Method Article
Generating Primary Cultures for Keratinocyte Live Cell Imaging
In This Article
Summary
This protocol outlines the culture of freshly obtained (passage 0) keratinocytes for use in live cell imaging.
Abstract
Tracking stem cells and committed progenitor behavior at the single-cell level in human skin has been challenging both in vivo and in vitro. Live-cell imaging has allowed for significant advances in the ability to identify differences between keratinocyte stem cells and committed progenitor behavior. Live-cell imaging is an evolving and somewhat challenging method to study keratinocyte behavior in vitro. This protocol has been developed to culture keratinocytes at low seeding density, enabling relatively long-term time-lapse photography and monitoring of individual cell behavior. Passage 0 keratinocytes are grown at clonal density, and time-lapse photography allows documentation of individual cell divisions and the time of their occurrence. For maximum biological relevance, freshly isolated human keratinocytes are placed in vitro. This approach focuses on proliferation. However, this protocol can be adapted for use in other live cell imaging applications measuring individual cell behavior, such as measuring cell migration, wound healing, and motility.
Introduction
The goal of this protocol is to provide the ability to track individual keratinocytes in culture over a relatively long period (several weeks) enabled by low seeding densities and time-lapse photography. Live cell imaging at low density affords investigators the opportunity to visualize cell behavior in vitro at a single cell level. While not possible in conventional cell culture, live cell imaging makes it possible to develop lineage trees in real-time, without the use of labeling, from which one can ascertain information regarding division kinetics, such as cell cycle duration and the proportion of proliferation and differentiation divisions in a colony. It also allows for the study of cell migration and motility. It can be technically challenging, with aspects that need optimization, depending on the cell type and on the data that is to be captured. Other labs have utilized neonatal keratinocytes and/or passaged keratinocytes that are easier to grow1.
However, cell lines that undergo repeated passaging are significantly different in behavior and morphology from their in vivo state2. For the greatest biological relevance in order to obtain the closest to in vivo behavior, passage 0 cells are utilized. In keratinocyte populations, it is possible to distinguish between the stem cell and committed progenitor without sorting or labels because there are distinct differences in division behavior that can be observed via live cell imaging3.
We utilize live cell imaging to study keratinocyte proliferation kinetics. A similar approach has been used to study the motility of cutaneous melanoma cells cocultured with keratinocytes4, adherent cell migration of scratch assays5, and how ROCK inhibitors affect keratinocyte proliferation6. This protocol is specifically designed for culturing cells to facilitate lineage tracing by live cell imaging, although it may be adapted for other purposes.
This protocol outlines the isolation of passage 0 keratinocytes for the purpose of live cell imaging, discusses complications that may arise, and provides considerations that may require alterations to the protocol (Figure 1).
Protocol
Approval from the institution's Committee on Human Research is required for the use of human tissue. Human samples for research are often obtained from commercial sources (for example, ATCC, Lifeline cell technology, and Zen-Bio). Our studies use discarded tissue at the time of surgeries or skin biopsy samples taken specifically for study purposes. These situations require different approvals and consenting procedures. All tissues are obtained after approval from the UCSF Committee on Human Research, utilizing written informed consent and following the tenets of the Declaration of Helsinki.
1. Separation of epidermis from the dermis
- Ensure that appropriate approvals are obtained to use human tissue for the study.
- Obtain skin sample and transport it in an insulated container with an ice pack. Place the sample in collection media containing 5x concentration of penicillin, streptomycin, and amphotericin.
NOTE: The quantity required will depend on the application. In our experience, approximately 1,000,000 keratinocytes per cm2 are obtained from adult and aged skin. However, this can vary significantly for each sample.- Place neonatal foreskins in cell culture media and start the keratinocyte isolation procedure no longer than 48 h after circumcision to minimize cell death. Store the tissue at 4 °C until processing. Processing of adult and aged samples typically begins within a few hours of collection.
- Obtain access to a biosafety cabinet with BS2-level equipment designed for work with agents that cause disease by ingestion, mucous membrane, or percutaneous exposure7. Ensure that the biosafety cabinet is UV sterilized for at least 15 min and that all surfaces are sprayed with 70% alcohol prior to use.
- Put on gloves, spray the collection container with 70% alcohol, and place the container in the fume hood. Place a cooling pad beneath the tissue to prevent it from warming to room temperature and to maintain its integrity. If the tissue is extremely large, cut a portion off for processing and keep the rest moist in the collection container.
NOTE: As many of the aged specimens are large abdominal or breast skin from surgeries, it takes time to cut and process the tissue. Tissue should never be placed directly on the pad, as the extreme cold can cause it to freeze. Instead, it should be placed on an intermediate surface, such as a marble slab. - Place two 100 mm x 15 mm Petri dishes and a sterile #23 surgical scalpel in the hood.
- Open the Petri dishes and place them on the surface of the cabinet such that the inside surfaces of each dish face upwards. Add 10 mL of 10% chlorhexidine gluconate to one of the Petri dishes. Add 10 mL of 5x penicillin/streptomycin/amphotericin/gentamycin in Hank's Balanced Salt Solution (HBSS; 5x) to 2 other Petri dish halves (See Table of Materials).
- Prepare a 6-well plate for the Dispase step. For neonatal tissue, use 3 mL of Dispase per well. For aged tissue samples, use 4 mL. Using inadequate amounts of Dispase for the volume of tissue can result in difficulty separating epidermis from dermis.
- Use a scalpel to cut the tissue into smaller pieces so that it will be better penetrated by Dispase. Dispase only penetrates up to 5 mm from the edges of the tissue, so cut long strips of tissue 0.5-1 cm wide. If not cut small enough, separation of the epidermis from the dermis will not occur.
- Cut thicker tissue into smaller pieces. Cut neonatal foreskins into 3 or 4 pieces (~5 mm x 7 mm). Use a maximum of two entire foreskins per well of Dispase. Superficial abdominal flaps tend to be much thinner, with minimal to no subcutaneous tissue, cut these into longer (~5 mm x 15 mm strips).
- Place a maximum of four strips per well of Dispase. If the tissue obtained has a lot of subcutaneous fat attached, trim off the subcutaneous tissue to help Dispase fully penetrate the tissue. If the tissue is cut too small, a dissecting microscope may be required to determine which side is epidermal and which side is dermal while peeling off the epidermis. Pulling the wrong side of the skin can result in additional tissue trauma.
- Place the tissue epidermal side down in the first Petri dish prepared in step 1.6, containing 10% chlorhexidine gluconate, for 10-20 s Then transfer the tissue epidermal side down to the second Petri dish, containing 5x penicillin/streptomycin/amphotericin (PSA) with HBSS, and agitate for 10-20 s. Transfer the tissue epidermal side down to the third Petri dish, again containing 5x PSA with HBSS, and agitate for another 10 s. Processing multiple samples with the same solution is acceptable.
- Transfer tissue epidermal side down to the 6-well Dispase plate prepared in step 1.7.
NOTE: We recommend the epidermal side down by our convention. Other protocols recommend dermis down8. All Dispase is not equally efficacious. Peeling the epidermis from aged skin proved challenging with certain products, even at concentrations up to 4x the manufacturer's recommended level. This resulted in the loss of time and specimens. Companies use non-standardized units for Dispase, making it difficult to identify how concentrations differ between brands. Due to these challenges, the use of one of the Dispase brands listed in the Table of Materials is highly recommended. - Label the 6-well plate containing the Dispase and samples, and transfer to 4 °C. Incubate neonatal samples for 16-18 h in Dispase and adult/aged samples for 24 h.
NOTE: There is an alternate 2 h protocol that can be used for samples, where the sample is placed in Dispase at 37 °C for 2 h, and then the epidermis is peeled. Unfortunately, this does not always work, and the integrity of the tissue can be compromised after incubation, resulting in a lost specimen. Overnight incubation is preferred whenever possible.
2. Isolation of keratinocytes
- Place 0.05% Trypsin-EDTA (or TrypLE), Trypsin neutralizing solution, and keratinocyte media in a 37 °C water bath 20 min prior to beginning the next set of steps
- Remove a 6-well plate from 4 °C, spray it with 70% ethanol, and place it in a prepared biosafety cabinet (see step 1.3).
- Place sterile toothed forceps and untoothed forceps in a 50 mL conical tube containing 20 mL of 70% ethanol. If outside the hood initially, spray forceps down with 70% ethanol prior to putting it in the hood.
- Using chemically sterilized forceps, grasp a piece of tissue and put it on a sterile surface epidermal side up (use a Petri dish or the inside of the top half of the 6-well plate). Hold the dermal portion of the tissue with toothed forceps using the non-dominant hand and simultaneously firmly grasp the epidermis with the nontoothed forceps and pull it off from edge to edge. It should come off smoothly as one piece.
- Place the epidermis on the inside of the lip of a 15 mL conical tube. Each 15 mL conical tube should have an epidermis of two foreskins maximum.
- Work efficiently; if the tissue dries out, the yield will be compromised. If the Dispase did not appropriately penetrate the tissue, there may be difficulty peeling off the epidermis. In such a case, pull/scrape off the epidermis from the underlying dermis. This also results in a much lower yield and is not recommended.
- Add 4 mL of 0.05% Trypsin-EDTA in the 15 mL conical tube, then close and invert the tube ensuring that all the epidermis is suspended in the Trypsin and not stuck on the side of the tube/the lid. Place in a 37 °C water bath for 10 min. Agitate cells every 2 min by hand.
NOTE: Trypsinizing for more than 10 min may result in a lower cell yield. Also, if the integrity of cell surface proteins is a concern, use TrypLE as it is more specific for its cleavage site, resulting in less damage to cells and has been shown to not significantly decrease surface antigen expression as compared to Trypsin-EDTA9. Furthermore, the water bath is a common source of contamination, so ensure that it is cleaned regularly. Change the water regularly with deionized water containing aquaguard-2 solution. - Place a 50 mL conical tube in the biosafety cabinet. Unscrew the lid and place a 100 µm cell strainer on the opening of the conical tube.
- Remove the 15 mL conical tube containing the digested sample from the water bath, dry thoroughly, and spray down with 70% alcohol prior to placing it back in the biosafety cabinet. Tap the conical tube 3x against the surface of the fume hood then invert 6x. Repeat tapping-inversion cycle 3x.
- Add 6 mL of trypsin-neutralizing solution into the conical tube. Pour through cell strainer into the 50 mL conical tube from step 2.7.
- Rinse the 15 mL conical tube containing the sample with 5 mL of trypsin-neutralizing solutions (TNS). Pour through a cell strainer into the 50 mL conical tube. Centrifuge at 300 x g for 5 min.
- Immediately remove from the centrifuge and pipette the supernatant to prevent cell loss into the medium. There should be only the pellet left with a small collection of fluid. It can be difficult to see the pellet from smaller tissue samples.
NOTE: There is Trypsin present in the supernatant, so it is best to remove as much as possible without losing the pellet. - Resuspend the keratinocyte pellet in 2 mL of keratinocyte media.
NOTE: There are two considerations here. First, which media to use. For this purpose, serum-free media without collagen is recommended. Historically, the standard for keratinocyte cell culture has been Dulbecco's Modified Eagle's Medium (DMEM) +10% Fetal Bovine Serum (FBS) +Ham's F-12 on a fibroblast feeder layer (3t3-J2s)10. However, with the advent of serum-free media and the availability of collagen, multiple other possibilities remain viable (see discussion)11. The second consideration is whether the use of antibiotics in the culture medium is appropriate for live cell imaging application. This will depend on the imaging time duration and experience with the imaging system. Colonies are imaged over multiple weeks using a shared imaging system, making the use of antibiotics a preferred approach despite the potential impact on cultures (see discussion). Penicillin, streptomycin, amphotericin B, and gentamicin-containing keratinocyte medium are used during resuspension, followed by a switch to penicillin/streptomycin-containing media after the initial media change. This method has effectively prevented contamination during extended live-cell imaging. - Count cells and seed the plate (see discussion for comments on selecting seeding density). To ensure uniform seeding distribution, before plating, resuspend keratinocytes cells in the total amount of medium to be plated. After gentle agitation by inversion, vortex lightly for 1-2 s, and plate. Then, move the microplate in a cross-like pattern (up-down, left-right) 3x in the incubator.
- After 24 h, place the plate in the incubator of the live cell imaging system. Perform live cell imaging at 10x with images taken every 20 min. Place the samples at 37 °C and 5% CO2 in the incubator attached to the microscope.
Results
Given the multiple variables that may be manipulated in this method that ultimately result in a variety of outcomes, here we outline common missteps and their resultant outcomes, as well as provide exemplar outcomes and the circumstances behind them.
As with any cell culture technique, contamination is a possibility (Supplementary Video 1). Along with careful sterile techniques, consider using antibiotics in media, especially if culturing for extended periods of time. Frequent contamination was observed when cultured cells were transported to an imaging system at another location without the use of antibiotics. Please see the discussion section for the negatives of antibiotic usage.
As stated in the protocol, media selection is critical. DMEM with 10% FBS and Ham's F-12 on a fibroblast feeder layer was initially used. However, there were problems with this selection (Supplementary Video 2). The initial seeding density of the fibroblasts used was too high, and as time passed, the fibroblasts appeared to expand, obscuring the view of the keratinocytes. Reduction of the seeding density of fibroblasts solved the issue of obscuring fibroblasts. However, additionally, the keratinocytes appeared to differentiate more rapidly than with serum-free medium, and this resulted in non-uniform areas of keratinocytes, which were difficult to track for lineage tracing purposes (Supplementary Video 3). Other issues with DMEM-based media are expanded upon in the discussion.
After encountering issues with traditional DMEM/FBS/Ham's F-12, serum-free media was chosen as an alternative. With culture media supplemented with HKGS and keratinocytes at clonal density there were uniform colonies that were trackable (Supplementary Video 4, Supplementary Video 5). With serum free media, differentiation occurs more slowly (possibly due to lower calcium), and many adherent cells never divide but do not detach (as they do in other media; Supplementary Video 4, Supplementary Video 3).
Seeding density can affect the ability to track cells. Too many cells can result in an inability to track cells accurately, and too few cells, conversely, result in insufficient growth. Many manufacturers of serum-free media recommend pairing their defined medium with collagen, allowing for lower seeding densities (Figure 2). See the discussion for more on this topic.
Keratinocyte differentiation is evident morphologically by large, flattened cells and stacked morphology. See Supplementary Video 2. The assessment of terminal differentiation is a lack of division over 48 h3, 12, 13.

Figure 1: Keratinocyte isolation steps. This diagram illustrates the steps of keratinocyte isolation as described in this protocol. Created in BioRender.com Please click here to view a larger version of this figure.
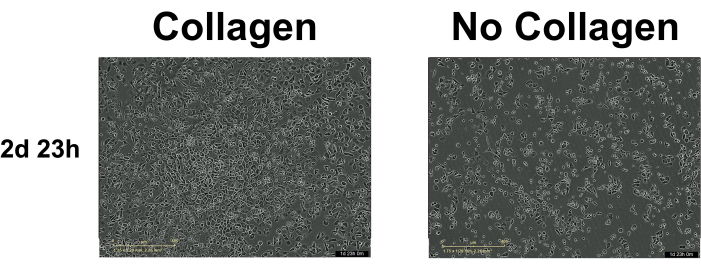
Figure 2: Cell adherence and colony formation. The difference in cell adherence and colony formation at 2 days 23 h with and without collagen. Collagen improves initial keratinocyte adherence significantly. Created in BioRender.com Please click here to view a larger version of this figure.
Supplementary Video 1: Contamination of keratinocyte colony. Images taken every 20 min by live cell imaging collated into video. Please click here to download this File.
Supplementary Video 2: Rapid growth of keratinocytes using traditional feeder and DMEM based media. Images taken every 20 min by live cell imaging collated into video. Please click here to download this File.
Supplementary Video 3: Increased keratinocyte differentiation using traditional feeder and DMEM based media. Images taken every 20 min by live cell imaging collated into video. Please click here to download this File.
Supplementary Video 4: Trackable keratinocyte colony. Images taken every 20 min by live cell imaging collated into video. Please click here to download this File.
Supplementary Video 5: Trackable keratinocyte colony. Images taken every 20 min by live cell imaging collated into video. Please click here to download this File.
Discussion
Here, we have detailed a method for the primary culture of human keratinocytes for the purpose of live cell imaging. This method adapts existing cell culture techniques using low-density plating and long-term culture to allow for successful live cell imaging studies. The preferred method for dissociation of this cell type involves a two-step enzymatic digestion, which has been shown to result in keratinocytes, of which 3%-4% are capable of becoming colony-forming units14. Adult and aged keratinocyte growth in vitro is challenging, as many samples simply fail to grow.
Multiple steps are critical to the goal of maximizing yields to improve growth. One should isolate these cells as soon as possible after tissue collection. A direct line of communication with the surgeons is maintained, and specimens are collected immediately after being obtained from the patient. Several other recommendations have been made on ways to preserve tissue integrity using this method. Keep tissue cool, keep tissue in collection media, and process tissue efficiently. Consider pooling samples, if small, to maximize growth in vitro. Always warm reagents for at least 20 min. Utilize Dispase of demonstrated efficacy and consider cutting smaller strips of tissue or extending the incubation period if there are problems peeling off the epidermis. Use the minimal amount of time possible for digestion with an enzymatic treatment of choice and neutralize immediately after incubation. Resuspend the pellet as soon as possible after centrifugation. Even after doing all of this, expect that there will be occasional adult and aged human samples that do not grow, even under what seems to be the most ideal of circumstances.
The goal is to plate keratinocytes at or near clonal density and track them using live cell imaging; this method aligns well with those objectives. If the goal is to isolate keratinocytes and observe motility under different conditions or for a functional assay like a scratch assay, an alternative method to consider is the skin explant technique. This enzyme-free approach for isolating keratinocytes may result in higher cell yields14,15,16.
Modifications in the media used can be important for live cell imaging. The medium selected to promote keratinocyte growth will impact all phases of the experiment. DMEM, 10% FBS, and Ham's F-12 with 3T3-J2 feeder cells allowed for lower seeding densities but resulted in several issues. Feeder cells, unless used at low density, obscured the visual plane the keratinocytes were on, making tracking more difficult. Using DMEM, the fibroblasts remained for the duration of live cell imaging (~2 weeks). Also, there are other problems with serum-based media that are important, including the promotion of differentiation (which prevents the use of IncuCyte software for live cell imaging due to the lack of uniform appearance of the cells) and the risk of possible contamination from the serum17. In serum-free media, a higher seeding density is needed than with DMEM, as fewer cells adhere. The combinations of 154 CF + HKGS, Epilife + HKGS, and Epilife + S7 have all been effective. As a reminder, always use fresh medium when using growth supplements as they degrade over time. Always test the medium with at least three samples when beginning a new protocol to verify that it can support the cells being cultured. Manufacturers of serum-free media often recommend the use of rat tail collagen (type 1)18. Collagen helps with initial attachment (allowing for lower seeding densities) and proliferation19.
Along with the type of medium, another factor that may affect results is the seeding density and cell distribution. Keratinocyte culture is helped by cell-cell interaction for proliferation, and thus, higher seeding densities result in more prolific growth20. Differing media and cell ages require varying seeding densities to achieve clonal growth. Experiments are usually run with multiple seeding densities to obtain appropriate growth rates/colony sizes for the desired live cell imaging application. Densities for clonal growth and lineage tracing may be different than those needed for other applications. Make sure that the seeding distribution is uniform prior to allowing cells to adhere.
Antibiotic usage during isolation and growth is controversial. It has previously been shown that commonly used antibiotics can have an inhibitory effect on keratinocyte proliferation21. Given this, many opt not to use antibiotics after cleansing the skin initially prior to processing. This live cell imaging application involves multiple weeks of colony growth, increasing the risk of contamination. The risk of losing weeks of time/valuable samples due to contamination needs to be weighed with the risk of possible decreased keratinocyte proliferation. Shorter applications may not need antibiotics, whereas extended periods of live cell imaging do.
A limitation of culturing cells for live cell imaging, as for other in vitro techniques, is the unknown in vivo relevance. Additionally, the use of freshly obtained versus passaged cells can produce different results as cell characteristics evolve as the passage number increases20. Furthermore, results may be influenced by the uniform extrinsic environment. This uniform environment may represent either a strength or a limitation, depending on the objectives of the study, particularly when distinguishing between intrinsic and extrinsic changes in cell behavior.
There is a plethora of factors to be considered when culturing keratinocytes for the purpose of live cell imaging. The presented method can help simplify the process. Being able to successfully perform keratinocyte live cell imaging in future studies will allow a more granular understanding of the proliferative and differentiative behavior of keratinocytes and the mechanisms of epidermal maintenance.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Special thanks to T. Richard Parenteau, MD/PhD, and Alexandra Charruyer, PharmD/PhD, for teaching me how to culture keratinocytes. Thanks to Merisa Piper MD for providing us with samples to isolate keratinocytes from. Final thanks to Michael Rosenblum MD for allowing us access to his live cell imaging system to complete the experiments.
Materials
| Name | Company | Catalog Number | Comments |
| .05% Trypsin-EDTA (1X), 100 mL | Gibco | 25300-054 | |
| 100 um cell filter | Corning | 352360 | |
| 15 cc Falcon | Corning | 352096 | |
| 50 cc conical Falcon | Corning | 352070 | |
| 6 well microplate | Corning | 3516 | |
| 70% reagent alcohol, 4 L | VWR Chemicals | BDH1164-4LP | Can alternatively dilute your own |
| Amphotericin B, 50 ml | Corning | 30-003-CF | Dilute to 5X (50 ug/mL) |
| Dipase, 100 ml | Corning | 354235 | Dilute 1:1 with HBSS, add 1x gentamycin, filter sterilize |
| Epilife, 50 mL | Gibco | MEP1500CA | Add HKGS, consider antibiotics |
| Fetal Bovine Serum Value Heat Inactivated FBS, 500 ml | Gibco | A52568-01 | FBS |
| Forceps | |||
| Gentamicin, 10 ml | Gibco | 15750-060 | Dilute to 50 ug/ml for 5X |
| Hank's Balanced Salt Solution (1X), 500 ml | Gibco | 14170-112 | (HBSS) |
| HBSS 5X PSAG | This is HBSS + 5X concentrations of Pen/Strep/Ampho/Gent | ||
| Hibiclens, Gallon | Molnlycke | 57591 | Dilute to 10% using deionized H20 |
| Human Keratinocyte Growth Serum, 5 mL | Gibco | S-001-5 | Added to epilife |
| Penicillin/Streptomycin, 100 ml | Corning | 30-002-Cl | Dilute to 5X (comes in 100x stock) for 5X PSA - 1X for media changes |
| Petri Dish 100 mm x 15 mm | Fisher Scientific | FB0875713 | |
| Scalpel Blade NO 23 | VWR | 76457-480 | |
| TrypLE | Gibco | 12604021 | A less caustic alternative to regular Trypsin |
| Trypsin Neutralizing Solution | (TNS), this is HBSS with 10% FBS (some use less serum, 5%) |
References
- Mateu, R. et al. Functional differences between neonatal and adult fibroblasts and keratinocytes: Donor age affects epithelial-mesenchymal crosstalk in vitro. Int J Mol Med. 38 (4), 1063-1074 (2016).
- Handl, J., Čapek, J., Majtnerová, P., Báčová, J., Roušar, T. The effect of repeated passaging on the susceptibility of human proximal tubular HK-2 cells to toxic compounds. Phy Res Aca Sci Boh. 69 (4), 731-738 (2020).
- Xiao, T. et al. Short cell cycle duration is a phenotype of human epidermal stem cells. Stem Cell Res The. 15 (1), 76 (2024).
- Noujarède, J. et al. Sphingolipid paracrine signaling impairs keratinocyte adhesion to promote melanoma invasion. Cell Rep. 42 (12), 113586 (2023).
- Sun, M. et al. An image-based dynamic high-throughput analysis of adherent cell migration. Bio-prol. 11 (6), e3957 (2021).
- Chapman, S., McDermott, D. H., Shen, K., Jang, M. K., McBride, A. A. The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res The. 5 (2), 60 (2014).
- U.S. Department of Health and Human Services. Biosafety in microbiological and biomedical laboratories. 6th edition. Centers for Disease Control and Prevention, National Institutes of Health, USA (2020).
- Poumay, Y., Roland, I. H., Leclercq-Smekens, M., Leloup, R. Basal detachment of the epidermis using dispase: tissue spatial organization and fate of integrin alpha 6 beta 4 and hemidesmosomes. J Inv Der. 102 (1), 111-117 (1994).
- Tsuji, K. et al. Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. CellTra. 26 (6), 1089-1102 (2017).
- Rheinwald, J. G., Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 6 (3), 331-343 (1975).
- Boisseau, A. M. et al. Production of epidermal sheets in a serum free culture system: a further appraisal of the role of extracellular calcium. J Der Sci. 3 (2), 111-120 (1992).
- Roshan, A. et al. Human keratinocytes have two interconvertible modes of proliferation. Nat Cell Bio. 18 (2), 145-156 (2016).
- Nanba, D. et al. EGFR-mediated epidermal stem cell motility drives skin regeneration through COL17A1 proteolysis. J Cell Bio. 220 (11), e202012073 (2021).
- Ścieżyńska, A. et al. Isolation and culture of human primary keratinocytes-a methods review. Exp Der. 28 (2), 107-112 (2019).
- Guo, A., Jahoda, C. A. B. An improved method of human keratinocyte culture from skin explants: cell expansion is linked to markers of activated progenitor cells. Exp Derm. 18 (8), 720-726 (2009).
- Orazizadeh, M., Hashemitabar, M., Bahramzadeh, S., Dehbashi, F. N., Saremy, S. Comparison of the enzymatic and explant methods for the culture of keratinocytes isolated from human foreskin. Bio Rep. 3 (3), 304-308 (2015).
- Usta, S. N., Scharer, C. D., Xu, J., Frey, T. K., Nash, R. J. Chemically defined serum-free and xeno-free media for multiple cell lineages. Ann of tra med. 2 (10), 97 (2014).
- Lenihan, C., Rogers, C., Metcalfe, A. D., Martin, Y. H. The effect of isolation and culture methods on epithelial stem cell populations and their progeny-toward an improved cell expansion protocol for clinical application. Cyt. 16 (12), 1750-1759 (2014).
- Bernstam, L. I., Vaughan, F. L., Bernstein, I. A. Keratinocytes grown at the air-liquid interface. In Vitro Cell Dev Biol. 22 (12), 695-705 (1986).
- Ponce, L. et al. Isolation and cultivation of primary keratinocytes from piglet skin for compartmentalized co-culture with dorsal root ganglion neurons. J Cell Bio. 2 (2), 93-115 (2017).
- Nygaard, U. H. et al. Antibiotics in cell culture: friend or foe? Suppression of keratinocyte growth and differentiation in monolayer cultures and 3D skin models. Exp Der. 24 (12), 964-965 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved