Method Article
An Efficient Protocol to Assess ERK Activity Modulation in Early Zebrafish Noonan Syndrome Models via Live FRET Microscopy and Immunofluorescence
In This Article
Summary
RASopathies are multisystem genetic syndromes caused by RAS-MAPK pathway hyperactivation. Potentially pathogenic variants awaiting validation emerge continuously while poor preclinical evidence limits therapy. Here, we describe our in vivo protocol to test and cross-validate RASopathy-associated ERK activation levels and its pharmacological modulation during embryogenesis by live FRET imaging in Teen-reporter zebrafish.
Abstract
RASopathies are genetic syndromes caused by ERK hyperactivation and resulting in multisystemic diseases that can also lead to cancer predisposition. Despite a broad genetic heterogeneity, germline gain-of-function mutations in key regulators of the RAS-MAPK pathway underlie the majority of the cases, and, thanks to advanced sequencing techniques, potentially pathogenic variants affecting the RAS-MAPK pathway continue to be identified. Functional validation of the pathogenicity of these variants, essential for accurate diagnosis, requires fast and reliable protocols, preferably in vivo. Given the scarcity of effective treatments in early childhood, such protocols, especially if scalable in cost-effective animal models, can be instrumental in offering a preclinical ground for drug repositioning/repurposing.
Here we describe step-by-step the protocol for rapid generation of transient RASopathy models in zebrafish embryos and direct inspection of live disease-associated ERK activity changes occurring already during gastrulation through real-time multispectral Förster resonance energy transfer (FRET) imaging. The protocol uses a transgenic ERK reporter recently established and integrated with the hardware of commercial microscopes. We provide an example application for Noonan syndrome (NS) zebrafish models obtained by expression of the Shp2D61G. We describe a straightforward method that enables registration of ERK signal change in the NS fish model before and after pharmacological signal modulation by available low-dose MEK inhibitors. We detail how to generate, retrieve, and assess ratiometric FRET signals from multispectral acquisitions before and after treatment and how to cross-validate the results via classical immunofluorescence on whole embryos at early stages. We then describe how, via examining standard morphometric parameters, to query late changes in embryo shape, indicative of a resulting impairment of gastrulation, in the same embryos whose ERK activity is assessed by live FRET at 6 h post fertilization.
Introduction
RASopathies are genetic syndromes that impair normal development and affect various organs and tissues. These conditions are often caused by germline gain-of-function (GoF) mutations in the key genes and players involved in RAS/MAK signaling, resulting in a hyperactivation (increased phosphorylation) of the extracellular signal-regulated kinase (ERK). ERK regulates some fundamental processes important during development-tissue growth-by translocating to the nucleus1,2. Somatic mutations in genes involved in the RAS-MAPK pathway are the most common events leading to cancer3. Thus, not surprisingly, cancer predisposition is also observed in RASopathies. Noonan syndrome (NS), characterized by developmental delay, short stature, cognitive deficits with variable severity, and cardiomyopathy, is the most common form of RASopathy2. In most cases, the disease is caused by GoF mutations in PTPN11, the first RASopathy gene to be discovered in early 20004 encoding for the protein tyrosine phosphatase SHP2, which acts as a positive regulator of the pathway.
Since then, thanks to the exponential use of exome sequencing approaches in undiagnosed patients, potentially pathogenic variants affecting factors involved in the RAS-MAPK, and likely linked to various forms of RASopathies, continue to be discovered and await functional characterization for efficient patients' stratification2. To achieve this goal, experimental protocols that guarantee fast and informative functional validation at the organismal level are required. Employing classical and standardized mammalian models to test variants with unknown significance would be costly, extremely time-consuming, and require invasive methods in non-transparent large animals. Such a strategy is clearly not compatible with the requirement for fast testing, given the societal burden represented by poor or undiagnosed RASopathy patients, currently without management or treatment. Protocols for quantitative assessment of key phenotypic traits and molecular correlates in entire organisms would also serve to accelerate the possible clinical translation of drugs possibly available to RASopathy patients by repurposing/repositioning.
Zebrafish is an ideal vertebrate model to study diseases that affect early development. As a start, zebrafish share a high level of genetic homology with humans. The high fecundity of adult fish results in a large production of embryos that are small and develop fast. Embryos are transparent at early stages, such that major developmental processes-epiboly, gastrulation, axes, and body plan formation-can be visualized effortlessly using standard microscopy. In addition, the availability of transgenic lines that can be used to track specific cellular behavior and dynamic molecular events in space and time during development, in conjunction with advanced techniques to generate genetic models, is unbeatable. Furthermore, phenotypic readouts can be assessed at multiple levels in zebrafish (from organismal to cellular defects), and dedicated assays are already established for several diseases, including RASopathies5. Moreover, relatively simple bath-immersion methods for drug administration during the early stages, at least for water-soluble compounds, permit high-throughput drug screening in vivo in a 96-well format.
From a molecular point of view, studies using standard approaches, such as immunohistochemistry and immunoblot, robustly demonstrate the correlation between ERK activation and RASopathy-associated developmental defects in fish embryos6,7. The recently developed EKAR-type FRET biosensor in zebrafish (Tg[ef1a:ERK biosensor-nes], Teen) provides a reliable in vivo tool to register ERK activation during embryogenesis in a spatiotemporally resolved manner. Hence, it could be valuable for better assessment of dynamic ERK alterations and pharmacological modulations in RASopathy fish models.
In the Teen sensor, a specific ERK substrate in the reporter is phosphorylated upon ERK activation, triggering a conformational change that brings in close vicinity the fluorescent CFP donor (D) and the fluorescent Ypet (improved YFP) acceptor (A). If the D emission spectrum overlaps considerably with the absorption spectrum of the A, FRET can occur (energy absorption from D to A). This is proportional to the distance between D and A and, therefore, in Teen, to the ERK activation status. Different imaging protocols can be set up using both standard and advanced imaging modules of standard or confocal microscopes in both live and fixed samples. Upon D excitation, the acquisition of multispectral scans along a defined spectrum of emission (λ) from CFP to YFP followed by spectral "unmixing" algorithms is among the most reliable methods to register and quantify FRET data8. It can be applied also to live zebrafish specimens to record in vivo tissue dynamics.
Following previous reports6,9 and our recent application7, here, we detail the step-by-step workflow using Teen fish to assess ERK activation in cells at the margin of the animal pole of NS models at the beginning of gastrulation and correlate it with characteristic body axes defects visible only later in development. We show how to obtain and examine quantitative FRET data from live NS gastrulae before and after treatment with an available MEKi and how to cross-validate the results via standard immunohistochemistry against phosphorylated (active) ERK or perform correlative morphometric analysis of embryo elongation defects.
The workflow could be applied to boost the functional test of emerging variants and disease genes putatively associated with RASopathies and to get insights into the correlation of ERK activation dynamics spatially and temporally during vertebrate development and the morphological defects in embryos. We show that this protocol can also be used to test the efficacy of candidate drugs acting to modulate ERK activation.
Protocol
All experimental procedures involving animals' housing and breeding were conducted according to ARRIVE guidelines for the use of zebrafish in animal research and authorized by the Italian Ministry of Health (Direzione Generale della Sanità Animale e dei Farmaci veterinari - DGSAF). All the DNA/RNA reactions and imaging sessions may be scaled up or down as desired, depending on the final material required or the number of genes and variants tested.
1. Generation and drug treatment of transient zebrafish RASopathy models
NOTE: To monitor the expression of RASopathy-associated variants, specific constructs harboring the desired coding sequence (cds) of the protein of interest in frame with the cds of small non-fluorescent tags (such as myc or similar) can be used. This way, expression levels of the mutant protein can be assessed by standard western blot against the tag. If antibodies against the specific protein of interest are available, tags can be avoided. Immunofluorescence can also be used to assess protein expression within embryo tissue following standard protocols. This type of a control experiment can be useful to correlate mutant protein expression with induced ERK activation levels. The use of fluorescent tags is not advisable in combination of FRET imaging, given the possible fluorescence emission cross-talks during microscopy.
- Plasmid linearization (Days 1-2) (Figure 1A)
NOTE: When generating a transient disease model in zebrafish (RAsopathy here) caused by GoF mutation affecting a specific gene, a rapid approach is to prepare the mRNA encoding the wild-type (WT) and the mutant protein of interest, which should then be injected in zebrafish embryos following the steps below.The plasmid used as template to transcribe the mRNA should contain the desired full-length CDS (coding sequence) for the gene of interest cloned downstream a T7, Sp6, or T3 polymerase promoter and upstream of polyadenylation (polyA) signal. If not available, the first step is to produce it by cloning the zebrafish or human desired CDS in a suitable vector10 and validate by SANGER sequencing. For the cloning, consider extra time (on average 1 week including cloning, screening colonies, and isolating and expanding the correct clones).- Linearize the plasmid with suitable restriction enzymes cutting only once downstream the polyA signal (here KpnI). To achieve efficient plasmid linearization, mix 3 γ (µg/µL) of plasmid DNA, 2 µL of restriction enzyme (20,000 Unit/mL), and 10 µL of 10x reaction buffer in a final volume of 100 µL in nuclease-free water. Mix the components thoroughly by pipetting a few times and incubate the reaction mix at 37 °C for 2-4 h.
- Check the outcome of the linearization by electrophoresis on a 1.5% agarose gel.
- Dilute 10x stock solution of TBE (48.5 g of Tris, 11.4 mL of glacial acetic acid, 20 mL of 0.5 M EDTA [pH 8.0]) with nuclease-free water.
- Prepare the agarose gel by dissolving in the microwave 1.5 g of agarose in a final volume of 100 mL of 1x TBE solution. Then, add 3.5 µL of dye gel stain.
- Cast the gel by pouring the agarose solution into chambers of suitable size, depending on the number of conditions/plasmids. Set the combs and wait approximately 1 h until the gel is solidified; then, remove the combs and position the polymerized gel in the appropriate gel tray for the electrophoresis.
- Mix a few µL of the digested plasmid ("cut") and 1.5 µL of 6x loading buffer (containing a dye to monitor the electrophoresis) in a final volume of 10 µL of nuclease-free water. Prepare in the same manner also a separate control sample with the undigested plasmid ("uncut"). Load the samples in the agarose lanes, and in the first lane, load a 1 Kb DNA ladder. Perform the DNA electrophoresis at 100 V for 30 min.
- Visualize and document the DNA bands resulting from the run on a standard UV transilluminator. Check the efficiency of plasmid linearization by inspecting the pattern of DNA bands comparing "cut" and "uncut."
NOTE: Sufficiently linearized plasmids should run faster as one sharp band. Make sure that the DNA cleavage is complete because initiation of the transcription reaction is a key limiting step that can be hindered by the presence of circularized plasmid forms, resulting in poor mRNA yield.
- Proceed to purify the linearized plasmid using commercially available spin column-based DNA purification kits and store the purified DNA preparation at -20 °C until required.
NOTE: Instead of linearization, it is possible to use a PCR product as the template for mRNA transcription, provided it includes the polymerase promoter sequence upstream and the polyA signal sequence downstream the stop codon. PCR products should also be purified and inspected on an agarose gel before proceeding.
- In vitro mRNA transcription, purification, and quality control (Days 2-3) (Figure 1A)
NOTE: clean carefully the working area and the pipettes with RNAse decontamination solutions. Use nuclease-free materials and reagents; wear gloves. This will minimize RNase contamination and allow a better yield of full-length mRNA.
Transcribe capped and polyadenylated mRNAs encoding WT and mutant proteins from linearized plasmids using standard kits for in vitro mRNA transcription and following manufacturer's instructions.- Centrifuge the purified linearized plasmid for a few seconds at 17,949 × g to ensure that debris is collected at the bottom of the tube and not transferred into the transcription reaction.
- Thaw all the components at room temperature (RT) except the mixture of NTP and CAP analogs, which are kept on ice. Keep the polymerase at -20 °C until use.
- Centrifuge briefly all the reagents at 17,949 × g before use to prevent reagent loss or accidental contamination and briefly vortex the 10x reaction buffer and the ready-to-use NTP/CAP analog mixture until they are completely in solution. Keep the 10x Reaction Buffer at RT.
- Prepare the transcription reaction at RT by adding 600-800 ng of the linearized and purified plasmid to a solution containing 10 µL of 2x NTP/CAP analog mixture, 2 µL of 10x reaction buffer, 2 µL of SP6, T7, or T3 enzyme (depending on the specific promoter upstream the CDS in the plasmid) in a final volume of 20 µL made up with nuclease-free water. Mix thoroughly by pipetting up and down a few times.
- Incubate the transcription reaction at 37 °C in a thermocycler. Make sure to also set the temperature of the lid to 37 °C and run the reaction for 2 h.
- To remove residual plasmid that was not transcribed during the reaction, add 1 µL of standard DNAse solution (2 Units/µL). Mix the reaction thoroughly by pipetting a few times and incubate at 37 °C for an additional 30 min.
- Before proceeding, check the quality and integrity of in vitro synthesized mRNA by TBE/formamide agarose electrophoresis. Dissolve 1.0 g of agarose in 50 mL of 1x TBE solution, add 5.5 mL of 37% formamide and 3.5 µL of dye gel stain, mix, and cast the gel as explained above.
CAUTION: When handling formamide, wear Personal Protective Equipment (PPE) and use a chemical hood. - Mix 1 µL of the transcribed mRNA with 2.5 µL of 2x Formamide Dye Loading Buffer in nuclease-free water in a total volume of 5 µL. Mix the components thoroughly by pipetting a few times and load the samples in the gel lanes. Run the gel at 100 mV for 10-15 min in prechilled 1x TBE buffer. Run also a 1 kb DNA and/or RNA Ladder.
- Optional: Denature the ladder and the mRNA at 70 °C for 10 min.
- Visualize and document the resulting mRNA bands on a standard UV transilluminator.
NOTE: Long runs and high temperatures increase the likelihood of RNA degradation. - If the integrity and size of the synthesized capped RNA are optimal, proceed with polyadenylation of the C-terminal by adding to the reaction: 36 µL of nuclease-free water, 20 µL of 5x denaturing loading buffer, 10 µL of 25 mM MnCl2, 10 µL of 10 mM ATP, and 4 µL of polyadenylation enzyme (such as purified E. coli Poly(A) Polymerase). Mix the reaction thoroughly by pipetting a few times and incubate the 100 µL of the final reaction at 37 °C for an additional 1 h.
NOTE: It is not advisable to check the RNA quality via gel electrophoresis after polyA reaction because the RNA will appear smeared due to the polyA tails. - Precipitate and recover the synthesized capped and polyadenylated mRNA using appropriate salts such as LiCl. Briefly, mix equal volumes of nuclease-free water and standard 2.5 M LiCl solution (30 µL) and incubate for >30 min at -20 °C.
NOTE: Efficient precipitation can be obtained after 2 h, but overnight incubation typically increases the final yield of mRNA. - To pellet the purified mRNA, centrifuge the reaction for 30 min at 4 °C at 17,949 × g and remove the supernatant. Now, wash the pellet with ~1 mL of 70% ethanol (EtOH), and centrifuge for 15 min at 4 °C at 17,949 × g to remove contaminants and residual nucleotides from the reaction.
- Air dry the pellet and then re-suspend it in 20 µL of nuclease-free water. Dissolve the RNA well by gently and repeatedly pipetting at RT and incubate at 10 min.
NOTE: Check the pellet every 5 min to ensure EtOH evaporation. When resuspending, given the stickiness of the RNA, pipette it until it is properly dissolved in water. - Assess the concentration and purity of the prepared mRNA by measuring the absorbance at 260 nm and 280 nm (260/280 ratio) in a spectrophotometer. Measure different scalar dilutions of the RNA preparation to ensure correct concentration estimation.
NOTE: Normally, the concentration of the synthesized RNA ranges from 1 to 2 µg/mL, but the final amount can differ depending on the CDS length and amount of starting material. - While assessing the quality, keep the mRNA stock in ice, then aliquot it into 5 µL aliquots and store at -80 °C until required for injection.
- Preparation of standard solutions and materials for embryo injection and drug treatment (Days 2-3, Figure 1B)
- Pull microinjection needles for zebrafish embryos using sterile capillaries (standard capillary dimensions: 1.0 OD x 0.58 ID x 100 L mm). Use a commercially available puller apparatus and apply the desired settings, in terms of output voltage, pulling mode (one step or two steps), and pulling force to obtain variable tip length, diameter, and needle shape. For commonly used needle features for zebrafish, refer to Abdelrahman and colleagues11.
NOTE: Optimal settings might vary across laboratories because environmental conditions (such as humidity or room temperature) can influence the needle-pulling results. Settings should be regularly re-tested and calibrated. Pulled needles can be stored at RT in clean containers for several months. - Prepare the 30x "Danieau" stock solution at pH 7.6 by dissolving 101.7 g of NaCl, 1.56 g of KCl, 2.96 g of MgSO4 x 7H2O, 4.25 g of Ca(NO3)2, and 35.75 g of HEPES in 1 L of double-distilled (DD) water for preparing the microinjection solution. Prepare also a stock solution of Phenol red at 5% in DD water for use as visible tracer of the injection mixture.
- Prepare E3 medium solution for embryo growth by diluting 4 mL of NaCl (5 M), 680 µL of KCl (1 M), 1.32 mL of CaCl2 x 2H2O (1 M), and 1.32 mL of MgSO4 (1 M) in a final volume of 4 L of reverse-osmosis water.
- Prepare stock solutions of the desired drugs (in this example: the MEK inhibitor [MEKi] PD032590) as stock solutions according to the product datasheet and solubility (10 mM of the MEKi of choice by dissolving 5 mg in 1.036 mL of DMSO). Mix thoroughly and generate small aliquots to store at -80 °C until use.
NOTE: DMSO is a common solvent used to dissolve both polar and nonpolar compounds and shows solubility in a wide range of organic solvents as well as water. - Prepare a live brine shrimp suspension by allowing Artemia salina cysts to hatch in hatching solution (2 g/L of Artemia salina cysts in 30 g/L of ocean salt previously dissolved in reverse-osmosis water) at 28-30 °C for at least 18 h, providing constant aeration.
NOTE: Culturing conditions and hatching time might be influenced by facility environmental conditions and cysts lots and should be re-tested regularly and re-set if necessary.
- Pull microinjection needles for zebrafish embryos using sterile capillaries (standard capillary dimensions: 1.0 OD x 0.58 ID x 100 L mm). Use a commercially available puller apparatus and apply the desired settings, in terms of output voltage, pulling mode (one step or two steps), and pulling force to obtain variable tip length, diameter, and needle shape. For commonly used needle features for zebrafish, refer to Abdelrahman and colleagues11.
- Preparing breeding pairs for Tg[ef1α:ERK biosensor-nes] (Teen) fish (Day 3)
- The day of mating, feed the adult pairs regularly according to the approved animal facility protocol.
- Clean the culture containing hatched brine shrimps by filtering out the non-hatched or empty cysts with two strainers of different filter sizes (filter step I: 180 µm, filter step II: 112 µm).
- Collect and wash the filtered brine shrimps in 200 mL of reverse-osmosis water to feed 10-15 adult fish with approximately about 5-10 mL of the brine shrimp solution.
NOTE: Check the quality of the hatched brine shrimp solution looking at their vitality, motility, size, and color and be sure to remove unhatched or empty cysts, which are indigestible for fish and can cause harm to the fish gastrointestinal tract. For breeding pairs, it is preferable to provide the last meal of the day at least 3 h before isolating pairs in the breeding tank to allow food digestion and keep high water quality levels.
- Select fish pairs who have not been paired for mating in (at least) the previous 2 weeks, to minimize distress and allow gamete development. Acclimate the selected adult Teen fish pairs (normally with this composition: 1 ♂: 2 ♀) in appropriate breeding tanks. Separate females from males by using a tank divider until the next morning. Ensure a standard light/dark cycle of 14/10 h.
NOTE: Group crosses can also be arranged. However, in this case, spawning can occur at different times and selecting embryos at the same stage from mixed clutches becomes more difficult. The transition from dark to light is critical for successful mating.
- The day of mating, feed the adult pairs regularly according to the approved animal facility protocol.
- Embryo collection and microinjection of WT and mutant mRNAs (Day 4)
- As soon as the light is switched on in the facility, remove the tank divider separating the males and females and, if necessary, change approximately 20% of the tank water with fresh water from the recirculating aquaculture system to keep the water quality high without diluting the hormones released by the fish excessively.
- Leave the fish undisturbed and check regularly for egg laying (usually in the first 30 min to 1 h of daylight). The eggs (2-3 mm) are laid and fall to the bottom of the tank separated by a grid such that adults cannot eat them. Isolate the adults who have successfully bred and strain the tank water containing the eggs through a standard strainer (such as a tea strainer) to retain the eggs.
NOTE: It is possible to put the same fish back in the breeding tank for another mating round or return the fish to the original housing tanks, recording the mating pair, date, and performance. - Wash the collected embryos with fresh E3 medium and remove unfertilized, degenerated eggs, feces, and other kinds of debris from the pairs by using a Pasteur pipet.
NOTE: Collect ~n = 100 fertilized Teen eggs in 90 mm diameter Petri dish to avoid embryo overpopulation. - Arrange and align fertilized Teen eggs in a custom microinjection plate obtained by molding 2% agarose in E3 medium to generate appropriate lanes that must contain and restrict the eggs during microinjection.
NOTE: To this aim, custom-made or commercial molds can be used. - Prepare fresh microinjection mix to generate mutant embryos as follows: 30-60 pg of capped and polyadenylated mRNA (here shp2) dissolved in 0.3x Danieau solution (diluted from stock solution) for a final volume of 20 µL. Add 0.2 µL (0.05%) of Phenol Red stock solution as microinjection tracer.
- Backload the needle with 2 µL of injection material using a microloader pipette. Inject the solution into one-cell stage of Teen zebrafish embryos using a commercially available pressure microinjection device, manually adjusting the pressure and time settings to calibrate each injection based on the needle and embryo quality. Refer to standard zebrafish microinjection protocols available in the literature11,12.
- Raise microinjected Teen embryos under controlled husbandry conditions (temperature: 28 °C, humidity: 70%, light/dark cycle: 14/10 h) for optimal development and clean out any eggs looking cloudy or degenerated in the next 3 hours after deposition.
- At ~4 h post fertilization (hpf), screen the embryos for fluorescence (reporter expression) using a standard fluorescence stereomicroscope with appropriate lamp and filter settings (465-500 nm). Use Teen-positive fish for FRET imaging and negative siblings for immunohistochemical (IHC) assessments (see below).
NOTE: Given the known variability in the expression of Tol2-based transgenes13, embryos can show variable levels of Teen fluorescence. It is recommended to inspect not-injected siblings to control for inter-individual variability in fluorescence within a batch and, considering the low dynamic range of FRET imaging, discard embryos with extremely low levels of basal fluorescence.
- Treatment of zebrafish embryos with the MEK inhibitor (MEKi) (Day 4)
- Prepare an intermediate solution of MEKi PD0325901 (1 mM) by diluting 1 µL of 10 mM stock solution with 9 µL with E3 medium. Use the intermediate solution to obtain final low dose solutions. Prepare a low-dose (0.25 µM) of the MEKi PD0325901 in a total volume of 3 mL by mixing 0.75 µL of the 1 mM intermediate solution with 2.25 µL of E3 medium. Dilute 0.75 µL of 10% DMSO with 2.25 mL of E3 medium to obtain the control (Ctrl) solution.
- Place embryo pools of equal numbers in different wells of a 6-well plate and start treatment by bath immersion: for each well, exchange the E3 medium with E3 medium containing vehicle control (0.0025% DMSO) or diluted drugs at the desired concentration (0.25 µM, 0.0025% DMSO) as mentioned before. Use 3 mL of drug solution per well.
NOTE: To eliminate any effects caused by varying concentrations of the carrier (DMSO), it is important to ensure that both control and treatment solutions have the same final DMSO concentration. - Keep the treated embryos at 28 °C before collecting them until the desired developmental stage for followup assays (here: morphometric analysis and IHC validation).
2. Live multispectral FRET imaging of RASopathy zebrafish models at gastrula stage and data analysis
- Mounting gastrulae for live imaging (Day 4) (Figure 2A)
- Prepare mounting medium for alive embryos by dissolving 1.5 g of low-melting agarose (LMA) in 1x PBS to make 1.5% LMA/E3.
NOTE: Choose the agarose concentration from 0.8 to 1.5% depending on the developmental stage and specific need to restrain the fish. Distribute freshly made LMA solution in aliquots of the desired format and store LMA in a polymerized state at RT (stable for a few months). This minimizes bacterial and fungal contamination but check the quality of the LMA aliquot regularly before use. - Before sample mounting, melt the 1.5% LMA aliquot in a thermomixer or sterile water bath at 50 °C. Once dissolved, decrease the temperature of the thermomixer to approximately 30 °C.
- Position a single injected Teen+ embryo (here a gastrula expressing ShpD61G) at the center of a glass bottom dish of 35 mm diameter for live imaging and orient it using a thin hair. Remove the excess E3 medium and immobilize the embryo by putting a drop of LMA on top and leaving it to polymerize at RT.
NOTE: In the beginning of the polymerization process, the fish can be oriented to the desired position. It is strictly recommended to use the LMA at a temperature not higher than 35 °C to avoid tissue damage.
- Prepare mounting medium for alive embryos by dissolving 1.5 g of low-melting agarose (LMA) in 1x PBS to make 1.5% LMA/E3.
- Setting microscopy parameters and performing multispectral FRET imaging of gastrulae (Day 4) (Figure 2B)
NOTE: The protocol applies to the use of any confocal microscopy platforms equipped with all the necessary hardware and software to perform FRET imaging. Here we used confocal microscope equipped with an argon-ion laser with the wavelength lines of 458-476-488-496-514 nm, a programmable acousto-optical beam splitter (AOBS) that separates excitation and emission light, two spectral Hybrid (HyD) detectors, and a stage incubator to maintain stable conditions of temperature (at 28 °C) and humidity during live imaging of samples.
The live multi-spectral FRET imaging protocol as described can be applied to embryos at different developmental stages beyond early gastrulae, as shown by Fasano et al.7. A larger z-stack should be considered for later developmental stages (e.g., 24 hpf) as well as appropriate z- and t- intervals' settings to permit multi-spectral detection steps during spectral FRET image acquisition.- Turn on the incubator controller at least 1 h before starting the acquisition and set the temperature to 28 °C to keep zebrafish embryos in a healthy state. Once the incubator temperature has stabilized, place the imaging dish with the embryo on the sample holder and use a 10x/dry objective (numerical aperture of 0.4) to quickly visualize the sample.
- In the Laser Configuration of the referenced software, switch on the argon-ion laser and use the slider to adjust the laser power to 50%. In Hardware Settings, choose 8-bit depth resolution at which the images will be acquired.
- Select the spectral detection XYλZ scanning mode in the acquisition panel and set the following acquisition parameters: format image: 512 x 512 px; scanning speed of 400 Hz; optical zoom of 0.75.
- Activate the 458 nm laser line of the argon-ion laser and set its intensity value, normally <10% (here 8.5%).
- Select a HyD detector and adjust the sensitivity of the detector (gain) by entering the desired value (here 500).
NOTE: For live, weakly fluorescent samples and to accumulate less background noise, it is advisable to use hybrid detectors rather than Photomultiplier Tubes (PMTs).
In the referenced software, displaying the emission spectrum of a dye requires activating a detector. - To begin, activate the first detector (HyD) and select the cyan fluorescence protein (CFP) to display its emission curve. Open the dropdown menu of the detector bar of the software to open the selection list for the CFP emission curve. Now, activate a second detector to display the emission curve of the second dye yellow fluorescence protein (YFP). To display also the Ypet emission curve, activate a second detector, then select the YFP emission curve that will appear in the spectrum image, after turning off the second detector.
NOTE: Once this step is complete, the second detector should be deactivated, as only one detector is utilized in spectral acquisition mode. - To start live acquisitions, position the detection cursor in the range of the most intense signal emission (in this case, that of YFP) to visualize the sample, decide and set the start and end positions of the sample thickness in the z-stack LAS X window.
- For λ-scan range properties, set the following parameters: begin (460 nm) and end (570 nm) of the detection range; Detection Band Width: 5 nm; λ-scan Stepsize: 5 nm.Start z-stack acquisition.
NOTE: The parameters indicated allow users to obtain a relatively fast imaging with acceptable resolution, but different settings can be used. In the Fluorifier Disc Settings, the automatically inserted NOTCH filter can be deselected to avoid single intensity loss. - To assess real-time signal changes upon MEKi exposure, mount a single mutant embryo (here Shp2D61G) in a glass dish and image before and after drug treatment. For these live experiments, directly perform drug treatment by bath immersion during imaging session with a maximum of 2 mL of the solution containing the dissolved drug.
- Image postprocessing and multispectral dye separation to obtain ratiometric FRET images (Day 5) (Figure 2C)
- To separate the donor (CFP) and acceptor (Ypet) emission, be sure to eliminate the contribution of the fluorescence emission of both molecules in the FRET channel, called spectral bleedthrough (SBT).
- Refer to publicly available dye/fluorescent proteins databases to display and download the .cvs file relative to a standard CFP emission spectrum (excitation and emission spectra).
- In the Data panel of the .cvs file, choose the Text to columns option and split the data into columns using a comma as the delimiter. The resulting file will include additional information (e.g., CFP excitation, CFP 2P) that should be removed. Retain only the column data relative to wavelength and emission.
- In the CFP emission column, remove all intensity data from 501 nm onwards.
- Save the adapted CFP emission spectrum file from 460 to 500 nm in .xls format (called "eCFP modified" in Figure 2C).
- Repeat the same procedure to download and process the YFP protein emission spectrum and retain only the column data relative to wavelength and emission. Remove all emission values up to 524 nm. Save the adapted YFP emission spectrum file from 525 to 650 nm in.xls format (called "Ypet from 525 nm" in Figure 2C).
- To allow exclusion of spectral bleed-through (SBT) in the dye database available in the Configuration window of the software, insert and save two adapted reference emission spectra for CFP and YFP.
- Select the resulting spectral image file from the imaging session, open the Process window, and select Spectral Dye Separation in the Dye Separation tool. Configure the settings for the dye separation as follows: in the dropdown lists on the left side of the dialog, select the new CFP emission spectrum (eCFP modified) in the first position and the new YFP emission spectrum (Ypet from 525 nm) from the spectrum database.
- Under Rescale, select Per Channel to scale the channels individually then, in the λ-scan of the images, choose the one with the greatest signal intensity (corresponding to step 14 of the spectral scan at the level of the FRET channel emission peak, indicated by white arrow in the panel showing detection steps on the right of Figure 2B). Then, move along the Z scan of the sample, and choose the optical section that highlights the area of interest on margin zone.
- Use the ROI selection mode on the margin zone of the animal pole to define the area with the best spectrum. Click on ROICrosshair indicated at the top in the display window to call up the crosshair and adjust the size of the reference ROI by entering the value of 40 voxels in the Measurements Area. Define precisely the area of interest by the ROICrosshair. In the image diagram, choose data normalization, then click Apply to perform the dye separation with the configured settings.
- Open this newly generated file obtained with the two channels separated and produce a two-dimensional projection image from the three-dimensional image series (maximum intensity projection) for data visualization.
- In the Process window, select Crop to separate channels into two separate files, the CH1 and CH2 (or FRET) channels.
- In the Process window, select Combine Images, select the CH2 file and insert it in the first option, and then select the CH1 file and insert it in the second option. Set a rescale with factor 5 and choose the Ratio operation. Then, click on Apply to generate the new file containing the ratiometric image (YFP/CFP). Save the file for followup data analysis.
- To separate the donor (CFP) and acceptor (Ypet) emission, be sure to eliminate the contribution of the fluorescence emission of both molecules in the FRET channel, called spectral bleedthrough (SBT).
- Quantitative analysis of FRET signals and image rendering (Days 5-6) (Figure 2D)
- For quantitative measurements of FRET signals from FRET/CFP ratiometric images, perform the experiment on N>2 embryos (replicates), depending on an a priori statistical assessment based on the expected effect. Employ an image processing package such as the open-source software Fiji. Import the image files obtained from spectral imaging and dye separation into an image analysis package such as the open source Fiji.
- Before starting with the ROI selection on the gastrulae images, set the parameters as readout measurements by using the Analyze function | Set Measurements | select the parameters of interest such as Area, Integrated density, and Mean grey value.
- Select the region of interest (in this specific study: the margin of gastrula) using the polygon selection tool from the toolbar. Save the ROI x,y specifications by clicking on Analyze | Tools | ROI Manager.
- To perform measurements in a selected ROI, click on Analyze | Measure. Repeat these steps for each ROI and image/condition.
NOTE: If needed, save the image as .TIFF format. It is possible to save all ROI measurements deriving from multiple images as one ROI file. Rename each measure in the ROI manager list with the correct name relative to each sample/image. - For each condition (mutant and mutant + treatment here), extract all the values of FRET/CFP ratio for each selected ROI obtained and organize them in a worksheet by organizing, for example, experimental groups in columns and each raw value in rows.
- Use any suitable software to assess statistical differences of FRET signals among different experimental conditions and for graph generation and data visualization.
NOTE: Open-source and licensed software are available for data analysis and graphing solutions. - Organize a specific project for the statistical assessment considering all raw measurements and the number of replicates for each experimental condition.For the statistical analysis of a single biological replicate, create a column table having one grouping variable, with each group defined by a column. For the statistical analysis of more than one biological replicate, create grouped tables having at least two grouping variables, one defined by columns (e.g., experimental conditions) and the other defined by rows (e.g., mean values of replicates).
- After appropriately arranging the experimental groups in the worksheet, assess data distribution (Normality test, e.g., D'Agostino-Pearson, Anderson-Darling) and based on the result, choose the most appropriate statistical test.
- Perform a One-Way ANOVA for parametric data (following Gaussian distribution) and Kruskal-Wallis test for non-parametric data. If more factors are present (genetic and drug concentrations), consider a Two-Way ANOVA. Compare the mean FRET value for each condition against each other (multiple comparisons) and correct for multiple comparisons using a post hoc test (e.g., Tukey's test and Dunn's for parametric and non-parametric data, respectively).
3. IHC validation of the FRET results and correlative morphometric analysis of gastrulation defects
- Preparation of solutions and embryo fixation and mounting for IHC against tERK and pERK (Day 7) (Figure 3A)
- Prepare working solutions required for IHC.
- Prepare a stock solution of 10x PBS by dissolving 80 g of NaCl, 2 g of KCl, 14.4 g of Na2HPO4, and 2.4 g of KH2PO4 in 1 L of DD water. Autoclave the solution to keep it sterile.
- Prepare a 0.8% PBS-Triton (PBSTr) solution by dissolving 8 mL of 10% Triton X-100 in a final volume of 100 mL of 1x PBS.
- Prepare 20%, 50%, and 80% glycerol solutions by diluting 3 mL, 7.5 mL, and 12 mL of 100% glycerol, respectively, in a final volume of 15 mL of 1x PBS.
- Prepare 4% paraformaldehyde (PFA)/0.25% PBSTr (fixative solution) by mixing 2.5 mL of 16% PFA in 250 µL of 10% Triton X-100 previously dissolved in a final volume of 10 mL of 1x PBS. Use this solution to fix embryos at 6 hpf overnight at 4 °C. Use 2 mL tubes and a maximum of five embryos per tube to allow efficient fixation and washes.
NOTE: It is recommended to prepare fresh fixative for each fixation round. CAUTION: When handling formaldehyde at 16%, wear Personal Protective Equipment (PPE) and under a chemical hood. - Wash the embryos in the 2 mL tubes by filling the tubes up with 1.5 mL of 0.8% PBSTr for a few times. Transfer the embryos to new 2 mL tubes and store the embryos in 1x PBS until use.
NOTE: If not familiar with the handling of early zebrafish embryos, work under a stereomicroscope to avoid embryo loss during pipetting. Use "low binding" plasticware to reduce possible stickiness of the embryos to the tube wall.
- Prepare working solutions required for IHC.
- Tissue permeabilization and IHC against pERK and tERK (Days 7-9)
NOTE: IHC outcomes depend upon multiple variables that can differ across labs and specific optimization of the protocols might be necessary.- Wash the fixed zebrafish embryos in 1 mL of 0.8% PBSTr for 3 x 10 min at RT.
- Permeabilize the samples with 2 µL of Proteinase K diluted in 0.8% PBSTr (1:1,000) for 2 min at RT.
- Stop the permeabilization of the tissue by washing the samples in 1 mL of 0.8% PBSTr for 3 x 10 min, and postfix the samples in 500 µL of 4% PFA/1x PBS for 20 min at RT. Then, wash the samples in 1 mL of 0.8% PBSTr for 3 x 10 min.
NOTE: The method and the time used for tissue permeabilization are critical steps that can be influenced by the antigen type, the quality of tissue preservation, and environmental factors. Optimize the protocol before applying it on precious samples. - Incubate the samples with 1x blocking buffer (BB) containing 5% normal goat serum (NGS), 1% Bovine Serum Albumin (BSA), 1% DMSO, prepared as follows: 75 µL of 100% NGS, 150 µL of 10% BSA, and 15 µL of 100% DMSO in a final volume of 1.5 mL of 0.8% PBSTr. Use 200 µL of the solution per tube and incubate the embryos for 20-30 min at RT in the solution.
- Remove the 1x BB from the previous step and incubate the samples in a solution containing a mix of the desired primary antibodies (here: diluting 1 µL of primary mouse monoclonal antibody p44/42 MAPK, total ERK, tERK, + 1 µL of rabbit polyclonal phospho-p44/42 MAPK, phosphorylated ERK, pERK) in 250 µL of freshly prepared 1x BB solution. Use 200 µL of the solution per tube and incubate the embryos in the solution overnight at 20 °C.
- To eliminate nonspecific binding, wash the samples with 1 mL of 0.8% PBSTr several times (first wash every 10 min and then every 30 min).
- Incubate the samples in freshly prepared 1x BB for 20 min at RT. Use 200 µL per tube.
- Remove the 1x BB from the previous step and incubate the samples with a mix of the secondary antibodies (1 µL of fluorescently 488 conjugated-goat anti-mouse, + 1 µL of fluorescently conjugated 633 goat anti-rabbit in 600 µL of 1x BB solution). Leave the embryos gently shaking overnight at 20 °C.
NOTE: We performed IHC on negative (not transgenic) Teen fish from the same batch used for FRET imaging. Alternatively, Teen+ fish can also be used, but in this case, secondary antibodies conjugated to fluorophores that do not overlap with the emission spectra of CFP or YFP should be employed instead. - Eliminate nonspecific binding as described above.
- Wash the samples in a gradient of glycerol/1x PBS solutions (20%, 50%, 80%) for 15 min per each solution at RT. Store the samples in 90% glycerol/1x PBS at 4 °C.
NOTE: To minimize possible fluorescence decay, store the samples in the dark.
- Confocal microscopy of immunostained tissue and quantitative analysis (Days 10-11) (Figure 3B-E)
- Mount the embryos as described before.
- Set the confocal microscopy acquisition parameters as follows: white laser at 80%, fluorescence emission range at 507 - 551 nm for fluorescently conjugated goat anti-mouse (in this case with the emission spectrum of 488 nm, used for tERK), 644 - 740 nm for fluorescently conjugated goat anti-rabbit (in this case with the emission spectrum of 633 nm, used for pERK). Select sequential mode of acquisition, a format scanning of 512 x 512 px, and a speed of 400 Hz. Define start and end of the scan with a z-step size thickness of 4 - 5 µm and standard digital zoom of 1. Use water immersion 25x objective (with a 0.05 numerical aperture) to acquire the whole gastrula at 6 hpf
CAUTION: This protocol includes the application of lasers that can be harmful; therefore, it requires personnel training according to the specific national requirements. - After confocal image acquisition, inspect the raw image file obtained using an image processing package such as the open-source software Fiji.
- In Fiji, use the command Split channels from the menu Image and submenu Color to obtain a single channel image (total ERK: channel = 0, C = 0; pERK: channel =1, C = 1) and generate a maximum intensity z-projection image for both channels by clicking on Image | Stacks | Z Project.
- Repeat the procedure for ROI intensity measurements as described before and extract the data to a worksheet.
- To infer p-ERK signal intensity changes in the desired ROI among experimental groups, calculate the p-ERK/t-ERK ratio by dividing the raw integrated density values of p-ERK staining by raw integrated density of the t-ERK signal. Proceed to the assessment of statistical significance as in step 2.4.
NOTE: Imaging parameters' settings can be modified based on the specific needs and considering the time duration for each sample, the desired quality of images, and the number of planes to be acquired.
- Axes measurements in 11 hpf embryos (Day 4 for embryo fixation, Day 12 for data acquisition and analysis) (Figure 4A-D)
- At the end of gastrulation (11 hpf), fix the embryos with 4% PFA in 1x PBS for 20 min at RT, wash several times in 1x PBS, and store at 4 °C until image acquisition.
- Orient the embryos laterally using a Pasteur pipette in a single well of a 12-well plate containing fresh 1x PBS.
- To evaluate the presence of an oval shape (Y/X embryo axes ratio > 1), a characteristic Rasopathy phenotype in zebrafish, and morphological rescue of embryo axes ratio as a result of the treatment efficacy, acquire images of whole embryos using a stereo microscope with a 3.4x magnification objective (0.63x scanning objective x 8.60 zoom factor) by simply capturing images in a brightfield mode.
- Import the image file into an image analysis package such as the open source Fiji.
- Measure the embryos' axes length (x, minor axis; y, major axis) by selecting the straight tool from the toolbar and clicking on Analyze | Measure. After performing selected measurements, add them to the ROI manager list and save the ROI file as explained for FRET imaging analysis above.
NOTE: Repeat these steps for each embryo image.
- Measure the embryos' axes length (x, minor axis; y, major axis) by selecting the straight tool from the toolbar and clicking on Analyze | Measure. After performing selected measurements, add them to the ROI manager list and save the ROI file as explained for FRET imaging analysis above.
- Proceed to export the data in a worksheet and calculate the axes ratio by dividing the length of the major axis (y) by the length of the minor axis (x). Calculate the mean, standard deviation of mean, or standard error of mean of different replicates.
- Proceed to statistical data analysis as in step 2.4.
Results
This protocol shows a simple workflow to quickly generate transient RASopathy models in zebrafish embryos and assess ERK fluctuations in early mutants with a standard live FRET imaging method applied to a recently established ERK zebrafish sensor6,9. As recently shown6,7 within the same experimental workflow, FRET results can be cross-validated by standard IHC against phosphorylated and total ERK on whole embryos. Impairment of body axes formation correlating with increased ERK activation can be followed at later stages, even in the same embryos used for FRET7. Here, typical ERK fluctuations in early gastrulae registered by FRET are shown, which are linked to the expression of NS-causing Shp2D61G. Data were also acquired from mutants in which the signal was negatively modulated by low-dose treatment with a MEKi, inhibiting the RAS/MAPK signaling cascade.
To generate NS models, we first produced high-quality capped and polyadenylated mRNA encoding Shp2 (wild-type and mutant form) by in vitro transcription from a suitable linearized plasmid harboring shp2 full-length CDS. As shown in Figure 1A, when most of the plasmid is successfully linearized ("cut"), a sharp band can be observed running in a standard 1-1.5% agarose gel (here in the example of the size of 7,500 bp) while the "uncut "DNA, used here as a control of undigested plasmid, displays different possible conformations, typical of undigested circular DNA. Capped and polyadenylated mRNA can be then produced in vitro. On the agarose gel containing formamide,intact RNA should look like a conspicuous band around the expected size (Figure 1A, right) without noticeable smear (indicative of mRNA degradation) or presence of smaller fragments. It is advisable not to proceed if fragmented or degraded RNA is obtained. The preparation should be repeated upon troubleshooting. As shown in the schematics of Figure 1B, good quality capped and polyadenylated mRNA encoding the desired RASopathy allele and a WT control (in our case Shp2D61G and Shp2WT as control) can then be injected into one-cell stage zebrafish embryos expressing the Teen reporter. Embryos are then raised until the desired stage (here early stages during gastrulation).
Figure 2 illustrates schematics and representative results of the workflow used to register FRET signals in our RASopathy model in vivo. Teen+ gastrulae expressing the mutant NS allele Shp2D61G raised and collected at the desired stage (4 hpf) were mounted in 1.5% low-melting agar (LMA) in E3 medium (Figure 2A). An example of general microscope settings and the lambda (λ) stack acquisition settings we used to obtain multispectral FRET imaging from donor excitation (405 nm) in Teen embryos is shown in Figure 2B. Importantly, to ensure enough spectra collection, we set the λ bandwidth at 5 nm. We used a relatively high step size (here 8 nm) to obtain a x, y, λ, z scans in a reasonable time, considering the live acquisition mode (acquisitions with approximately 13 min time interval).
After image acquisition, the recorded signal intensity was inspected along the entire λ spectrum to assign the best emission windows and proceed to spectra CFP (D) and Ypet (A) unmixing (Figure 2C, left). Here, by inspecting the margin region of the animal pole of the embryo by an ROI-based approach built-in in the software Spectral dye separation wizard, we focused on assigning the best emission spectral windows for the two fluorophores, avoiding any spectral overlapping between D and A molecules. Representative raw single channels (CFP or Ypet, green and red, respectively) obtained by this approach and the FRET/CFP ratiometric image (gray) resulting from applying the Ratio function are shown in Figure 2C, right top panel. The bottom panel shows a representative suboptimal result from Teen reporter embryos, which occasionally show insufficient signal intensity, as often observed within a population of transgenic fish. These embryos should be discarded beforehand, and care should be used to assess embryos with similar levels of Teen expression in the analysis.
After signal inspection at the margin of the animal pole, where ERK activity is concentrated during epiboly and gastrulation cell movements, image rendering was performed using "smart" LUT pseudocoloring in Fiji. This allows for better visualization of spatial signal intensity differences on the resulting ratiometric image. The resulting ratiometric FRET images in Figure 2D already show a clear trend of signal intensity reduction in Shp2D61G in the margin region after approximately 13 min of treatment with the selected MEKi, PD0325901 (here PD) at low doses (0.25 µM)7. The data demonstrate the suitability of the Teen sensor and the multispectral FRET imaging protocol used here to detect live dynamic ERK changes in a RASopathy model during early stages of development, as well as the fine modulation of disease-associated signal upon low-dose MEKi treatment.
In parallel, we validated the results of our FRET approach applied to RASopathy fish models by fluorescence IHC against total ERK (t-ERK) and its phosphorylated (p-ERK) form. By normalizing the signal obtained (p-ERK/t-ERK) within an ROI, cells with high ERK levels emerge. Figure 3 shows an example of ERK activity validation via IHC for Shp2WT, Shp2D61G, and Shp2D61G treated with low-dose PD from 4 hpf to 6 hpf (longer treatment as compared to the 13 min treatment window shown during live FRET imaging). Immunostaining was performed using fluorescent secondary antibodies with non-overlapping emission spectra for t-ERK and p-ERK (488 and 633 nm, respectively).
The 6 hpf embryos were mounted in 1.5% LMA dissolved in PBS and standard confocal settings were set to obtain x,y,z scans of the entire embryo volume (Figure 3A,B). Standard confocal settings with 512 x 512 px resolution at 400 Hz allow us to inspect signal localization, important to determine the good outcome of the immunofluorescence prior to signal quantification. As expected, and as shown in the representative result, for t-ERK (green), we observed a near-ubiquitous staining within the cells of the margin, while p-ERK (red) signal was mostly confined to the nuclei (Figure 3C). Raw images, as single-channel and merged images, were processed initially by performing a z-stack projection to obtain whole-embryo images. Pseudocoloring of p-ERK (cherry) was applied to better resolve the contrast with t-ERK (green) (Figure 3D). Compared to Shp2WT, in Shp2D61G an increase in the number of p-ERK+ nuclei within the margin region could be observed, which was rescued in Shp2D61G embryos treated from 4 hpf to 6 hpf with 0.25 µM PD. The data confirm the results obtained by FRET imaging and demonstrate the effective molecular modulation obtained by the low-dose MEKi of choice. Similar to FRET images, the signal from single channel z-stack acquisitions was then inspected in the animal pole margin region using ROI-based analysis using Fiji, and p-ERK and t-ERK values were measured separately and extracted for follow-up normalization (p-ERK/t-ERK) and statistical analysis (Figure 3E).
Last, Figure 4 provides representative morphometric data that can be obtained and measured, ideally on the same samples from live FRET imaging and subsequentially raised from 6 to 11/12 hpf or on their siblings. Altered measurements of the minor and major axes (major/minor axes ratio) at this embryonic stage can indeed highlight gastrulation movements defects associated with RASopathy. The data can be used to examine the phenotypic outcome of new variants discovered in patients with uncertain pathogenicity and to correlate the strength of the phenotype with that of the molecular alteration (ERK activation) measured with FRET. In the example here, siblings of the Teen embryos (control group 1: Shp2WT as well as groups 2 and 3: Shp2D61G and Shp2D61G treated with low-dose PD between 4 and 6 hpf) were raised separately until 11 hpf and then fixed in 4% PFA (Figure 4A) to avoid further embryo development that could interfere with data comparison across individuals. Images of lateral embryos were acquired using a simple brightfield modality available for standard settings. Embryo elongation was assessed in terms of major and minor axes measurements (Figure 4B,C). The results measures (Figure 4D) reflect and corroborates the data obtained with FRET imaging and demonstrate a correlation between dynamic ERK activity at the margin of the animal pole and gastrulation outcomes. Altogether, the representative dataset shown here for the NS-associated allele shp2D61G with or without treatment MEKi PD0325901 shows the utility of the multispectral FRET imaging in Tg[ef1α:ERK biosensor-nes] (Teen) reporter fish to predict variants' pathogenicity for NS in early embryo.

Figure 1: Analysis of plasmid and mRNA preparations required to generate transient NS zebrafish models. (A) The panel depicts a schematic and representative results of the DNA electrophoresis outcome visualized at a UV transilluminator, used to assess efficient plasmid linearization and good-quality mRNA transcription. Linearized plasmids ("cut") are obtained by digestion with a restriction enzyme (in this case KpnI) and compared with the undigested control plasmid ("uncut"). A single band running faster than the "uncut" should be ideally observed upon plasmid linearization. Plasmid size: ~ 7,500 bps: (backbone plasmid + shp2 CDS). Note how the "uncut" circular plasmid displays different DNA forms of relaxed and supercoiled molecules. A 1 kb ladder (lane 1), whose molecular weight fragments ranged from 0.5 to 10 kilobases (kb), was loaded on the same gel for DNA fragments' size inspection. On the right, a representative gel run of freshly transcribed mRNAs is shown (in this example, transcribed mRNAs encoding Rasopathy-associated human SPRED2). Two ladders that run slightly differently (DNA ladder, lane 1 and RNA ladder, lane 2) with similar molecular range from 0.5 to 10 kb are loaded for RNA fragments' size inspection. (B) Schematic overview depicting the use of the transcribed mRNAs encoding RASopathy-associated variants to generate transient disease models by injection into one-cell stage zebrafish embryos. mRNA encoding wild-type form of the protein of interest is injected as control. The mRNA mixture is prepared in Deneau's solution with the vital dye Phenol Red. Embryos are raised and assessed using microscopy at 6 hpf (for FRET and IHC assays) or at 11/12 hpf (for body axes). Abbreviations: NS = Noonan syndrome; CDS = coding sequence; FRET = Fluorescence resonance energy transfer ; IHC = immunohistochemistry ; hpf = hours post fertilization . Please click here to view a larger version of this figure.

Figure 2: Workflow and representative results of live multispectral FRET imaging in early Teen Shp2D61G embryos before and after MEKi treatment. (A) Overview of the steps required for live spectral FRET imaging in zebrafish samples. Embryos raised and staged in E3 medium were mounted in a glass-bottom dish in 1.5% LMA for live spectral FRET acquisition. FRET imaging is performed before and after low-dose PD0325901 administration. A schematic of the Teen reporter system developed by Sari et al. is shown9. Ratiometric images (FRET/CFP) are obtained in postprocessing. (B) Details of the microscope and multispectral imaging acquisition settings in LAS X software for both fluorophores (CFP, D; Ypet, A). (C) Commands and method to obtain spectral dye separation and ratiometric images applied to a Shp2D61GTeen gastrulae. On the left, a strong signal from the animal pole margin was selected for spectral separation with an ROI selection tool. On the top right, settings and representative outcomes of resulting images for both D and A channels as maximum intensity z-projections from a single fish are shown, as well as settings to obtain the ratiometric image and representative results. The margin region at the embryo animal pole with high FRET signal is indicated (dotted yellow line). (D) Left panel: schematics of the ROI selection on the margin (dotted yellow line) from the raw ratiometric image for data analysis in Fiji. Representative FRET/CFP images generated via the "smart" LUT plugin of Fiji are shown to visualize changes in ERK activation in embryo expressing Shp2D61G compared to Shp2WT (middle panel) or in the same Shp2D61G embryo before and after PD treatment (right panel). For this experiment, raw intensity density values of the selected ROI are shown. Scale bar is indicated. Abbreviations: FRET = Fluorescence resonance energy transfer; MEKi = Mitogen-activated protein Kinase Enzymes inhibitor; LMA = low-melting agarose; CFP = cyan fluorescence protein ; Ypet = yellow fluorescence protein; D = donor; A = acceptor; ROI = region of interest; ERK = Extracellular signal-Regulated Kinase. Please click here to view a larger version of this figure.

Figure 3: Workflow and representative results of IHC against t-ERK and p-ERK in Shp2D61G early embryos with or without MEKi treatment. (A) Schematics illustrating the collection of zebrafish samples immunostained against t-ERK (488) and p-ERK (633) and mounted in 1.5% LMA/PBS for confocal z-stack acquisitions. (B) Confocal microscope settings for two laser lines (488, 633 nm), used to capture sequentially the fluorescent signals deriving from the fluorescently conjugated secondary antibodies. The inset illustrates Z-stack parameters for the acquisition of multistack raw images with acceptable resolution. (C) Schematics (left panel) and representative raw confocal images (right panel) of correctly stained zebrafish gastrulae at 6 hpf showing ubiquitous and mostly nuclear localization of t-ERK (green) and p-ERK (red), respectively, at the margin of the animal pole. (D) Representative maximum intensity z-projections from confocal stacks of 6 hpf embryos expressing Shp2WT (control), Shp2D61G , as well as Shp2D61G after treatment with 0.25 µM of PD0325901 between 4 and 6 hpf. Images are shown as single 633 nm channel (here p-ERK, pseudocolored in cherry) and combined (p-ERK in cherry and t-ERK in green). (E) In the small inset schematics of the ROI selection on the margin region (dotted yellow line) from the raw z-stack images for data analysis in Fiji is shown. Zooms of the z-stacks images showing the margin region are included, as well as the raw intensity density measures retrieved and assessed to infer ERK activation in the ROI from siblings expressing Shp2WT or the Shp2D61G with or without 0.25 µM PD0325901 treatment. The scale bar is indicated in the image panels. Abbreviations: IHC = immunohistochemistry; ERK = Extracellular signal-Regulated Kinase; t-ERK = total Extracellular signal-Regulated Kinase; p-ERK = phosphorylated Extracellular signal-Regulated Kinase; FRET = Fluorescence resonance energy transfer; MEKi = Mitogen-activated protein Kinase Enzymes inhibitor; LMA = low-melting agarose; PBS = phosphatase buffer saline; hpf = hours post fertilization; ROI = region of interest. Please click here to view a larger version of this figure.
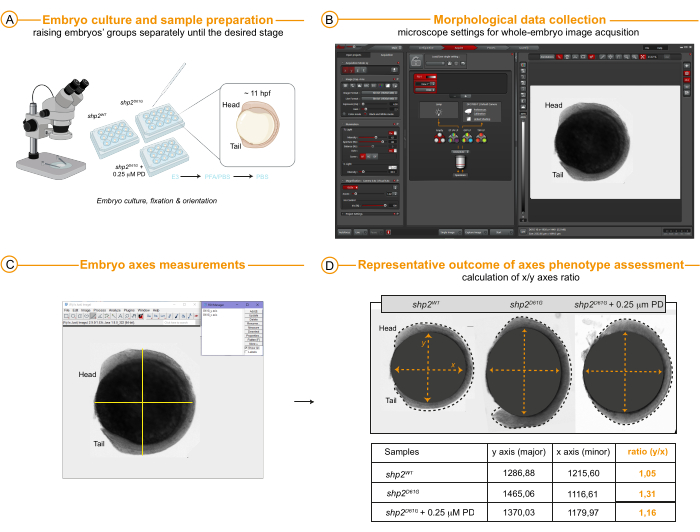
Figure 4: Morphological analysis at 11 hpf. (A) Embryos were raised in E3 medium until the desired developmental stage, here 11 hpf, then fixed in 4% PFA, kept in 1x PBS, and arranged laterally in wells for microscope image acquisition and axes assessment. (B) Image acquisition settings in a standard stereomicroscope and one example of the resulting image showing a whole embryo (here Shp2WT) assessed with bright-field mode. (C,D) Representative results and axes measurements outcomes in Shp2WT (control), Shp2D61G , and Shp2D61G after treatment with 0.25 µM PD0325901 between 4 and 6 hpf. Embryo outline is indicated by a dotted black line. The scale bar is indicated in the image panels. Abbreviations: hpf = hours post fertilization; PFA = paraformaldehyde; PBS = phosphatase buffer saline. Please click here to view a larger version of this figure.
Discussion
Despite decades of research and myriads of mutations leading to highly heterogeneous forms of RASopathies now mapped, genetic variants with unknown significance continue to emerge from sequencing efforts on undiagnosed patients. Indeed, in many cases, diagnosis based solely upon clinical features can be challenging and functional genomic approaches to validate sequencing results remain crucial. Moreover, despite some available anticancer molecules (i.e., MEK inhibitors) being proposed to treat a subset of RASopathies and some success stories starting to emerge, limited consensus exists. This is caused by poor preclinical evidence for most of the available drugs also when it comes to the effective doses of potent anticancer drugs and therapeutic windows for pediatric patients.
Functional analysis in animal models represents an important step for disease sub-classification, patients' stratification, and initial drug evaluation. Zebrafish can be an efficient in vivo model for functional validations of potentially pathogenic variants causing pediatric diseases, including RASopathies14. State-of-the-art phenotypic assessment of the impact of RASopathy-associated variants exist for zebrafish RASopathy models and are based mainly on morphological readouts -- the embryo "oval test", where body axes are measured at the end of gastrulation. However, correlation with ERK activation is commonly performed mainly by standard post-fixation methods -- immunoblots/IHC.
The pipeline we set up aimed to assess ERK fluctuation live that can rapidly test the potential efficacy of available drugs at the beginning of gastrulation, anticipating analysis of the resulting morphometric alterations. The protocol presented here begins with the generation of the desired transient zebrafish RASopathy model by overexpression of the GoF allele of interest as mRNA into the one-cell stage of the ERK in vivo reporter (Tg[ef1a:ERK biosensor-nes] Teen)6. In this EKAR-type FRET sensor, multispectral FRET imaging is a non-invasive, non-disruptive method that can be used to accurately detect ERK signal fluctuation spatially and temporally in live embryos that are physiological during development, aberrant in RASopathy models, and corrected by pharmacological signal modulation6,7.
Different critical steps of the presented pipeline should be considered to obtain informative results from embryos' samples. Given the known low sensitivity of FRET sensors15 and possible variable effects of the mutations affecting ERK signaling (especially for mutations not previously validated by standard methods), before setting the experiment, it is advisable to decide the type of experimental design (i.e., n. of groups and conditions) and thereby make an estimation of the required number of embryos (sample size, n) for the expected effect size and given standard values for type I and type II errors. It is also important to conduct pilot experiments to estimate the actual minimum n required to observe relevant differences between WT and mutant protein. Cross-validation of the FRET results using alternative and complementary approaches, such as IF or morphological assays (as indicated here) is also essential.
In general, a standard sample size of n = 30 can be appropriate to detect large effects of normally distributed values16. In detail, for a priori analysis of sample size, freely available programs such as “G-power”17 can be used, setting these standard parameters: significance level (α): typically set to 0.05; confidence level (1 - β): typically set to 95%; power of a hypothesis test: typically set to 80% (1 - β, where β is the probability of committing a Type II error); effect size d (the expected magnitude of differences among experimental groups) that can have different values depending on whether is large, medium or small, according to Cohen18; statistical variation (dispersion in a frequency distribution).
In the specific example provided a well-known large effect of the Shp2D61G mutant was also previously validated in zebrafish using morphological readouts and pilot live FRET experiments, including complementary assays such FRET on fixed samples as IF and morphological readouts were used to validate the observed effect (see in Fasano et al.7).
Moreover, mRNA of high quality is a key starting factor to generate appropriate transient disease models. Indeed, while it would be ideal to employ models genetically modified to recapitulate the heterozygosity of the patients, generation of these models is not compatible with the requirement for speedy test of emerging variants as it would necessitate to raise the line. Transient models that are generated and assessed only during embryogenesis are to be preferred for large screening.
However, to ensure the informativeness and reproducibility of the results special care should be taken in assessing the quality of the RNA. Besides classical formamide gels, RNA can be also checked using instruments such as the Bioanalyzer that provide direct quantification and visual electropherograms for quality inspection. High-quality RNA preparations look like a single sharp peak of the desired size. It is advisable to not proceed with microinjection if the RNA is conspicuously degraded. Importantly, when it comes to microinjection, for newly synthetized batches of mRNA, an initial experiment to calibrate the correct injection dose is a must. At this ideal dose, expression of the WT form of the mRNA of interest in siblings from the same batch should not cause visible phenotype.
As a general recommendation, when preparing embryos to inject, it would be best to obtain, collect, and assess embryos from mating single pairs (1 female + 1 male) to be sure to minimize batch variability. However, to increase embryo yield, fish spawning can be set up from group crosses with multiple males and females. In this case, collected embryos likely derive from more fertilization events occurring with slight delay within the tank and it is, therefore, important to accurately select individuals at the same developmental stage (early gastrulation) by inspecting embryos several times in the first 2 h after collection. To allow good embryo growth in the delicate initial phase, particular attention should be paid also to use all freshly prepared solutions. In addition, given the possible presence of fecal material and debris from the mating event, embryo batches’ contamination from fungi and bacteria can occur if embryos in E3 medium are not washed and cleaned starting immediately after collection. Viability of the embryos’ batch should be monitored before, during, and after the experiment. If mortality rate is above the standard rate (normally 20–30%), the experiment should be discontinued.
Regarding drug treatments, at least for the class of MEKi tested here, it is best to avoid treatments before 4 hpf, as these might irreversibly affect embryo development (toxicity), as described earlier19. Another critical step for the reliability of the experiment is to use the same concentration of drug vehicle (in this case DMSO) for all experimental conditions and avoid overcrowding of embryos per well during treatment to keep good levels of oxygenation (a maximum of 20 embryos should be kept in a 6-well plate).
Optimization of imaging parameters is also extremely important for the success of the pipeline that includes especially multispectral FRET imaging but also confocal imaging of IHC specimens. First, given the variability in transgene expression, a decisive aspect is to accurately select Teen embryos and discard those with very low expression, that, provided the low dynamic range of FRET sensors, might impede sufficient signal detection and quantification (see example Figure 2). Transgene expression can be visible under a standard stereomicroscope with fluorescent lamps and appropriate filter wheels ~2 h after injection.
In our experience for multispectral data acquisition of Teen sensor fish, laser settings and spectral dye definition acquisition mode parameters (x, y, λ, z) are critical initial steps of the microscope and hardware configurations and should be optimized based on the specific need. In principle, parameters should be set such that a good compromise between signal collection and speed of acquisition is reached. This is particularly important for real-time registrations on near-entire embryos, as shown here. Acquisition speed of 400–600 Hz with a step-size between 8 µm and 10 µm and setting a single scan per z plane might not provide cellular resolution but may be sufficient to capture dynamic ERK activity changes across embryos’ tissues. It is, in principle, possible to obtain images with near-cellular resolution with ad hoc microscope settings but at the expense of speed and 3D sampling. This might be interesting if only a certain cell population is investigated within the embryos also at later stages6.
Spectral dye separation can be performed with algorithms different from the one indicated here. Regardless, the selection of the reference region required to assign the best emission spectra for both CFP and YFP is crucial. To this purpose, it is advisable to check the consistency of the results obtained by selecting and averaging different ROIs, where the tissue/cell signal is clearly visible. At the end of the spectral acquisition and once dye spectral separation is obtained, ratiometric images can also be obtained starting from raw CFP and YFP scans and freely available Ratio functions in Fiji. Nevertheless, for visualizing and highlighting differences in FRET signal levels spatially, rescaling of the images might be advisable. In our hands, pseudocoloring using “smart” Look up tables (LUT) encoded within the free Fiji software works well to better show the ERK activation levels. Other LUTs and other scales can be chosen based on the user’s preference.
It should be stressed that the experimental pipeline presented here is limited to Teen sensors harboring CFP as donor (D) and Ypet (similar to YFP) as acceptor (A). Generally, when deciding the FRET reporter, one should consider the dynamic range of the sensors (the ability to detect small but significant changes) and the FRET efficiency (E), influenced by the D-A distance and orientation within the senor, as well as the spectral overlap between D emission and A absorption. Teen is an EKAREV sensor that, through optimized construct design, offers improved dynamic range and sensitivity (E) compared to previous versions. However, the relatively low-dynamic range, normally worse in vivo, can be considered the major limitation to this approach. Nevertheless, Sari et al. and Wong et al. showed that dynamic ERK changes physiologically occurring during development are nevertheless visible and measurable in Teen embryos and we observe signal fluctuations in a pharmacologically treated NS fish model6,9. However, we expect that extremely low ERK signal fluctuations might not be easy to assess using the method shown here. It will be important to test the performance of our pipeline with improved ERK biosensors that will likely be available in the future.
For the cross-validation of the results with IHC, a critical step is the quality of tissue fixation. Embryo tissue at 6 hpf is extremely delicate and thin and, therefore, sensitive to poor fixation and overfixation. Given the lot-to-lot quality and performance variability for fixative solutions, as well as for antibodies, fixation time and the success of the entire IHC protocol should be evaluated in a pilot experiment shortly before assessing experimental samples. Freshly prepared and sterile fixative is always advisable.
In summary, the experimental protocol shown here in the convenient zebrafish model represents a robust and relatively fast pipeline to assess the impact of selected NS alleles on ERK activation in gastrulating cells of early embryos. The method, which involves multispectral FRET imaging in the newly developed Teen sensor fish to detect live molecular fluctuations, surpasses classical IHC. Nevertheless, IHC can be used as a complementary approach to validate the results obtained. The approach anticipates classical phenotypic readouts, namely the major/minor axes ratio (“oval embryo” test), a gold-standard for RASopathy fish models, but measurable only by the end of gastrulation. The protocol can also be applied to test the ability of the proposed MEKi to correct signal and axes alterations in embryos.
Considering that we tested only the NS-associated shp2D61G allele and one drug, further development of the assay should include the assessment of the performance with respect to other RASopathy-associated mutations with different impacts on ERK activation and additional proposed drugs, doses, and treatment windows. It will be interesting to evaluate the sensitivity of this multilevel approach for other emerging variants affecting molecules at different levels of the RAS-MAPK signal cascade. Last, future standardization of a dedicated high-throughput method for ratiometric FRET imaging and quantification in dedicated high-content analysis systems would be required to boost variant testing.
Acknowledgements
We thank Dr. Jeroen den Hertog (Hubrecht Institute, Utrecht, Netherlands) for kindly providing pCS2+_eGFP-2a-Shp2a from which the shp2 full-length CDS was extracted to generate the plasmid template we used7. We thank Nara Institute of Science and Technology (Takaaki Matsui), National Institute of Genetics (NIG/ROIS) (Koichi Kawakami), for providing the transgenic Teen reporter line. This work was supported by the Italian Ministry of Health - Current Research Funds 2021 and Current Research Funds 2024 and Ricerca Finalizzata Giovani Ricercatori GR-2019-12368907 to AL; Current Research Funds 2019, PNRRMR1-2022-12376811, 5x1000 2019, AIRC (IG-21614 and IG-28768) and LazioInnova (A0375-2020-36719) to MT.
Materials
| Name | Company | Catalog Number | Comments |
| Plasticwares | |||
| 1.7 L Breeding Tank - Beach style Design | Tecniplast | 1.7L SLOPED | Breeding tank |
| Capillaries GC100F-10 | Harvard apparatus | 30-0019 | One-cell stage embryo microinjection |
| Cell and Tissue Culture Plates - 12 well | BIOFIL | TCP011012 | Embryo collection and treatment |
| Cell and Tissue Culture Plates - 6 well | BIOFIL | TCP011006 | Embryo collection and treatment |
| Cell Culture Dish | SPL Life Sciences | 20100 | Embryo collection |
| Nunc Glass Dishes 12mm | Thermo Fisher | 150680 | Embryo FRET spectral imaging |
| Pipette Pasteur | Corning | 357524 | Embryo transfer |
| Protein Lobind Tubes 2ml | Eppendorf | 30108450 | IHC assay |
| Reagents and others | |||
| Caviar 500-800 µm | Rettenmaier Italia | BE2269 (500-800) | Dry fish food |
| Great Salt Lake Blue Artemia Cysts | Sanders | 00004727 | Live fish food |
| Instant Ocean salt | Tecniplast | XPSIO25R | Dehydrated sea salt for live food preparation |
| Tg(EF1a:ERK Biosensor-nes) (Teen) | Contacts for ordering*: National BioResource Project Zebrafish, Support Unit for Animal Resources Development, RRD, RIKEN Center for Brain Science, Japan. https://shigen.nig.ac.jp/zebra/index_en.html *upon MTA signature. | - | Supplier of ERK Reporter zebrafish line. Fish embryos can be obtained upon MTA signature from National BioResource Project of Japan for Zebrafish (RIKEN, Japan). The zebrafish line is deposited by Nara Institute of Science and Technology (Takaaki Matsui) and the National Institute of Genetics (NIG/ROIS) (Koichi Kawakami, patent for Tol2 system) (Wong et al., 2018, Urasaki et al., 2006, Okamoto and Ishioka, 2010). |
| 6x loading dye | Cell Signaling | B7024S | Gel Elecrophoresis |
| 100 bp DNA ladder | NEB | N3231S | Gel Elecrophoresis |
| Agarose | Sigma-Aldrich | 1,01,236 | Gel Elecrophoresis |
| Agarose, low gelling temperature | Sigma-Aldrich | A9414-10G | Embryo mounting for FRET spectral imaging and IHC assay |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A8022 | IHC assay |
| Calcium chloride | Sigma-Aldrich | 223506 | E3 medium component |
| Calcium nitrate | Sigma-Aldrich | 237124 | Danieau stock solution component |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | D8418-100ML | IHC assay |
| EDTA | Sigma-Aldrich | E9884 | TBE buffer component for gel preparation |
| Ethanol 99%+ | Fisher Scientific | 10048291 | In vitro RNA purification |
| Formaldeide 16% | Thermo Fisher | 28908 | Embryo fixation |
| Formamide | Sigma-Aldrich | F9037 | Gel Elecrophoresis |
| Gel Loading Buffer II (Denaturing PAGE) | Thermo Fisher | AM8546G | In vitro RNA transcription |
| Glacial Acetic Acid | Sigma-Aldrich | 695092 | TBE buffer component for gel preparation |
| Glycerol | Sigma-Aldrich | G6279-1L | IHC assay |
| Goat anti-mouse Alexa Fluor 488 | Thermo Fisher | A11001 | IHC assay |
| Goat anti-rabbit Alexa Fluor 633 | Thermo Fisher | A21070 | IHC assay |
| HEPES | Sigma-Aldrich | H3375 | Danieau stock solution component |
| KpnI - HF (Enzyme + rCutSmart Buffer) | NEB | R3142 | Plasmid linearization |
| Magnesium sulfate | Sigma-Aldrich | 230391 | E3 medium component/Danieau stock solution component |
| Millennium RNA Markers | Thermo Fisher | AM7150 | Gel Elecrophoresis |
| Monarch Genomic DNA purification Kit | NEB | T3010L | Plasmid linearization |
| Mouse monoclonal p44/42 MAPK | Cell Signaling | 4696S | IHC assay |
| mMACHINE SP6 Transcription Kit | Thermo Fisher | AM1340 | In vitro RNA transcription |
| Normal Goat serum (NGS) | Sigma-Aldrich | G9023 | IHC assay |
| Nuclease-free water Ambion | Thermo Fisher | AM9937 | In vitro RNA transcription |
| PD0325901 | Sigma-Aldrich | PZ0162 | MEK inhibitor |
| Phenol Red solution | Sigma-Aldrich | P0290 | Microinjection mix component |
| Poly A Tailing Kit | Thermo Fisher | AM1350 | In vitro RNA transcription |
| Potassium chloride bioxtra | Sigma-Aldrich | P9333 | E3 medium component/Danieau stock solution component/PBS stock solution component |
| Potassium dihydrogen phosphate | Sigma-Aldrich | P0662 | PBS stock solution component |
| Proteinase K | Sigma-Aldrich | P2308 | IHC assay |
| Rabbit polyclonal phospho-p44/42 MAPK | Cell Signaling | 4695S | IHC assay |
| SYBR safe DNA gel staining | Thermo Fisher | S33102 | Gel Elecrophoresis |
| Sodium Chloride | Sigma-Aldrich | 31434-M | E3 medium component/Danieau stock solution component/PBS stock solution component |
| Sodium phosphate dibasic | Sigma-Aldrich | 71643 | PBS stock solution component |
| Trizma base | Sigma-Aldrich | T1503 | TBE buffer component for gel preparation |
| Triton X-100 | Sigma-Aldrich | T8787 | PBSTr buffer component |
| Equipment | |||
| Alliance Mini HD9 | Uvitec | - | Imaging system |
| Centrifuge 5430 R | Eppendorf | 5428000205 | Microcentrifuge |
| Eppendorf ThermoMixer C | Eppendorf | - | Embryo mounting |
| FemtoJet 4x | Eppendorf | - | Microinjection system |
| Infinite M Plex | Tecan | - | Multimode plate reader |
| Leica M205FA | Leica Microsystems | - | Fluorescence stereo microscope |
| Leica TCS-SP8X equipped with incubator (OkoLab) | Leica Microsystems | - | Confocal microscope |
| Mini-sub Cell GT Horiziontal Electrophoresis System | Bio-Rad | 1704406 | Gel Elecrophoresis |
| PC-100 Vertical puller | Narishige | - | Needle puller |
| PowerPac Universal Power Supply | Bio-Rad | 1645070 | Gel Elecrophoresis |
| Stellaris 5 | Leica Microsystems | - | Confocal microscope |
| Vortex MiniStar silverline | VWR | - | Plasmid preparation |
| Softwares | |||
| Biorender | Biorender | CC-BY 4.0 license | Cartoon elaboration for Figures |
| Excel | Microsoft Office Professional Plus 2019 | - | Data analyses |
| Fiji software | ImageJ | 15.3t | Imaging rendering and quantitative analyses (FRET signals measurements, ERK fluorescence intensity in IHC assay, embryo axes lenght) |
| GraphPad Prism | GraphPad Software LLC | v. 9 | Statistical data analyses |
| iControl spectrophotometer software | Tecan | v. 2.0 | RNA quantification |
| Illustrator | Adobe | 26.0.3 (64-bit) | Figure assembling |
| LASX software | Leica Microsystems | v. 4.5 (Stellaris 5), v. 3.0 (M205FA), v. 3.5 (TCS-SP8X) | Imaging acquisition for spectral FRET experiments and embryo imaging for axes lenght measurements |
| Q9 Mini 18.02-SN software | Uvitec | - | Gel image acquisition |
References
- Iroegbu, J. D., Ijomone, O. K., Femi-Akinlosotu, O. M., Ijomone, O. M. ERK/MAPK signalling in the developing brain: Perturbations and consequences. Neurosci Biobehav Rev. 131, 792-805 (2021).
- Tartaglia, M., Aoki, Y., Gelb, B. D. The molecular genetics of RASopathies: An update on novel disease genes and new disorders. Am J Med Genet C Semin Med Genet. 190 (4), 425-439 (2022).
- Tate, J. G., et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47 (D1), D941-D947 (2019).
- Tartaglia, M., et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 29 (4), 465-468 (2001).
- Jopling, C., van Geemen, D., den Hertog, J. Shp2 knockdown and Noonan/LEOPARD mutant Shp2-induced gastrulation defects. PLoS Genet. 3 (12), e225 (2007).
- Wong, K. -. L., Akiyama, R., Bessho, Y., Matsui, T. ERK activity dynamics during zebrafish embryonic development. Int J Mol Sci. 20 (1), 109 (2019).
- Fasano, G., et al. Assessment of the FRET-based Teen sensor to monitor ERK activation changes preceding morphological defects in a RASopathy zebrafish model and phenotypic rescue by MEK inhibitor. Mol Med. 30 (1), 47 (2024).
- Zimmermann, T., Rietdorf, J., Girod, A., Georget, V., Pepperkok, R. Spectral imaging and linear un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair. FEBS Lett. 531 (2), 245-249 (2002).
- Sari, D. W. K., et al. Time-lapse observation of stepwise regression of Erk activity in zebrafish presomitic mesoderm. Sci Rep. 8 (1), 4335 (2018).
- Wang, H., Li, J., Xue, T., Cheng, N., Du, H. Construction of a series of pCS2+ backbone-based Gateway vectors for overexpressing various tagged proteins in vertebrates. Acta Biochim Biophys Sin (Shanghai). 48 (12), 1128-1134 (2016).
- Abdelrahman, D., Hasan, W., Da'as, S. Microinjection quality control in zebrafish model for genetic manipulations. MethodsX. 8, 101418 (2021).
- Yuan, S., Sun, Z. Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J Vis Exp. (27), e1113 (2009).
- Rossant, J., Nutter, L. M. J., Gertsenstein, M. Engineering the embryo. Proc Natl Acad Sci USA. 108 (19), 7659-7660 (2011).
- Jindal, G. A., et al. In vivo severity ranking of Ras pathway mutations associated with developmental disorders. Proc Natl Acad Sci USA. 114 (3), 510-515 (2017).
- Leavesley, S. J., Rich, T. C. Overcoming lmitations of FRET measurements. Cytometry A. 89 (4), 325-327 (2016).
- Mascha, E., Vetter, T. Significance, errors, power, and sample size: Theblocking and tackling of statistics. Anesth Analg. 126 (2), 691-698 (2018).
- Faul, F., Erdfelder, E., Lang, A. -. G., Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 39 (2), 175-191 (2007).
- Cohen, J. . Statistical power analysis for the behavioral sciences. , (1988).
- Anastasaki, C., Rauen, K. A., Patton, E. E. Continual low-level MEK inhibition ameliorates cardio-facio-cutaneous phenotypes in zebrafish. Dis Model Mech. 5 (4), 546-552 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved