Method Article
Development and Assessment of Intracellular Infection Models for Staphylococcus aureus
In This Article
Summary
Staphylococcus aureus (S. aureus) has the capability to disseminate throughout the body, causing persistent and recurrent infections. To better understand these processes, this study establishes an intracellular infection model for S. aureus. This model will provide a crucial foundation for investigating the mechanisms behind intracellular infections.
Abstract
S. aureus can invade and persist within host cells, including immune cells, which allows it to evade immune detection and clearance. This intracellular persistence contributes to chronic and recurrent infections, complicating treatment and prolonging the disease. Consequently, there is a critical need for an intracellular infection model to better understand, prevent, and treat infections caused by S. aureus. This study indicated that antibiotics effectively eliminated extracellular bacteria but could not eradicate those that had entered the cells. Thus, a stable intracellular infection in vitro was established by RAW264.7 infected with S. aureus and co-culturing them with antibiotics. Subsequently, an intracellular infection model in mice was established by injecting peritoneal macrophages containing the intracellular infection. Vancomycin effectively cleared bacterial loads in mice challenged with planktonic S. aureus; however, it was ineffective against mice infected with equal or lower levels of intracellular bacteria within the peritoneal macrophages. This indicates that the intracellular infection model of S. aureus was successfully established, offering potential insights for the prevention and treatment of intracellular infections.
Introduction
S. aureus is a highly contagious pathogen that can cause a range of infections, including skin and soft tissue infections, sepsis, meningitis, pneumonia, and endocarditis1. The clinical misuse of antibiotics has led to increased resistance in S. aureus and the emergence of methicillin-resistant Staphylococcus aureus (MRSA), which poses a significant public health threat in many countries2.
Although S. aureus is not traditionally classified as an intracellular pathogen, emerging evidence suggests that it can persistently colonize host cells following invasion3. The ability of S. aureus to survive intracellularly within host phagocytes is increasingly recognized as a mechanism that facilitates metastatic infection and dissemination throughout the host4,5,6. S. aureus secretes various virulence factors, which create an immune environment that promotes its survival and complicates the host's ability to fully eliminate it7. The accessory gene regulator (agr) and Staphylococcal helper elements (Sae) are two important virulence regulators that are closely related to the survival of Staphylococcus aureus in phagocytes8,9. The agr system is a quorum-sensing mechanism that regulates the expression of numerous virulence factors in S. aureus. It controls the production of toxins and other factors that facilitate bacterial survival and dissemination. During intracellular infection, the agr system plays a critical role in the regulation of virulence factors that are essential for the bacterium's ability to evade host immune responses and survive within host cells. Studies have shown that the agr system influences the bacterium's ability to escape from phagosomes and persist within macrophages. The absence of agr can lead to reduced bacterial survival within host cells and decreased virulence10,11. The Sae system is a two-component regulatory system that controls the expression of several virulence factors in S. aureus. It is involved in the regulation of toxins and enzymes that contribute to the bacterium's ability to invade and damage host tissues. The Sae system also plays a crucial role in S. aureus intracellular survival. It influences the bacterium's ability to resist killing by host phagocytes and evade autophagic degradation12,13.
When pathogens invade, macrophages have phagocytic functions, which can engulf and kill foreign pathogens and activate adaptive immune response14. Most invading bacteria are phagocytosed by macrophages, which then activate various killing mechanisms to eliminate them. However, some S. aureus bacteria can survive within macrophages, leading to persistent infection of the host. In addition to bacterial proteins, the host also impacts the survival and proliferation of S. aureus within macrophages by secreting cytokines15,16,17. Some studies indicate that S. aureus can evade degradation by residing in autophagosomes, creating an intracellular niche that promotes dissemination18. S. aureus escapes autophagic degradation by blocking autophagy flux (e.g., LC3-II, p62) and increasing pH within autolysosomes after macrophage invasion19. This immune evasion is achieved through the regulation of S. aureus virulence factors and autophagy, leading to persistent, hidden infections.
Clearing intracellular infections of S. aureus is crucial for managing persistent and latent infections in clinical practice. Currently, antibiotics are the primary treatment for S. aureus infections, with vancomycin serving as the last line of defense for MRSA infections20,21. However, numerous studies have shown that existing antibiotics are ineffective at eliminating intracellular S. aureus, both invivo and in vitro22,23,24.
There is currently no unified standard for the various intracellular infection models of S. aureus25,26,27, as the conditions of each model differ significantly. Consequently, the same criteria cannot be applied to assess the effectiveness of these models. In this study, we established a universal intracellular infection model of Staphylococcus aureus by optimizing the experimental conditions. This model offers greater convenience compared to others, as it allows for the initial infection of bacteria into cells in vitro, followed by the delivery of these infected cells into the body.
To better understand the mechanisms of intracellular S. aureus infection and develop related drugs, we established both in vitro and in vivo models. A stable intracellular infection model was successfully created in vitro by infecting RAW264.7 and co-culturing them with antibiotics. Then, peritoneal macrophages were extracted and formed into intracellular infections. An intracellular infection model in mice was established by injecting these peritoneal macrophages.
Protocol
Experimental animals, 6-8 weeks old specific pathogen-free (SPF) female BALB/c mice, were purchased from the Beijing HFK Bioscience Co., Ltd (Beijing, China). All animal studies were approved by the Laboratory Animal Welfare and Ethics Committee of Third Military Medical University and were performed in accordance with the institutional and national policies and guidelines for the use of laboratory animals. The mice were kept and vaccinated in SPF facilities and provided free access to sterile food and water. Animals were randomly divided into groups and conceded an adaptation time of at least 7 days before the beginning of the experiments.
1. Preparation for S. aureus
- Strain recovery: Obtain a tube of frozen MRSA252, cauterize and cool the inoculation ring to obtain a ring of bacterial solution, inoculate the bacteria on Tryptic Soy Agar (TSA) plates (see Table of Materials) by the streak plate method28, and culture overnight at 37 °C.
- Bacterial activation: The next day, take one 50 mL shaker flask and add 20 mL of Tryptic Soy Broth (TSB) medium (see Table of Materials). Pick one single colony with a 10 µL pipette tip and add it to the shaker. Incubate the colonies overnight at 220 rpm, 37 °C.
- Secondary activation: The next day, take one 50 mL shaker flask containing 15 mL of TSB medium. Remove 200 µL of bacterial solution from the shaker containing the first activated bacteria, add to the one new flask containing 20 mL of TSB medium, and incubate at 220 rpm, 37 °C for 4 h.
- Collect the secondary activated bacterial solution in a 50 mL centrifuge tube, centrifuge at 3,000 x g for 10 min, and discard the supernatant.
- Resuspend the precipitated bacteria in 10 mL of saline and centrifuge at 3,000 x g for 10 min. Repeat this step 2X.
- Measure the bacterial solution's OD600 value and adjust it to 1.0 with saline. At this time, the bacterial quantity of MRSA252 in this experiment was 1.0 x 109 CFU/mL.
NOTE: The bacterial solution is added to a medium without antibiotics and diluted to the desired concentration; All bacteria used in this experiment were prepared by the above method.
2. Establishment of intracellular infection model in vitro
- Preparation of RAW264.7 cells
NOTE: The cell lines used in this study were obtained from ATCC. All cell lines tested negative for mycoplasma contamination. All experiments were performed on cells in the logarithmic growth phase.- Remove the cells from the liquid nitrogen and thaw them in a 37 °C water bath.
- Put RAW264.7 cells into a 15 mL centrifuge tube, add 2 mL of Dulbecco's modified eagle medium (DMEM; see Table of Materials) supplemented with 10% fetal bovine serum (see Table of Materials), penicillin (100 units/mL) and streptomycin (100 µg/mL; see Table of Materials; complete medium) at 37 °C in 5% CO2. Centrifuge at 300 x g for 5 min.
- Seed 3 x 106 RAW264.7 cells in 100 mm dishes with 10 mL of complete medium. Keep for 12 h in a cell culture incubator at 37 °C in 5% CO2 so that the confluence of the cells is between 50%-60%.
- In vitro intracellular infection
- Take 10 µL of cell suspension and add it to the cell counter to calculate the cell concentration. Count and Seed 2 x 105 RAW264.7 cells in a 24-well plate (see Table of Materials) using 1 mL of complete medium for each well and keep it in a cell culture incubator for 10-12 h.
- Remove the supernatant and add 1 mL of complete medium containing 2 x 106 CFU bacterial solution (step 1.6) to each well. Place it in the cell culture incubator for 2 h. The MRSA252 infected multiplicity of infection
(MOI) is 10. (To verify the correlation between various multiplicities of infection (MOI) values and the phagocytosis of bacteria by cells, we established experimental groups with MOI values of 20, 30, 40, and 50.).
NOTE: The complete medium used when infecting cells does not contain penicillin and streptomycin. - Remove the supernatant and wash cells 2x with PBS (see Table of Materials). Add 800 µL per well of DMEM with 100 µg/mL gentamycin (see Table of Materials) and place in the cell incubator at 37 °C in 5% CO2 for 2 h.
- Evaluation of in vitro intracellular infection model
- Intracellular S. aureus killing assay
- Collect the RAW264.7 cells (steps 2.2.2 and 2.2.3) separately into centrifuge tubes. Wash the cells 2x with PBS, add 0.1% Triton X-100 (see Table of Materials) solution to each tube for 5 min, and collect the lysate in the centrifuge tubes.
- Use PBS to dilute the lysate serially. Take 20 µL of lysate and add it to a microcentrifuge tube containing 180 µL of PBS, then mix thoroughly by pipetting. Dilute step by step following this procedure. Spot each dilution of the lysate onto TSA plates and incubate overnight at 37 °C. Count the colonies on the TSA plates for lysate (Figure 1A).
NOTE: Furthermore, we investigated the optimal experimental conditions for intracellular infection of MRSA252 in peritoneal macrophages. The bacterial load in peritoneal macrophages infected with MRSA252 and treated with varying concentrations of gentamicin is shown in Figure 1B, while the bacterial load after treatment with 100 µg/mL gentamicin for different durations is shown in Figure 1C. - Dilute the supernatant and lysate serially with PBS as described in Section 2.3.1.2, and observe the colonies on the TSA plates for the supernatant and lysate (Figure 2A).
- Confocal laser scanning microscope (CLSM)
- Dilute RAW264.7 cells (step 2.2) into 2 x 105 cells/mL on a confocal petri dish (see Table of Materials) and place them in a cell incubator for adhesion overnight at 37 °C in 5% CO2. Add 1 mL of complete medium containing 2 x 106 CFU and 4 x 106 bacterial solution (step 1.6) to each confocal petri dish and place it in the cell incubator for 2 h, making the MOI 10 and 20, respectively.
- Remove the supernatant and wash cells 3x with PBS, add 100 µg/mL gentamycin DMEM for 2 h, and remove the extracellular bacteria. Then remove the supernatant and wash cells 2x with PBS, add DIL working fluid (see Table of Materials), and incubate at 37 °C for 5 min in the dark.
- Remove DIL working fluid and wash 3x times with PBS, and fix with 4% paraformaldehyde solution (see Table of Materials) for 20 min.
- Remove paraformaldehyde solution and wash 3x with PBS, add DAPI dye (see Table of Materials) and incubate for 5 min in the dark. Remove the DAPI dye solution and wash 5x with PBS. Use CLSM to observe cells (Figure 2B).
NOTE: This work used MRSA252 engineered with a green fluorescent protein (GFP), which was constructed using the homologous recombination method29.
- Intracellular S. aureus killing assay
3. Establishment of in vivo intracellular infection model
- Acquisition of peritoneal macrophages in mice
- Prepare 6% starch broth. According to the mass ratio 3:10:5 (w/w), add three kinds of excipients (beef extract powder, tryptone, sodium chloride; see Table of Materials) successively in the glass container. Add 1 L of ddH2O to a glass container, stir to melt, and heat in the microwave. Add starch soluble (see Table of Materials) with a mass ratio of 6, stir to melt, and ensure to autoclave the glass container at 121 °C for 30 min. Store in the refrigerator at 4 °C until it becomes a paste.
- Collect mouse peritoneal macrophages following the steps described below.
- Pick two Balb/c mice. Use the left hand to grab the neck of the mouse and control the tail. Flip the mouse and let the head down and abdomen up (Figure 3A). Administer intraperitoneal injection of 6% starch broth, 3 mL per mouse (Figure 3B).
NOTE: Due to gravity, the organs in the abdominal cavity will fall backward to the chest, preventing the syringe from injuring other organs. - After 72 h, anesthetize the mice with 3% isoflurane and then sacrifice them by cervical dislocation. Disinfect them with 75% ethyl alcohol. Use an ophthalmic scissor to cut open the abdomen of the mouse to fully expose its peritoneum (Figure 4A-C).
- Inject 10 mL of DMEM medium into the mice to lavage the abdominal cavity by intraperitoneal injection (Figure 4D). After injecting DMEM into the mice's abdominal cavity, rub the mice's abdomen gently for about 1 min to lavage the abdomen more thoroughly. Use a syringe to collect lavage fluid into a 50 mL centrifuge tube (Figure 4E).
- Centrifuge the lavage fluid at 300 x g for 5 min and discard the supernatant.
- Use 10mL DMEM supplemented with 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 µg/mL; complete medium) to blow and suction the collected cells, count them, and dilute cell concentration to 2 x 106/mL and plank onto 6-well plates (see Table of Materials), 1 mL per well.
- Place the 6-well plate at 37 °C in 5% CO2, incubate for 4 h and then remove the supernatant. Wash cells 2x with sterile PBS and culture overnight with 1 mL of DMEM per well at 37 °C in 5% CO2.
- Pick two Balb/c mice. Use the left hand to grab the neck of the mouse and control the tail. Flip the mouse and let the head down and abdomen up (Figure 3A). Administer intraperitoneal injection of 6% starch broth, 3 mL per mouse (Figure 3B).
- Preparation of intracellular bacterial
- Incubate the peritoneal macrophages (step 3.1.2) with MRSA252 for 2 h. Remove the supernatant and wash cells 2x with PBS. Add DMEM complete medium with 100 µg/mL gentamycin (1 mL per well) and incubate for 2 h at 37 °C in 5% CO2.
- Remove the supernatant and wash cells 3x with PBS. Use a cell scraper to collect mouse peritoneal macrophages in 50 mL centrifuge tubes, centrifuge at 300 x g for 5 min. The intracellular bacteria of MRSA252 were successfully prepared in peritoneal macrophages.
- For the calculation of intracellular bacteria, to the peritoneal macrophages infected with intracellular MRSA252, add lysozyme (10 µg/mL, see Table of Materials) at 37 °C for 10 min and then wash with PBS 2x. Then, add 1 mL of PBS to resuspend the cells. Lyse the cells by adding 0.1% Triton X-100 for 5 min.
- Dilute the lysis with series dilutions and drop it onto a TSA plate. Culture overnight at 37 °C. Calculate the intracellular bacteria of MRSA252 in peritoneal macrophages, which were 1.8 x 106 CFU per mouse, by counting the bacterial colonies.
NOTE: When the cell scraper collects cells, the action should be gentle. The cells are scraped down in one direction to avoid cell breakage, which could affect the subsequent experiment.
- Intracellular bacterial infection in mice
- Randomly divide 20 Balb/c mice into four groups (n=5, Figure 5A).
- For Group I, inject 3 x 106 CFU planktonic MRSA252 per mouse by intravenous injection. For Group II, inject 3 x 106 CFU planktonic MRSA252 and vancomycin (110 mg/kg; see Table of Materials) per mouse by intravenous injection. For Group III, inject the intracellular bacteria (prepared in step 3.2) by intravenous injection. For Group IV, inject the intracellular bacteria (prepared in step 3.2) and vancomycin (110 mg/kg) per mouse by intravenous injection.
- Place the mice under the infrared physiotherapy lamp until their tail veins become dilated.
- Intravenously inject 3 x 106 CFU planktonic MRSA252 per mouse of Group I and Group II. Intravenously inject the intracellular bacteria (prepared in step 3.2) per mouse of Group III and Group IV.
NOTE: When using a syringe to collect liquid containing intracellular bacteria, it is recommended to first gently mix the liquid evenly with a pipette to ensure consistent infection levels across all mice. - After 30 min, intravenously inject vancomycin to Group II and Group IV per mouse.
- Evaluation of in vivo intracellular infection model
- Evaluate the intracellular infection model of mice by counting the colonization of bacteria in the kidneys. After 24 h of injection, anesthetize the mice with 3% isoflurane and sacrifice them by cervical dislocation. Disinfect them in 75% ethyl alcohol.
- Immobilize the mice, lift the abdominal skin with tweezers with one hand, cut the abdominal skin of the mouse with ophthalmic scissors with the other hand, find the kidneys in the abdominal cavity, and completely strip the kidneys. Put them in PBS.
- Add 1 mL of PBS to the tissue grinding tube. Transfer the kidneys from each mouse into separate grinding tubes and grind until no solid tissue remains. Pour the homogenized tissue into individual EP tubes and label each tube with the corresponding mouse identification.
- Use PBS to serially dilute the tissue homogenate. Spot each dilution onto separate TSA plates and incubate overnight at 37 °C. Count the colonies and analyze the data (Figure 5B).
Results
Intracellular infection models of S. aureus were successfully established both in vitro and in vivo. By optimizing the experimental conditions for phagocytosis and extending both the concentration and duration of antibiotic treatment, some S. aureus survived within the macrophages (Figure 1). To further assess the antibiotic resistance of S. aureus, macrophages infected with the MRSA252 were treated with antibiotics for 2 h until the cell supernatant no longer contained S. aureus, while some bacteria persisted within the macrophages (Figure 2A). A confocal laser scanning microscope revealed that antibiotics could not kill S. aureus within the cells (Figure 2B). Additionally, bacterial colonization assays in mice showed that while vancomycin could eliminate planktonic S. aureus, it was ineffective against bacteria residing inside cells (Figure 5B). Thus, the intracellular infection model of S. aureus has been successfully established.
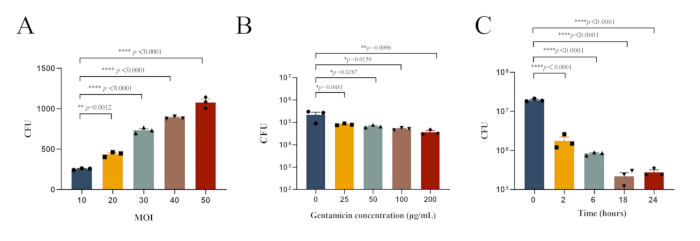
Figure 1: Optimal experimental conditions for intracellular infection of MRSA252 in peritoneal macrophages. (A) Bacterial load in peritoneal macrophages infected with MRSA252 at different MOI values. (B) Bacterial load in peritoneal macrophages infected with MRSA252 and treated with varying concentrations of gentamicin. (C) Bacterial load in peritoneal macrophages infected with MRSA252 after treatment with 100 µg/mL gentamicin for different durations. Data are expressed as mean ± SEM (n=3), and one-way ANOVA with Dunnett's post-hoc test was used to determine significance (**** p < 0.0001, ** p < 0.01, * p < 0.05). Please click here to view a larger version of this figure.
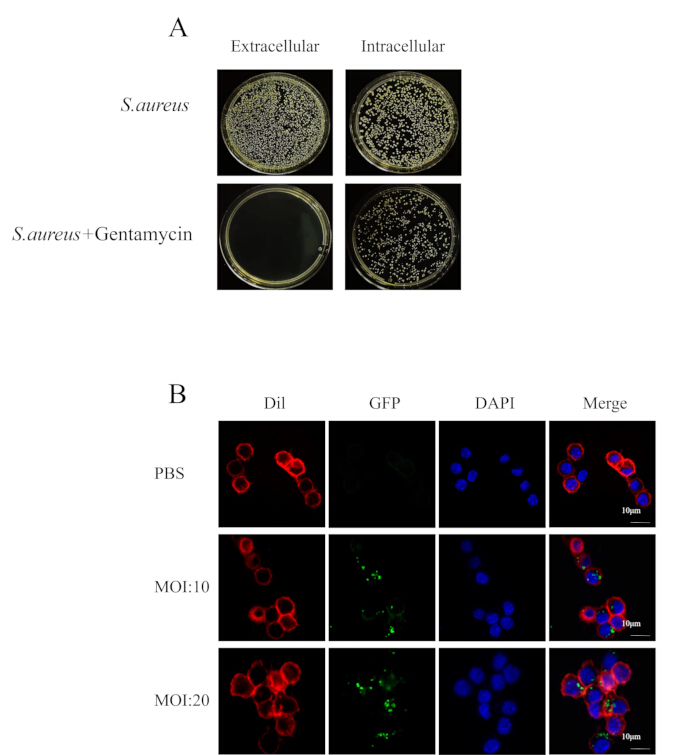
Figure 2: Intracellular S. aureus resists antibiotic treatment. (A) Image of bacterial plate coating of cell suspension and cytoplasm. (B) Confocal microscopy showed the growth of bacteria in RAW264.7 (red) infected by MRSA252 (green) at 4 h points in the presence of antibiotics (scale bar = 10 µm). Please click here to view a larger version of this figure.
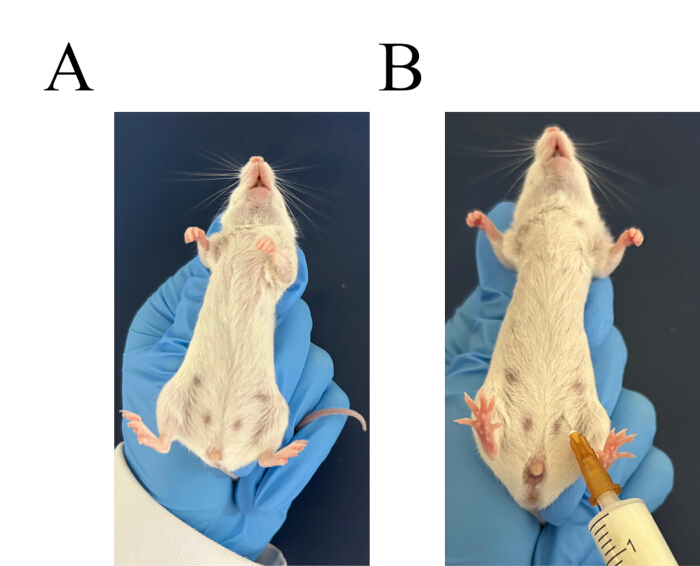
Figure 3: Procedure for intraperitoneal injection. (A) Grasp the mouse securely. (B) Identify the injection site for the intraperitoneal injection. Please click here to view a larger version of this figure.

Figure 4: Procedure for peritoneal macrophage extraction. (A) Cut the surface skin of the abdomen. (B) Perform blunt separation of the skin. (C) Skin separation exposes the peritoneum. (D) Lavage the abdominal cavity. (E) Collect and further lavage the abdominal cavity. Please click here to view a larger version of this figure.

Figure 5: Intracellular infection with MRSA252 is resistant to vancomycin killing. (A) Graphical abstract illustrating the experimental design for establishing infection models with planktonic versus intracellular bacteria. The graphical abstract was drawn by Figdraw. (B) Bacterial loads in the kidney: Bacterial loads were detected 1 day after infection. CFU, colony-forming units. Vanco represents vancomycin. Data were expressed as mean ±SEM (n=5 biologically independent samples), and one-way ANOVA with Dunnett's post-hoc test was used to determine significance (****p < 0.0001, ns represents no significance). Please click here to view a larger version of this figure.
Discussion
S. aureus, as a facultative intracellular pathogen, can invade and survive in various cell types, using this capability to evade antibiotics and immune responses during infection30. This study established an intracellular infection model of S. aureusin vivo to provide a foundation for investigating the pathogen's intracellular infection mechanisms. By exploring the impact of various MOI values on macrophage phagocytosis of S. aureus, as well as the efficacy of different antibiotic concentrations and treatment durations, the optimal conditions for establishing an intracellular infection model were determined (Figure 1).
When constructing the in vitro model of S. aureus intracellular infection, adjust the S. aureus at the logarithmic growth phase to the desired concentration and add it to the cell culture plate. Ensure that the medium does not contain antibiotics, as their presence can hinder the pathogen's ability to enter the cells. After 2 h of infection, a medium containing 100 µg/mL concentration of gentamicin was added to treat the infected cells. Cell colonization experiments (Figure 2A) demonstrated that gentamicin effectively eliminated extracellular bacteria while the remaining bacteria were internalized by the cells. Confocal microscopy further confirmed that this approach facilitated the entry of bacteria into cells, establishing an intracellular infection (Figure 2B).
In this study, mice were stimulated with starch broth to produce peritoneal macrophages, which were then extracted and infected with MRSA252 in vitro. These infected peritoneal macrophages were subsequently injected into mice. For control, mice were also injected with 3 x 106 CFU of planktonic MRSA252. After these injections, vancomycin treatment was administered to all mice.
Vancomycin was administered to mice 24 h prior to assessing bacterial levels, establishing a critical timeframe for intracellular bacterial infection. In mice treated with planktonic MRSA252, vancomycin nearly eradicated the bacterial load in the kidneys. However, the intracellular infection group still exhibited significant bacterial presence despite receiving a lower challenge dose of 1.8 x 106 CFU compared to the planktonic group (Figure 5B). Thus, we conclude that the model was successfully established. In comparison to other models25, we first stimulated mouse peritoneal macrophages for in vitro infection, resulting in an increased recovery of these cells.
In summary, the bacterial invasion and colonization of cells can be observed more directly using MRSA252 engineered with green fluorescent protein. The strain can also be used to evaluate the interaction between S. aureus and the host, for example, the invasion and adhesion of S. aureus to the host. Additionally, the model uses conditions from in vitro intracellular infection to refine and optimize the in vivo model. However, it does not fully explore bacterial colonization in other organs post-intracellular infection and may not entirely reflect clinical infection scenarios. A more in-depth investigation of intracellular infection patterns could improve the model's simulation of clinical situations.
The bacterial invasion and colonization of cells can be directly observed using MRSA252 engineered with green fluorescent protein. This strain also facilitates the evaluation of interactions between S. aureus and the host, including the invasion and adhesion processes. Additionally, the model leverages conditions from in vitro intracellular infection to refine the in vivo model. However, it falls short of fully exploring bacterial colonization in other organs post-intracellular infection in this work and may not accurately represent clinical infection scenarios. A more in-depth investigation of intracellular infection patterns is needed to enhance the model's simulation of clinical situations.
Disclosures
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No.32300779, NO.32270989), Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0156), Science and Technology Research Project of Chongqing Education Commission (KJQN202312802) and China Postdoctoral Science Foundation (2024M754250).
Materials
| Name | Company | Catalog Number | Comments |
| 24-well plate | Corning Incorporated, USA | 3524 | |
| 4 % paraformaldehyde solutione | BBI, UK | E672002-0500 | |
| 6-well plate | Corning Incorporated, USA | 3516 | |
| Beef extract powder | BBI, UK | A600114-0500 | |
| Biohazard safety equipment | Heal force, China | VS-1300L-u | |
| Cell incubator | ESCO, Singapore | CCL-170B-8 | |
| Cell scraper | Nest | 710001 | |
| Centrifuge M1416R | RWD, China | M1416R | |
| Centrifuge tube | Guanghou Labselect, China | CT-002-50A | |
| Confocal laser scanning microscope (CLSM) | Zeiss, Germany | 880 | |
| Confocal petri dish | Biosharp, China | BS-20-GJM | |
| DAPI dye | Shanghai Beyotime, China | C1006 | |
| DIL working fluid | Shanghai Beyotime, China | C1991S | |
| Dulbecco’s Modified Eagle Medium | Thermo Gibco, USA | C11995500BT | |
| Fetal Bovine Serum | Hyclone | SV30208.02 | |
| Gentamycin | Shanghai Sangon, China | B540724-0010 | |
| Incubator | Shanghai Hengzi, China | HDPF-150 | |
| Lysozyme | Beijing Solarbio, China | L9070 | |
| MRSA252 | Third Military Medical University, China | null | |
| MRSA252(GPF) | Third Military Medical University, China | null | |
| Penicillin and Streptomycin | Shanghai Beyotime, China | C0222 | |
| Phosphate Buffer Solution | Shanghai Beyotime, China | ST476 | |
| Saline | Sichuan Kelun, China | null | |
| Sodium chloride | Shanghai Macklin, China | S805275 | |
| Starch soluble | Shanghai Sangon, China | A500904-0500 | |
| Triton X-100 | Shanghai Beyotime, China | P0096-100ml | |
| Tryptic Soy Agar (TSA) plates | Beijing AOBOX Biotechnology Co., LTD,China | 02-130 | |
| Tryptic Soy Broth (TSB) medium | Beijing AOBOX Biotechnology Co., LTD,China | 02-102K | |
| Tryptone | OXOID, UK | LP0042B | |
| Vancomycin | Shanghai Beyotime, China | ST2807-250mg | |
| RAW264.7 cell | USA, ATCC | null |
References
- Sutton, J. A. F., et al. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 17 (3), e1009468 (2021).
- Yang, H., et al. Lateral flow assay of methicillin-resistant Staphylococcus aureus using bacteriophage cellular wall-binding domain as recognition agent. Biosens Bioelectron. 182, 113189 (2021).
- Howden, B. P., et al. Staphylococcus aureus host interactions and adaptation. Nat Rev Microbiol. 21 (6), 380-395 (2023).
- Guo, H., et al. Biofilm and small colony variants-an update on Staphylococcus aureus strategies toward drug resistance. Int J Mol Sci. 23 (3), 1241 (2022).
- Huitema, L., et al. Intracellular escape strategies of Staphylococcus aureus in persistent cutaneous infections. Exp Dermatol. 30 (10), 1428-1439 (2021).
- Pidwill, G. R., et al. Clonal population expansion of Staphylococcus aureus occurs due to escape from a finite number of intraphagocyte niches. Sci Rep. 13 (1), 1188 (2023).
- Wang, M., et al. Autophagy in Staphylococcus aureus infection. Front Cell Infect Microbiol. 11, 750222 (2021).
- Arya, R., et al. Identification of an antivirulence agent targeting the master regulator of virulence genes in Staphylococcus aureus. Front Cell Infect Microbiol. 13, 1268044 (2023).
- Münzenmayer, L., et al. Influence of sae-regulated and agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol. 18 (8), 1172-1183 (2016).
- Chin, D., et al. Staphylococcus lugdunensis uses the agr regulatory system to resist killing by host innate immune effectors. Infect Immun. 90 (10), e0009922 (2022).
- Podkowik, M., et al. Quorum-sensing agr system of Staphylococcus aureus primes gene expression for protection from lethal oxidative stress. Elife. 12, RP89098 (2024).
- Purves, J., et al. Air pollution induces Staphylococcus aureus usa300 respiratory tract colonization mediated by specific bacterial genetic responses involving the global virulence gene regulators agr and sae. Environ Microbiol. 24 (9), 4449-4465 (2022).
- Wittekind, M. A., et al. The novel protein scra acts through the saers two-component system to regulate virulence gene expression in Staphylococcus aureus. Mol Microbiol. 117 (5), 1196-1212 (2022).
- Pidwill, G. R., et al. The role of macrophages in Staphylococcus aureus infection. Front Immunol. 11, 620339 (2020).
- Lang, J. C., et al. A photoconvertible reporter system for bacterial metabolic activity reveals that Staphylococcus aureus enters a dormant-like state to persist within macrophages. mBio. 13 (5), e0231622 (2022).
- Li, M., et al. Interactions between macrophages and biofilm during Staphylococcus aureus-associated implant infection: Difficulties and solutions. J Innate Immun. 15 (1), 499-515 (2023).
- Sun, L., et al. Staphylococcal virulence factor hlgb targets the endoplasmic-reticulum-resident e3 ubiquitin ligase amfr to promote pneumonia. Nat Microbiol. 8 (1), 107-120 (2023).
- Mulcahy, M. E., et al. Manipulation of autophagy and apoptosis facilitates intracellular survival of Staphylococcus aureus in human neutrophils. Front Immunol. 11, 565545 (2020).
- Cai, J., et al. Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux. J Cell Mol Med. 24 (6), 3460-3468 (2020).
- Ahmad-Mansour, N., et al. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins. 13 (10), 677 (2021).
- Davis, J. S., et al. How I manage a patient with MRSA bacteremia. Clin Microbiol Infect. 28 (2), 190-194 (2022).
- Fait, A., et al. Staphylococcus aureus response and adaptation to vancomycin. Adv Microb Physiol. 85, 201-258 (2024).
- Kelly, J. J., et al. Measurement of accumulation of antibiotics to Staphylococcus aureus in phagosomes of live macrophages. Angew Chem Int Ed Engl. 63 (3), e202313870 (2024).
- Rowe, S. E., et al. Recalcitrant Staphylococcus aureus infections: Obstacles and solutions. Infect Immun. 89 (4), e00694-e00720 (2021).
- Lehar, S. M., et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 527 (7578), 323-328 (2015).
- Peyrusson, F., et al. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun. 11 (1), 2200 (2020).
- Pollitt, E. J. G., et al. Staphylococcus aureus infection dynamics. PLoS Pathog. 14 (6), e1007112 (2018).
- Sanders, E. R. Aseptic laboratory techniques: plating methods. J Vis Exp. 11 (63), e3064 (2012).
- Qin, L., et al. Antibody-antibiotic conjugate targeted therapy for orthopedic implant-associated intracellular S. aureus infections. J Adv Res. 65, 239-255 (2024).
- Andie, S. L., et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 31 (4), 18034 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved