Solutions and Concentrations
Genel Bakış
Source: Laboratory of Dr. Michael Evans — Georgia Institute of Technology
A solution is a homogeneous mixture containing some components in small amounts, called solutes, and one component in a large amount, called the solvent. Solid-liquid solutions contain one or more solid solutes dissolved in a liquid solvent. Solutions are ubiquitous in chemistry: they are used to store and handle small amounts of material, carry out chemical reactions, and develop materials with controllable properties.
The density of a solute in a solution is known as the concentration of the solute. Concentration can be expressed in several ways, differing in the units used to convey the amounts of solute, solvent, and solution.
This demonstration illustrates how to prepare a sucrose solution with a target concentration using precise analytical techniques. Additionally, various measures of the concentration of this solution are presented and explained.
İlkeler
When immersed in water, many solids break apart into particles (molecules or ions) surrounded by water molecules. This process of dissolution converts a heterogeneous mixture of solid and liquid into a single homogeneous mixture consisting of liquid water and dissolved solute particles. The dissolution process for sucrose can be written as a chemical equation using the solid and aqueous phase designators. The (aq) designator following a species implies that water molecules are surrounding and solvating that species.

Different solutions may contain different numbers of dissolved particles, and concentration is a measure that quantifies the density of solute particles within a solution. One fundamental measure of concentration is the mole fraction (x) of the solute: the number of moles of solute particles (nsolute) divided by the total number of moles of solution components (all solutes and solvent).

Multiplying the mole fraction by 106 gives the parts per million (ppm) concentration, the number of solute particles per million particles of solution. The number of moles of solute per liter of solution, or molarity (M), is a second common measure of concentration.
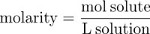
Concentration may also be expressed as parts by mass, the fraction of the solution mass due to the solute.
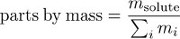
Multiplying the parts by mass concentration by 100% gives the mass percent.
Finally, molality is a measure of concentration that uses the mass of the solvent, rather than the volume of the solution, as a measure of the “size” of the solution. Molality is the ratio of the number of moles of solute to the mass of the solvent in kilograms.
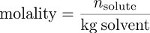
Precise and accurate preparation of a solution with a target molarity requires careful analytical technique. The solid solute must be carefully weighed and transferred quantitatively (completely) to a volumetric flask. The solvent can then be added carefully until the solution reaches the mark on the glassware. For best results, the solute should be allowed to dissolve completely in less than the total volume of solvent, and any remaining solvent should be added when no solid solute is visible.
Prosedür
1. Preparation of 100 mL of a 0.0100 M Sucrose Solution
- Determine the number of moles and mass of sucrose (C12H22O11) to be dissolved in 100 mL of solution.
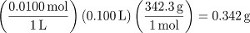
- Weigh out the mass of sucrose on the balance. First place a weigh boat on the balance and set the “tare weight”. Then using a scoopula, carefully transfer solid solute from the reagent bottle to the weigh boat until the desired amount is obtained.
- Place a powder funnel into a clean and dry 100-mL volumetric flask. Pour the solid from the weigh boat through the funnel into the flask.
- Using a wash bottle containing distilled water (the solvent), rinse any remaining solid from the weigh boat through the funnel into the flask.
- Add solvent using the distilled water faucet until the liquid level reaches the neck of the flask (but not the mark). Cap and swirl the flask gently to dissolve the solute.
- Once all of the solute has dissolved, use a wash bottle to carefully add solvent until the liquid level reaches the mark.
- Cap and invert the volumetric flask several times to ensure good mixing of the solution.
2. Making a Supersaturated Sucrose Solution
- Add 100 mL of distilled water to a 600-mL beaker.
- Add 220 g of sucrose to the beaker.
- Place a magnetic stir bar in the beaker and allow the mixture to stir for 15 min.
- Examine the mixture: not all of the sucrose has dissolved. Heat the mixture to 50 ºC and stir for an additional 10 min.
- Examine the mixture: all of the sucrose has dissolved at 50 ºC.
- Allow the solution to cool to room temperature. Examine the solution: the additional sucrose that dissolved at 50 ºC remains dissolved at room temperature. The solution at room temperature is supersaturated.
Sonuçlar
Procedure step 1 creates 100 mL of a 0.0100 M sucrose solution. To convert to measures of concentration other than molarity, determine the mass of water used to prepare the solution. Although this can be measured accurately, in the absence of a measurement it can be assumed that the volume of dissolved solute particles is negligible (i.e., the volume of water used was 100 mL). Using the density of water…

The molality of sucrose in this solution is thus:
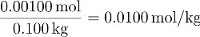
The parts by mass of sucrose is equal to:
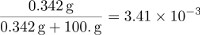
The mole fraction of sucrose can be calculated by determining the number of moles in 100 g of water and dividing the amount of sucrose by the total amount of particles in the solution.

Procedure step 2 illustrates that the solubility of sucrose in water is temperature dependent. Upon heating, undissolved sucrose resting in a saturated solution dissolves, forming a saturated solution of higher concentration at higher temperature. When this solution cools, sucrose does not precipitate out of the solution. The resulting cooled solution is supersaturated with sucrose. Adding even a small amount of additional sucrose powder into this solution can trigger rapid recrystallization of all the dissolved sucrose.
Başvuru ve Özet
Solid-liquid solutions are ubiquitous in chemistry. Most chemical reactions are run in solution because dissolved solutes are mobile enough to rapidly mix and bump into one another. Solutions can also be used to store small amounts of solutes in macroscopic and easily handled volumes. Solutions exhibit some interesting physical properties called colligative properties that can be attributed to the entropic effects of dissolving a solute in a solvent.
One may wonder why so many different measures of solution concentration exist. The answer lies in the many applications of solutions and the many orders of magnitude over which concentrations span. In samples of water from the environment, for example, concentrations of metal ions can be in the range of a few parts per million — it is impractical and potentially misleading to express this tiny concentration as a molarity or mole fraction. Although molarity is a convenient measure of concentration for stoichiometry calculations involving chemical reactions, molality is more appropriate in studies of certain colligative properties.
Perfecting the technique of solution preparation is important, because in many contexts, precise knowledge of concentration is essential. When running a chemical reaction, for example, use of too much or too little solute could result in wasted reactants or low product yields. Studies of empirical relationships involving concentration, such as Beer’s law, depend on precisely known concentrations. Oftentimes, imprecision in solution concentrations leads directly to uncertainty in calculated values, such as reaction enthalpies. Although it is impossible to completely eliminate imprecision, the use of analytical techniques for solution making ensures that uncertainty is minimized.
Atla...
Bu koleksiyondaki videolar:

Now Playing
Solutions and Concentrations
General Chemistry
273.4K Görüntüleme Sayısı

Common Lab Glassware and Uses
General Chemistry
654.9K Görüntüleme Sayısı

Determining the Density of a Solid and Liquid
General Chemistry
555.2K Görüntüleme Sayısı

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.2K Görüntüleme Sayısı

Determining the Empirical Formula
General Chemistry
180.5K Görüntüleme Sayısı

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.1K Görüntüleme Sayısı

Using a pH Meter
General Chemistry
344.7K Görüntüleme Sayısı

Introduction to Titration
General Chemistry
423.7K Görüntüleme Sayısı

Ideal Gas Law
General Chemistry
78.3K Görüntüleme Sayısı

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.3K Görüntüleme Sayısı

Le Châtelier's Principle
General Chemistry
264.7K Görüntüleme Sayısı

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.6K Görüntüleme Sayısı

Determining Rate Laws and the Order of Reaction
General Chemistry
195.8K Görüntüleme Sayısı

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Görüntüleme Sayısı

Coordination Chemistry Complexes
General Chemistry
91.3K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır