 ng), add loading buffer, and denature at 100 °C for 5 min.
- Resolve the protein (20 ng) with a 10% SDS-PAGE gel or a 7.5% SDS-PAGE gel, then transfer it to a cellulose membrane at a constant voltage.
- Block the membrane with 5% fat-free milk, then incubate with the primary antibody at 4 °C on a shaker overnight.
- Incubate with the secondary antibody at room temperature for 1 h after washing with TBST (Tris Buffered Saline with Tween 20) three times.
- Detect signals with the imaging system.
NOTE: The primary antibodies used in this study included mouse anti-αSMA (1:500), rabbit anti-vimentin (1:2000), rabbit anti-fibronectin (1:2000), and mouse anti-GAPDH (1:50000). The secondary antibodies used in this study included Peroxidase-conjugated Goat Anti-Rabbit IgG (H + L) (1:5000) and Peroxidase-conjugated Goat Anti-Mouse IgG (H + L) (1:5000).
- Immunofluorescence stain of murine MCs
NOTE: The reagents needed for the procedure are provided in Supplementary Table 2.- Trypsinize the cells following the same steps as in steps 3.3-3.8 and step 4.1.
- Resuspend the cells and count them. Take 1 x 105 cells and add them to the 15 mm glass bottom cell culture dish (1 mL culture media 2 per 15 mm glass bottom).
- Fix the MCs with 4% paraformaldehyde for 15 min (1 mL of 4% paraformaldehyde per 15 mm glass bottom), then wash three times with PBS.
- Treat the MCs with 0.1% Triton X-100 for 10 min to permeabilize the cell membranes.
- After three more washes with PBS, block the cells at room temperature with 3% BSA PBS solution for 30 min.
- Wash the cells and incubate them with primary anti-mouse αSMA and anti-rabbit Vimentin antibodies at 4 °C overnight.
- Incubate for 1 h with secondary donkey anti-mouse IgG conjugated to red Alexa 594 and donkey anti-rabbit IgG conjugated to green FITC at room temperature.
- Use DAPI for nuclear counterstaining for 1 min at room temperature.
- Add fluoromount media and cover with a glass slide. Analyze the cells using a confocal microscope.
NOTE: The primary antibodies used in this study included mouse anti-αSMA (1:200) and rabbit anti-vimentin (1:200). The secondary antibodies used in this study included donkey anti-mouse IgG conjugated to red Alexa 594 (1:400), donkey anti-rabbit IgG conjugated to green FITC (1:400), and DAPI (1:1000).
- Flow cytometry of murine MCs
- Trypsinize the cells following the same steps as in steps 3.3-3.8 and step 4.1.
- Resuspend the cells and count them. Take 5 x 105 cells and wash them once with 3 mL PBS.
- Resuspend the cells with 100 µL of PBS in a flow tube, then add 1-2 µL of anti-mouse PDGFRB (CD140b) antibody.
- Incubate the cells on ice for 30 min in the dark, then analyze by flow cytometry.
Results
This study developed an optimized protocol for murine MCs isolation and ex vivo cell culture. To our knowledge, no standard method exists for validating primary MCs ex vivo. According to previous publications, MCs are characterized by the expression of αSMA, Vimentin, and Fibronectin3,14,15. Western blot analysis was used to detect the expression levels of MCs. The MCs isolated in this study exhibited significantly high expression of αSMA, Fibronectin, and Vimentin (Figure 4A). The complete Western blot image is provided in Supplementary Figure 1.
Based on the scRNA-seq database CellMarker, the PDGFRB (CD140b) gene serves as a marker gene of MCs18. After cell selection and culture in media 2 for 5 days, the expression of PDGFRB (CD140b) was verified by flow cytometry (Figure 4F).
To further validate the purity, after cell selection and culture in Media 2 for approximately 7-10 days, the MCs were verified by immunofluorescence staining. As shown in Figure 4B-E, after 10 days of culture in Media 2, more than 95% of the cells expressed both αSMA and Vimentin. The positive immunofluorescence staining results (Figure 4B-E), along with the viability of the cells in D-valine-specific media16, indicate that the protocols described above successfully isolated mouse glomerular MCs. These data further confirm the high purity of the MCs obtained using the differential adhesion method.
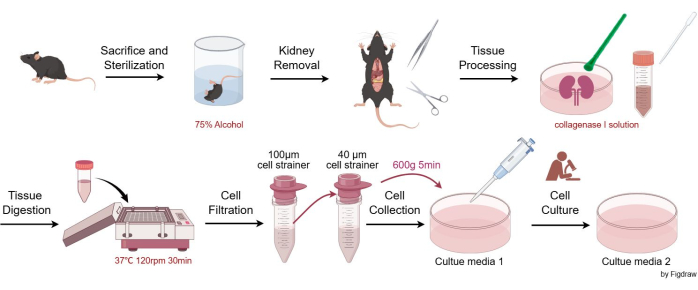
Figure 1: Procedure for isolating and purifying murine glomerular mesangial cells. Please click here to view a larger version of this figure.

Figure 2: Initial culture of isolated cells. (A) Cells on day 1 after isolation, with bright spherical structures representing glomeruli. (B) Cells on day 2 after isolation, showing polygonal cells characteristic of epithelial cells. (C) Cells on day 3 after isolation, with an increased number of epithelial-like cells surrounding the glomerulus. (D) Cells on day 6 after isolation. The red arrows point to the glomerulus. Red scale bars in lower right corner of each image represent 100 µm. Please click here to view a larger version of this figure.

Figure 3: Morphology of isolated cells after trypsinization and replacement of culture media 2 during the first passage. (A) Cells on day 1 after trypsinization, showing stellate-shaped cells adhering to the surface. (B) Cells on day 4 after trypsinization. (C) Cells on day 6 after trypsinization. (D) Cells on day 10 after trypsinization, exhibiting a spindle-shaped, multilayered growth pattern characteristic of mesangial cells. (E) Cells seeded at different densities in 96-well plates, with absorbance at 450 nm measured 2 h after the addition of CCK-8 reagent. Red scale bars in the lower right corner of each image represent 200 µm. Please click here to view a larger version of this figure.

Figure 4: Identification of isolated mesangial cells. (A) Western blot analysis of α-SMA, Vimentin, and Fibronectin expression in mesangial cells, with GAPDH as a loading control. (B-E) Immunofluorescence staining of isolated mesangial cells for Vimentin (green), α-SMA (red), and DAPI (blue). (F) Flow cytometry analysis of PDGFRB (CD140b) expression in mesangial cells cultured in media 2 for 5 days. The blue peak represents specific staining, while the isotype control is shown for comparison. Red scale bars in the upper left corner of each image represent 100 µm. Please click here to view a larger version of this figure.
Supplementary Figure 1: Complete Western blot image. Please click here to download this File.
Supplementary Table 1:Reagents used for Western blot analysis. Please click here to download this File.
Supplementary Table 2: Reagents used for immunofluorescence staining. Please click here to download this File.
Discussion
Kidney-related diseases are highly prevalent, and mice are widely used as the primary animal model for studying these conditions due to their genetic similarity to humans and the availability of well-established disease models. Mesangial cells play a crucial role in maintaining the normal structure and function of the glomerulus by providing structural support, regulating glomerular filtration, and participating in immune responses. While various experimental techniques exist for isolating mesangial cells from humans, rats, and chickens14,15,19, a standardized, efficient, and reproducible method for isolating these cells from mouse kidneys has not yet been established. The lack of such a method limits research progress and hinders the ability to investigate mesangial cell function in mouse models of kidney disease.
In this study, a time-efficient and effective method was developed for the isolation, purification, and ex vivo culture of murine mesangial cells. The optimized protocol ensures a high yield of viable cells, making it a valuable tool for kidney research. Several critical factors must be carefully considered during the experimental process to achieve successful cell isolation and maintain high cell viability: (1) Sterility maintenance: All cell harvesting steps must be performed under a clean bench to prevent microbial contamination, which could compromise cell viability and affect experimental outcomes. (2) Tissue disruption: The grinding rod should be used to thoroughly grind the kidney tissue, ensuring complete mechanical dissociation and facilitating enzymatic digestion. Inadequate grinding may lead to inefficient cell release and lower cell yield. (3) Tissue digestion optimization: During the enzymatic digestion process, periodic observation is necessary to assess the extent of tissue breakdown. Gentle inversion of the solution multiple times helps to enhance enzymatic action and promote better cell dissociation while minimizing damage to the cells. (4) Cell sieving and collection: At the sieving stage, rinsing the cell strainer multiple times is essential to maximize the number of cells collected. Incomplete rinsing may result in cell loss and reduced yield, affecting the overall success of the isolation process.
This method is currently optimized exclusively for the isolation of primary mesangial cells from mouse kidneys, and its applicability to other animal models has not yet been assessed. While successful isolation has been achieved using this protocol, further modifications may be required to adapt it for use in different species. The cultured primary mesangial cells remained viable up to the seventh generation; however, a decline in growth rate was observed in later passages. This reduction in proliferation capacity may be attributed to cellular senescence or alterations in the culture environment. Therefore, it is recommended that the cells be utilized for relevant experiments at the early stages of primary culture to ensure optimal functionality and experimental reproducibility.
Overall, this method provides a reliable, efficient, and reproducible approach for the rapid isolation of primary mesangial cells, utilizing commonly available laboratory equipment and reagents. By offering a straightforward and accessible protocol, this technique facilitates kidney research and supports studies investigating mesangial cell function in disease models. For researchers requiring primary mesangial cells from mice, this method serves as a valuable reference, enabling the establishment of high-quality cell cultures for various experimental applications.
Disclosures
The authors declare no conflicts of interest, financial or otherwise.
Acknowledgements
This work was supported by grants to Y.Z. from the National Natural Science Foundation of China (No. 82470196, No. 82070219 and No. 81870157), and the Sichuan University Faculty Start Fund.
Materials
| Name | Company | Catalog Number | Comments |
| 100 µm Cell Strainer | Biosharp | BS-100-CS | |
| 100 mm Petri Dish | Sorfa | 230301 | |
| 15 mL Centrifuge Tube | Sorfa | 411000 | |
| 15 mm glass bottom cell culture dish | Sorfa | 201200 | |
| 180 kDa Plus Prestained Protein Marker | Vazyme | MP201-01 | |
| 2 mM L-glutamine | BasalMedia | S210JV | |
| 4% Paraformaldehyde | Biosharp | BL539A | |
| 40 µm Cell Strainer | Biosharp | BS-40-XBS | |
| 50 mL Centrifuge Tube | Sorfa | 41000 | |
| 60 mm Petri Dish | Sorfa | 230201 | |
| 75% Alcohol | Knowles | 64-17-5 | |
| 96 Well Cell Culture Plates, TC-treated | Servicebio | CCP-96H | |
| Antibiotics (10 μg/mL ceftriaxone plus 100 μg/mL gentamicin) | NCM | C100C5 | |
| BCA (Bicinchoninic acid) Protein Assay kit | CWBIO | CW0014S | |
| Cell Counting Kit-8 | Oriscience | CB101 | |
| Confocal Microscope | FV-3000 | Olympus | |
| DMSO | Sigma | D2650 | |
| DMSO | Sigma | D2650 | |
| Earle's Balanced Salt Solution (1x EBSS) | Beyotime | C0213 | |
| Fetal Bovine Serum (FBS) | Excell | FSP500 | |
| Fluorescence Cell Analyzer | Mira FL | Countstar | |
| Fluoromount media | Southern Biotech | 0100-01 | |
| Insulin, Transferrin, Selenium Solution 100x (ITS -G) | Gibco | 41400045 | |
| Inverted Fluorescence Microscope | Olympus | IX83 | |
| Lysis Buffer | Adilab | PP1101 | |
| One-Step PAGE Preparation Kit (10%) | Oriscience | PB102 | |
| One-Step PAGE Preparation Kit (7.5%) | Oriscience | PB101 | |
| PE anti-mouse CD140b Antibody | Biolegend | 323605 | |
| Phosphate Buffered Saline (PBS) | Servicebio | G4202 | |
| Plastic Cell Pestle | Biofil | CC-4090 | |
| Proteinase Inhibitor Cocktail | Roche | 4693159001 | |
| PVDF Membrane | Vazyme | E802-01 | |
| Recombinant Human Insulin | Solarbio | 11061-68-0 | |
| Roswell Park Memorial Institute (RPMI) 1640 Medium | Corning | 10-040-CV | |
| SDS-PAGE Sample Loading Buffer (5x) | Servicebio | G2013 | |
| Specially customized Roswell Park Memorial Institute (RPMI) 1640 Medium (D-valine instead of L-valine) | Procell | WH3923U222 | |
| TBS (Tris Buffered Saline) | Servicebio | G0001-2L | |
| Triton X-100 | Biosharp | BS084 | |
| Trypsin-EDTA (0.25%) | Gibco | 25200072 | |
| Tween 20 | Biosharp | BS100 | |
| Type I collagenase | Solarbio | CB140 |
References
- Pollak, M. R., Quaggin, S. E., Hoenig, M. P., Dworkin, L. D. The glomerulus: The sphere of influence. Clin J Am Soc Nephrol. 9 (8), 1461-1469 (2014).
- Mené, P., Simonson, M. S., Dunn, M. J. Physiology of the mesangial cell. Physiol Rev. 69 (4), 1347-1424 (1989).
- Avraham, S., Korin, B., Chung, J. J., Oxburgh, L., Shaw, A. S. The mesangial cell - the glomerular stromal cell. Nat Rev Nephrol. 17 (12), 855-864 (2021).
- Chadban, S. J., Atkins, R. C. Glomerulonephritis. Lancet. 365 (9473), 1797-1806 (2005).
- Qian, Y., Feldman, E., Pennathur, S., Kretzler, M., Brosius, F. C. From fibrosis to sclerosis: Mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 57 (6), 1439-1445 (2008).
- Zhu, Y., et al. Iga Gut microbiome regulates the production of hypoglycosilated iga1 via the tlr4 signaling pathway. Nephrol Dial Transplant. 39 (10), 1624-1641 (2024).
- Kong, L. -. L., et al. Advances in murine models of diabetic nephropathy. J Diabetes Res. 2013, 797548 (2013).
- Schiffer, M., et al. Inhibitory smads and tgf-beta signaling in glomerular cells. J Am Soc Nephrol. 13 (11), 2657-2666 (2002).
- Gyarmati, G., et al. Sparsentan improves glomerular hemodynamics, cell functions, and tissue repair in a mouse model of FSGS. JCI Insight. 9 (19), e177775 (2024).
- Chafin, C. B., Regna, N. L., Hammond, S. E., Reilly, C. M. Cellular and urinary microRNA alterations in NZB/W mice with hydroxychloroquine or prednisone treatment. Int Immunopharmacol. 17 (3), 894-906 (2013).
- Lucero, C. M., et al. Tnf-α plus il-1β induces opposite regulation of cx43 hemichannels and gap junctions in mesangial cells through a rhoa/rock-dependent pathway. Int J Mol Sci. 23 (17), (2022).
- Schnaper, H. W., Hayashida, T., Hubchak, S. C., Poncelet, A. C. Tgf-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 284 (2), F243-F252 (2003).
- Mackay, K., et al. Glomerular epithelial, mesangial, and endothelial cell lines from transgenic mice. Kidney Int. 33 (3), 677-684 (1988).
- Wilson, H. M., Stewart, K. N. Glomerular epithelial and mesangial cell culture and characterization. Methods Mol Med. 107, 269-282 (2005).
- Menè, P., Stoppacciaro, A. Isolation and propagation of glomerular mesangial cells. Methods Mol Biol. 466, 3-17 (2009).
- Gilbert, S. F., Migeon, B. R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 5 (1), 11-17 (1975).
- Kreisberg, J. I., Venkatachalam, M., Troyer, D. Contractile properties of cultured glomerular mesangial cells. Am J Physiol. 249 (4 Pt 2), F457-F463 (1985).
- Adam, M., Potter, A. S., Potter, S. S. Psychrophilic proteases dramatically reduce single-cell rna-seq artifacts: A molecular atlas of kidney development. Development. 144 (19), 3625-3632 (2017).
- Sadovnic, M. J., Brand-Elnaggar, J., Bolton, W. K. Isolation and characterization of chicken mesangial cells. Nephron. 58 (1), 75-84 (1991).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved