Method Article
Investigating Anti-Obesity Potential of Papaver Somniferum Seeds in Obese Rat Model
In This Article
Summary
The study investigates the anti-obesity efficacy of Papaver somniferum seeds in obesity-induced Albino rats. Washed poppy seeds effectively reduced weight, lowered glucose levels, and improved lipid profiles without toxicity. In contrast, unwashed seeds altered blood parameters, suggesting potential toxicity and the need for further research.
Abstract
Obesity is a major global health issue, affecting nearly 30% of the population worldwide. Despite the prevalence of obesity, there is currently no data available on the anti-obesity and metabolic effects of Papaver somniferum. The objective of the study was to confirm the anti-obesity and metabolic effects of Papaver somniferum (poppy) seeds in high-fat diet (HFD)-induced obese rats, assessing their impact on weight reduction, lipid profile, and organ toxicity. The experiment was conducted in two phases: a 4 week poppy seed intervention and a 6 week obesity induction trial. Rats were separated into groups and given both washed or unwashed poppy seeds, HFD, and a prescription medication for weight loss. The findings demonstrated that washing poppy seeds significantly decreased weight gain and enhanced lipid profiles, particularly reducing triglycerides, low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL). Additionally, treated groups showed a decrease in glucose levels. However, higher doses of unwashed poppy seeds caused modest liver stress, indicated by raised alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and renal histopathology showed mild inflammation, but hematological parameters were constant. These results suggest that washed poppy seeds may have the potential to reduce obesity and enhance metabolic health without adverse harm, indicating the need for further research to explore their therapeutic potential.
Introduction
Obesity is a medical condition characterized by an excessively high body fat percentage despite a body mass index (BMI) of less than 201,2. When the body's controlled system fails to maintain the proper balance between nutrients and energy in the body's regulatory system, it results in the accumulation of excess body fat deposited in the body3. The BMI is the primary diagnostic tool used to determine normal weight, overweight, and obesity status. It is commonly used in clinical research to identify individuals with excess weight or obesity. Research indicates that obesity is a disease in and of itself rather than a major cause of any chronic disease4. It is primarily caused by the consumption of excessive calorie-containing food and a sedentary lifestyle5. Genetic weight-gaining mechanisms and prolonged and excessive exposure to food high in energy may also contribute to obesity. The global prevalence of obesity has increased rapidly in recent years, with an estimated 2.1 billion people globally, or 30% of the world's population, suffering from obesity and being overweight. This ratio is predicted to reach 40% by 2030 if the current trends continue to rise. Environmental factors like ecosystem and social issues also influence the development of obesity6,7. Obesity has been linked to several forms of cancer, including uterine, breast, and colon cancer, as well as comorbidities such as dyslipidemia, diabetes, and musculoskeletal disorders (especially osteoarthritis). Furthermore, obesity is also associated with cardiac risk factors, including hyperglycemia and a high body mass index. The most well-known factors that contribute to obesity include hormonal problems and compulsive eating8. While traditional treatments for obesity have not been extensively studied, they generally pose minimal risks. On the other hand, western medications often come with costly side effects that can pose significant health risks. Therefore, an alternate approach to creating safe, efficient anti-obesity medications can be to investigate natural products against obesity. The advancement of phytochemical studies supports the traditional use of therapeutic herbs9, which may be used as an alternative therapy for obesity. Several scientific investigations have shown the effectiveness of herbal medicines in treating obesity for centuries. Earlier studies have demonstrated that medicinal herbs, which contain a variety of pharmacological components, are consumed as food10. There has been discussion about the interest in employing natural herbs as medications. Using these herbs has been associated with very few negative consequences11; these plants can improve digestion and accelerate weight reduction12. Medicinal plants employ a multifaceted approach to address obesity, encompassing five primary mechanisms: appetite suppression, stimulation of thermogenesis and lipid metabolism, inhibition of pancreatic lipase activity, prevention of pathogenesis, and promotion of lipolysis13. Furthermore, natural herbs often contain bioactive compounds that act as digestive enzyme inhibitors, thereby hindering the hydrolysis and absorption of dietary carbohydrates and fats14.
Papaver somniferum, commonly known as the opium poppy or Khashkhash in the subcontinent, is a globally recognized traditional plant with a rich history of use. Various phytochemicals, including alkaloids such as morphine, codeine, noscapine, papaverine, and thebaine, have been isolated from opium15. While the poppy plant is primarily associated with its psychoactive and analgesic properties, its seeds are increasingly recognized for their potential health benefits. Poppy seeds are a rich source of polyunsaturated fatty acids, particularly omega-3 fatty acids, which have been linked to weight management16. Notably, poppy seeds contain α-linolenic acid, an omega-3 fatty acid whose anti-obesity potential has garnered considerable attention. The 10 and 12 isomers of α-linolenic acid have been specifically implicated in weight loss. Numerous human studies have demonstrated that supplementation with a combination of these isomers can decrease body fat percentage17. The primary objective of this investigation was to evaluate the effects of P. somniferum on weight reduction in an animal model. Additionally, the study aimed to assess its impact on lipid profile, hematological parameters, kidney and liver function, and adipose tissue histology.
Protocol
All procedures were conducted after the ethical committee of the University of Lahore Pakistan approved them in the meeting held on 21-04-2021 with Registration No: REG. # EPZOOL02193026
1. Housing of animals
- House 35 male Wistar albino rats (3 weeks old) individually in standard laboratory cages. Provide ad libitum access to rodent chow and water throughout the experiment.
- Maintain the vivarium environment at a constant temperature of 22 ± 1 °C and a relative humidity of 50% ± 10%. Implement a 12 h:12 h light-dark cycle using artificial illumination.
2. Grouping of animals
- At the start of the experiment, divide animals into 2 groups: obese and non-obese. Provide the obese group with a high-fat diet in addition to the normal diet. Feed the non-obese group a normal diet and use it as the control group (control negative) in the experiment.
3. Preparation of high-fat diet (HFD)
- Formulate a high-fat diet (HFD) by increasing the proportion of fats derived from both plant and animal sources. Combine the HFD with the standard chow to prepare pellets.
- Set the fat content in the HFD at 4 g per 30 g of feed. Place the pellets in the animal cages for 24 h. Weigh and record any remaining pellet after 24 h, then remove it. Provide fresh pellets each day. Maintain this HFD feeding regimen for 6 weeks to induce obesity.
- Monitor the body weight of the animals and record every week to check if it is increasing or not. To measure the body weight, put the jar on the weight machine and cover it. Add the rat inside the jar to check the weight.
- Supplement the same pellets with poppy seeds for the remaining 4 weeks to investigate their potential weight-loss effects.
4. Grouping of obese animals
- After 6 weeks, further divide obese animals into four groups: Group 1 is the obese control (control positive), which continued feeding on HFD but was not provided with any treatment. Group 2 (Standard) is treated with commercially available medicine (see Table of Materials) for obesity control. Group 3 (unwashed) and Group 4 (Washed) are fed with washed and unwashed poppy seeds, respectively.
5. Preparation of poppy seeds
- Obtain poppy seeds (P. somniferum) from a local commercial supplier as it is easily available in stores. Sun-dry the seeds for a few hours. Divide the seeds into two groups: unwashed and washed.
- Wash the washed group poppy seeds 7x with distilled water, followed by sun drying. Add 500 g of poppy seed to a 1 L beaker, add water and mix with hands. Then discard the water and repeat the procedure 7x to make sure to remove all the impurities, dust, and other particles from the seeds. After the washing, place the seeds in sunlight to dry.
6. Calculation of dosage and preparation of feed
- Determine the dosage based on the animal's feed intake, standardized at 30 g per individual. Administer poppy seeds at a concentration of 0.5 g per 30 g of feed.
- Incorporate the poppy seeds into the feed by mixing them with distilled water to form a pellet. Prepare the pellets fresh daily throughout the experimental period of 4 weeks.
7. Dissection of rats and blood collection
- Fast the rats for 24 h. Sacrifice the animal by using chloroform and collect the blood and organ samples for further procedures as described below.
- Anesthetize the rats using chloroform. Administer chloroform by a trained lab technician at the rate of 1% inhaled chloroform (0.05 mL/L) and ensure the rat before is properly anesthetized by pinching the foot of the rat.
- Perform dissection using standard dissecting box instruments. Disinfect a sharp knife, scissors, and forceps prior to starting the dissection. During dissection remove kidney, liver, and adipose tissue samples. Preserve the excised samples in formalin-filled centrifuge tubes.
- Collect blood samples in EDTA-coated vials. Extract 12 mL of blood from each animal and then further divide 3 mL each to test kidney function, liver functioning test, and lipid profiling.
- Centrifuge the collected blood samples at 1,957 x g for 5 min. Separate blood serum from the rest as a clear or yellowish liquid. Separate the serum and aliquot it into micro centrifuge tubes for further biochemical analyses.
8. Sample analysis
- Determine the total lipid profile, including total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and VLDL, using standard enzymatic colorimetric methods18. Base the determination of HDL cholesterol on a time-consuming precipitation procedure18.
- Monitor rat glucose levels regularly during the whole experiment using a blood glucose monitor GL-11019.
9. Evaluation of toxicity
- Assess the toxicological impact of Papaver somniferum on the parameters ALT, AST, ALP, TP, and bilirubin using liver function tests20. Measure serum creatinine levels following the methodology outlined in21.
- Determine hematological parameters, including red blood cell (RBC) and white blood cell (WBC) counts, using a Neubauer hemocytometer. Assess hemoglobin concentration using the cyanmethemoglobin method.
- Conduct a histopathological examination of the collected tissue samples. Stain sections with hematoxylin and eosin (H&E) for microscopic evaluation.
10. Statistical analysis
- Use SPSS software version 16 for statistical analysis. Apply an independent t-test to the first month's data for the obesity induction trial.
- Utilize repeated measures ANOVA with LSD post-hoc test for the second phase. Employ one-way ANOVA with LSD to analyze blood parameters for toxicity assessment
- Define statistical significance as p ≤ 0.05, with p ≤ 0.001 considered highly significant.
Results
Rats with an initial body weight of 40-45 g (weaning stage) were selected for the 70 day experiment, which was divided into two phases. The first phase, lasting 6 weeks, involved inducing obesity in the experimental group by supplementing their standard chow with a high-fat diet (HFD; Figure 1). In the subsequent 4 week phase, the obese rats were administered Papaver somniferum. Body weight was measured at the beginning and end of the experiment. Repeated measures ANOVA revealed a significant difference in body weight between the control and experimental groups (p < 0.001), as well as a significant effect of time (weeks) on body weight (p < 0.001). As illustrated in Figure 2, the final body weight of the obese rats was significantly higher (p < 0.001) compared to the initial body weight of the control group.
The poppy seed trial spanned 4 weeks (28 days) and involved dividing the obese rats into four experimental groups: Control Positive (HFD only), Control Negative (standard chow only), Group Standard (HFD + commercial weight-loss drug (see Table of Materials)), Group Washed (HFD + washed poppy seeds), Group Unwashed (HFD + unwashed poppy seeds).
The control positive group received only the high-fat diet (HFD) with their feed, while the control negative group received standard chow. The group standard animals were treated with a commercially available weight-loss drug (0.5 g). In the washed and unwashed groups, poppy seeds (0.5 g) were incorporated into the HFD feed.
During week 1, a significant weight increase was observed in both the washed and unwashed groups. In contrast, the group standard showed no significant weight change compared to the control positive group (p < 0.01). Week 2 saw a weight increase in the unwashed group compared to the others (p < 0.01). This trend continued in week 3, with the unwashed group exhibiting a significant weight gain. In week 4, both the unwashed and standard groups demonstrated a significant weight increase compared to the other groups (p < 0.05). These results are visually represented in Figure 3. Repeated measures ANOVA confirmed a highly significant effect of both time (weeks) and group on body weight (p < 0.001 for both).
Figure 3 shows glucose levels in different experimental groups. A represents the highest value, while D is the lowest value. Graph showing highly significant results with p-value (p < 0.001) indicates the inhibition of the alpha-amylase by poppy seeds. Figure 4A shows the density of Lipoprotein during the experiment, which is lowering in the experimental groups as compared to the control; statistically, the difference is not significant. Poppy seeds help reduce low-density and very low-density lipoproteins (LDL And VLDL) in blood, as shown in Figure 4B,C. Statistically, the difference is highly significant. There is no significant effect on triglycerides shown in Figure 4D.
The red blood cell count, white blood cell count, lymphocytes, ESR, eosinophils, platelets count, and mean corpuscular hemoglobin were studied in CBC studies. There is no statistically significant effect of poppy seeds on blood CBC and hematology, which indicates that poppy seeds do not have any side effects on the blood parameters. Alanine Transaminase (ALT), Aspartate Aminotransaminase (AST), bilirubin, and alkaline phosphate were studied in the liver functioning test to check for any negative effect on the liver by poppy seeds. There is no significant effect observed on bilirubin and alkaline phosphates. A significant increase in ALT (p < 0.01) and AST (p < 0.01) was observed in standard and unwashed groups, as shown in Figure 5. This exhibits the nontoxic effect of poppy seed as it does not increase in the washed group. Serum creatinine is studied for renal function and there was no significant effect on renal function (Figure 6). Histopathological analysis revealed a normal liver architecture in the control negative group. In contrast, all treated groups exhibited varying degrees of hepatic inflammation, ranging from mild to moderate. Hepatocytes displayed evidence of both microvesicular and macrovesicular steatosis, predominantly localized around the portal tracts and central veins. (Figure 7). Histopathological examination of kidney tissue of respective groups (Figure 8). Histopathological examination of adipose tissue from the various groups demonstrated normal adipose tissue architecture in the control group. In contrast, the HFD-fed groups exhibited evidence of vascular leakage and mild inflammation within the adipose tissue (Figure 9).
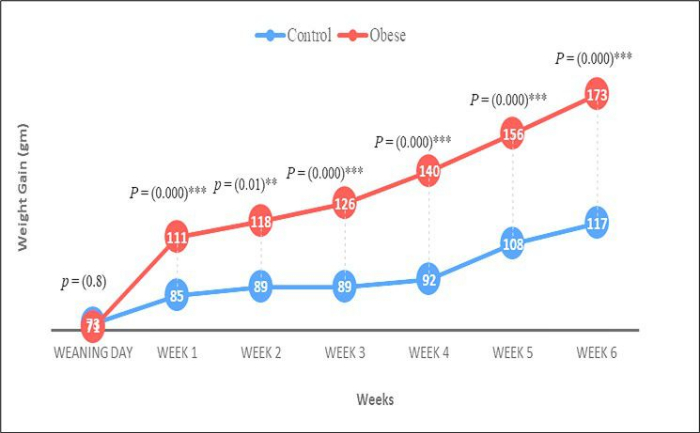
Figure 1: Weight gain. The graph shows a significant increase in the weight of rats as compared to the first week. The comparison was performed by applying an independent t-test. Data presented in terms of Mean ± SE, For p value * shows the least significant results; ** show significant results and *** shows highly significant results. Please click here to view a larger version of this figure.

Figure 2: Difference in weight gain. Graph represents multiple comparisons of a 4-week experiment by applying Repeated Measure ANOVA showing differences in weight gain in experimental groups while there is no effect of commercially brought drug and Papaver somniferum seeds (washed and unwashed) to make animals lean. Data presented in terms of (Mean ± SE), For P value one star (*) shows the least significant results. Two stars (**) show significant results. Three stars (***) show highly significant results. Please click here to view a larger version of this figure.
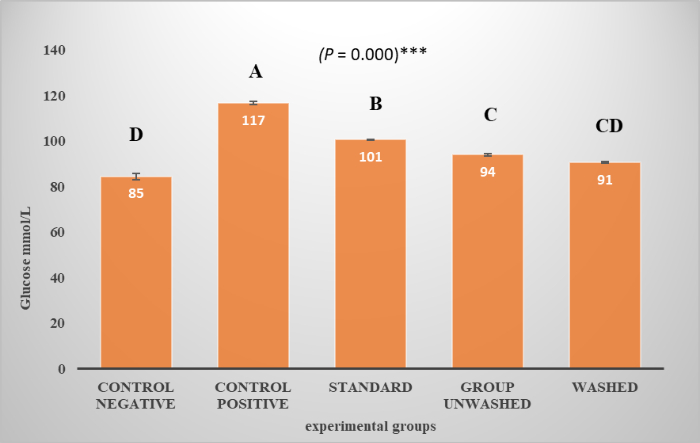
Figure 3: Glucose levels. Graph shows glucose levels in different experimental groups. P value is expressed in one-way ANOVA and compare-wise ANOVA shown by LSD. Data presented in terms of (Mean ± SE), For P value one star (*) shows the least significant results. Two stars (**) show significant results. Three star (***) shows highly significant results. Please click here to view a larger version of this figure.
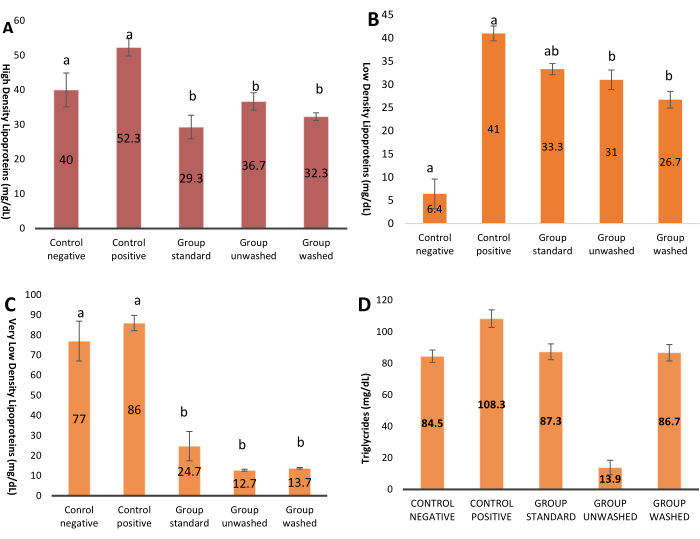
Figure 4: Lipid profile. (A) The graph shows an increase in High-Density Lipoprotein in experimental groups. P value is expressed in ONE WAY ANOVA, and pair-wise comparison is shown by LSD. Data presented in terms of (Mean ± SE). p=0.06. (B) Graph represents the level of Low-Density Lipoprotein in experimental groups. P value expressed in ONE WAY ANOVA and pair-wise comparison is shown by LSD. Data presented in terms of (Mean ± SE), p=0.05*. (C) Graph represents the elevation in VLDL level in experimental groups. P value expressed in ONE WAY ANOVA and pair-wise comparison is shown by LSD. Data presented in terms of (Mean ± SE), p=0.000***. (D) Graph representing the level of triglycerides in experimental groups.P value expressed in ONE WAY ANOVA and pair-wise comparison is shown by LSD. Data presented in terms of (Mean ± SE), p= 0.22. Please click here to view a larger version of this figure.
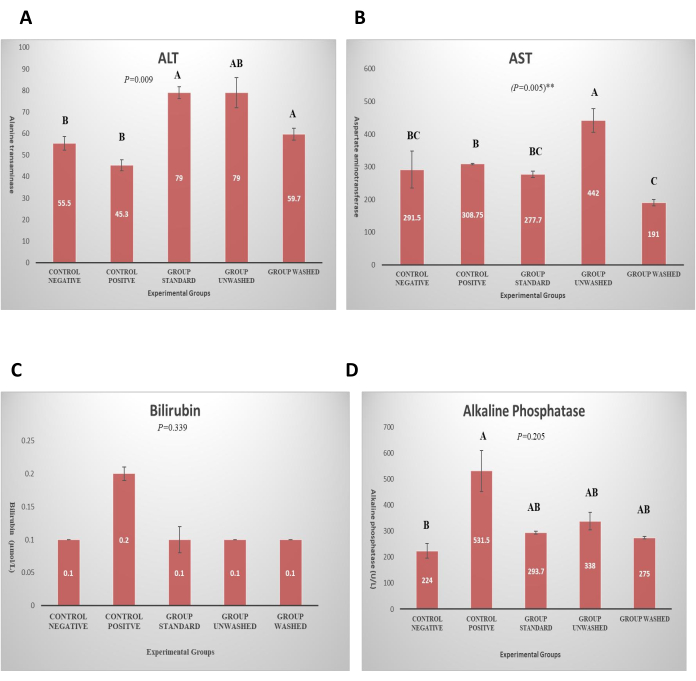
Figure 5: Liver function test. (A) Graph represents ALT in different experimental groups. The P value is expressed in one-way ANOVA, and pairwise comparison is shown by LSD. Data presented in terms of (Mean ± SE). (B) Graph shows AST in different experimental groups. The P value is expressed in one-way ANOVA, and pairwise comparison is shown by LSD. Data presented in terms of (Mean ± SE). (C) Graph shows Bilirubin level in different experimental groups. The P value is expressed in one-way ANOVA and pairwise comparison shown by LSD. Data presented in terms of (Mean ± SE). (D) Graph shows alkaline phosphatase level in different experimental groups. Please click here to view a larger version of this figure.
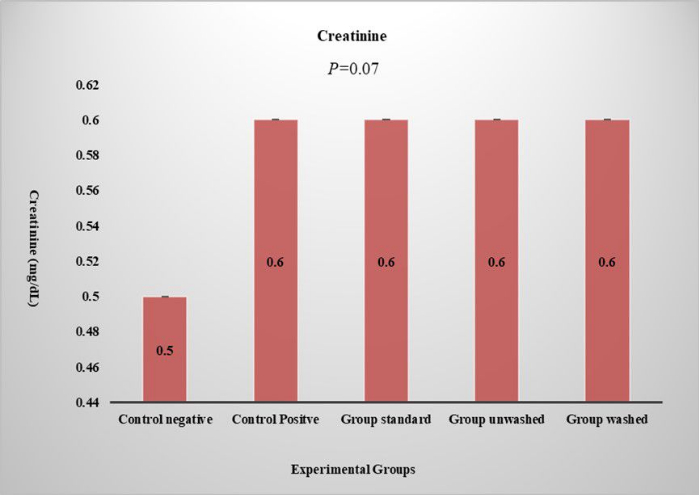
Figure 6: Serum creatinine level. Graph shows creatinine levels in different experimental groups. P value is expressed in one-way ANOVA and compare-wise ANOVA shown by LSD. Data presented in terms of (Mean ± SE). Please click here to view a larger version of this figure.

Figure 7: Histopathological examination of liver tissue. Histopathological examination shows normal looking liver in control negative, but all other treated groups show mild to moderate inflammation. Hepatocytes show micro and macro steatosis around the portal spaces and perivenular areas. 40X resolution of (A) Group control negative (B) Group control positive (C) Group standard (D) Group unwashed (E) Group washed reveals normal looking control negative liver while fatty and damaged in other groups. Please click here to view a larger version of this figure.
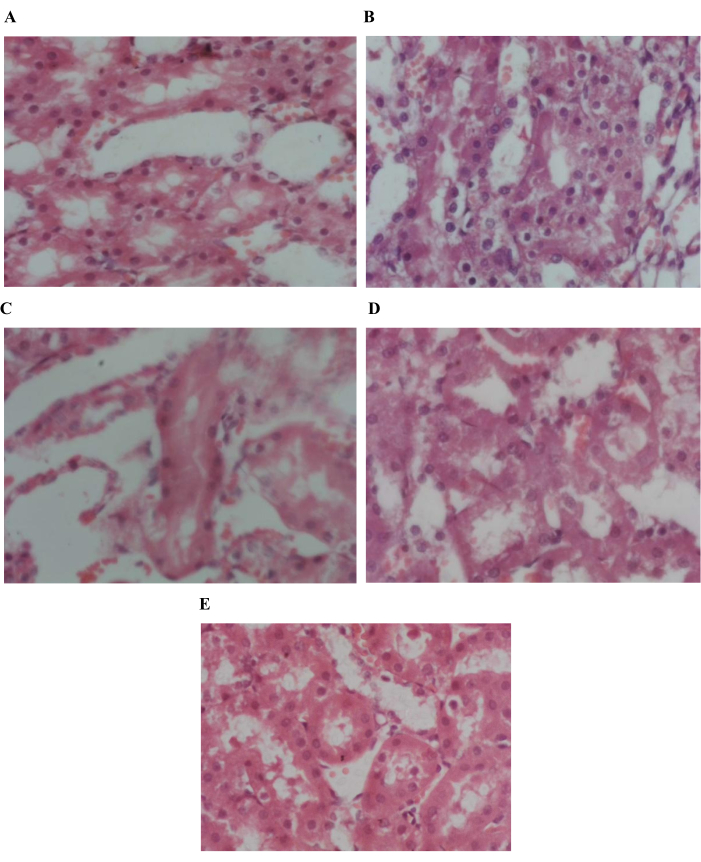
Figure 8: Histopathological examination of kidney tissue. 40X resolution of (A) Group control negative (B) Group control positive (C) Group standard (D) Group unwashed (E) Group washed reveals normal fatty tissues. Please click here to view a larger version of this figure.

Figure 9: Histopathological examination of adipose tissue. Histopathological examination of adipose tissue of respective groups reveals normal fatty tissues with leaky blood vessels and slight inflammation due to HFD. 40X resolution of (A) Group control negative (B) Group control positive (C) Group standard (D) Group unwashed (E) Group washed reveals normal fatty tissues. Please click here to view a larger version of this figure.
Discussion
The protocol has the following critical steps to be taken care of. The first critical step was to induce obesity in the animals by using High fat diet. The second step was to monitor animals which are on high fat diet that they do not develop diabetes or any other disease due to HFD.
The protocol also has the following limitations. Due to academic restrictions, the experimental time to study the exact effect of poppy seeds was only 10 weeks. A more detailed study of poppy seeds with different dosages should be studied. Due to the unavailability of advanced equipment, resources, and funding, we were only able to conduct basic tests, and this is the biggest limitation.
The results of this study reveal that there is no overall effect or loss of weight due to these seeds. Somehow, Papaver somniferum seeds help lower lipids by using washed Poppy seeds, which supports the statement that with more accuracy of dose and duration for experiment conduction, weight can be reduced by using washed Poppy seeds. Unwashed Poppy seeds have adverse effects on lipid profile and hematology due to morphine and sedatives present in them in large quantities. As no research has been done on these seeds regarding weight loss, it is suggested to conduct more experimental work to check their effects on weight control management by controlling lipids. A dyslipidemia profile, characterized by elevated serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides coupled with reduced high-density lipoprotein cholesterol (HDL-C) levels, is a well-established risk factor for cardiovascular disease. It is noteworthy that even in the presence of normal LDL-C and triglyceride levels, low HDL-C can pose a significant risk to coronary health, comparable to elevated LDL-C. HDL-C plays a crucial role in reverse cholesterol transport, thereby mitigating the atherosclerotic burden. Furthermore, HDL-C exhibits anti-inflammatory, anti-oxidative, anti-thrombotic, anti-apoptotic, and vasodilator properties22.
In the present study, the administration of Papaver somniferum seeds at the selected dosage did not elicit any significant alterations in lipid profile parameters, except triglycerides. Notably, the unwashed group exhibited the lowest triglyceride levels compared to the other groups.
The study's use of both washed and unwashed poppy seeds reveals that the preparation method significantly impacts the outcomes. The unwashed seeds, while showing some positive effects on triglyceride levels, also led to adverse effects, particularly on blood parameters and potential liver stress. The washing process likely removes or reduces the concentration of certain compounds present in the seeds, which could explain the difference in outcomes. The potential liver stress indicated by elevated ALT and AST levels in the groups receiving unwashed poppy seeds is a critical concern. The washing process might remove or dilute these toxic substances, as the group receiving washed seeds did not show the same level of liver enzyme elevation23. The study mentions that opium, which is derived from the poppy plant, contains various alkaloids, including morphine, codeine, and thebaine. These alkaloids are known to have potential hepatotoxic effects, especially when consumed in higher doses or over prolonged periods. Residual amounts of these alkaloids or other toxic compounds may remain on the unwashed seeds, contributing to the observed liver stress. The study's findings highlight the importance of proper preparation and processing of natural products, even those traditionally considered safe. The presence of potentially toxic compounds in unwashed poppy seeds underscores the need for further research to identify and characterize these substances and to establish safe consumption guidelines. The washing process appears to be an effective method for reducing the potential toxicity of poppy seeds, but more research is needed to determine the optimal washing procedures and to assess the long-term safety of consuming washed poppy seeds.
The study's findings highlight a clear distinction between the effects of washed and unwashed poppy seeds. Washed seeds appear to offer potential benefits in managing obesity and improving metabolic health without causing significant adverse effects24. In contrast, unwashed seeds, while showing some positive impacts on triglyceride levels, also exhibit potential toxicity, particularly concerning liver health. The observed hepatic inflammation, even if mild to moderate in the short term, raises concerns about the long-term implications of consuming unwashed poppy seeds. Chronic inflammation can lead to progressive liver damage, potentially resulting in fibrosis, cirrhosis, or even liver failure25. Therefore, it is crucial to exercise caution when consuming unwashed poppy seeds and to prioritize the use of washed seeds, which appear to have a safer profile. The study's findings underscore the need for further research to fully elucidate the safety profile of P. somniferum, particularly regarding the long-term effects of its consumption in various forms and dosages.
The observed increase in body weight in the unwashed seed group, coupled with potential liver toxicity, suggests that the treatment may have unintended and potentially harmful effects. It is essential to conduct more rigorous and long-term studies to comprehensively evaluate the safety and effectiveness of P. somniferum as an anti-obesity agent. These studies should investigate various preparation methods, dosages, and durations of treatment to establish safe and effective consumption guidelines. Additionally, further research is needed to identify and characterize the specific compounds responsible for the observed effects, both beneficial and adverse, to develop standardized and safe preparations of P. somniferum for potential therapeutic use.
It is postulated that a reduction in pancreatic amylase activity may lead to decreased glucose absorption by impeding digestion, consequently resulting in a lower caloric intake26. Several scientific investigations have demonstrated the potential of plant-derived α-amylase inhibitors to attenuate postprandial hyperglycemia, thereby contributing to the management of aberrant glucose metabolism and the prevention of metabolic disorders. For instance, oral supplementation with Bauhinia purpurea extract has been shown to decrease pancreatic lipase and amylase activities in hypercholesterolemia diet-fed rats19. Amylase and lipase, key enzymes involved in carbohydrate and lipid metabolism, are increasingly being explored as therapeutic targets for the treatment of various metabolic diseases, including obesity. Dietary interventions and natural products that act as amylase and pancreatic lipase inhibitors are gaining traction as potential strategies to impede intestinal fat absorption, which may facilitate weight management27.
In the current study, a decrease in glucose levels was observed in the treated groups compared to the control groups. Furthermore, triglyceride levels were reduced in the unwashed group. Further investigations employing varying dosages and concentrations of Papaver somniferum may shed light on its potential role in modulating lipid metabolism, particularly concerning lipase inhibition.
The treated groups also exhibited elevated HDL levels compared to the control groups, potentially attributable to dose differentiation. Similarly, LDL levels were higher in the treated groups compared to the negative control group, with the positive control group demonstrating the highest LDL values. Conversely, VLDL and triglyceride levels were lower in the treated groups compared to the control group. This variability in the results may be influenced by the high-fat diet regimen.
Leaky blood vessels and inflammation in treated groups, standard and control positive groups are shown due to the accumulation of HFD. According to a study, nutmeg extract decreases adipocyte numbers28. A study used Tamarindus indica L. pulp to reduce the total body weight by reducing the number of adipocytes29. Our result reveals that the untreated group has significantly higher epididymal as compared to the treated group, which shows a reduced size of adipocytes (p = 0.05; Figure 8). The results of this research presented that Papaver somniferum seeds (washed) help lower lipids levels in the body while unwashed Papaver somniferum seeds lower triglycerides. At the same time, Papaver somniferum seeds do not adversely affect the histopathology of adipose tissues. Unwashed seeds negatively affect blood parameters by raising their levels in the blood. Therefore, using washed Papaver somniferum seeds can control obesity, maybe with an increase in duration time for the conduction of the experiment and the calculation of dose. More studies should be conducted with the increase in dose and time duration for different results.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2025R986), King Saud University, Riyadh, Saudi Arabia.
Materials
| Name | Company | Catalog Number | Comments |
| Blood glucose monitor GL-110 | Certeza | GL-110 | A portable device used to measure blood glucose levels. |
| Centrifuge | Eppendorf | 22620005 | A laboratory equipment that spins samples at high speeds to separate components based on density. |
| EDTA-coated vials | BD Vacutainer | 367861 | Tubes used for collecting blood samples, preventing clotting by binding calcium ions. |
| Eppendorf tubes | Eppendorf | 0030 120.094 | Small plastic tubes used for storing and handling small volumes of liquid. |
| Falcon tubes | Corning | 352059 | Conical tubes used for centrifugation and various laboratory applications. |
| Microscope | Olympus | CX23RF100 | An optical instrument used for viewing small objects, typically magnified through lenses. |
| Neubauer hemocytometer | Hawksley | H.H1 | A specialized slide used for counting cells under a microscope. |
| Orlistat120 mg | Windlas Biotech Ltd | ||
| Pipette tips | Eppendorf | 0030 073.435 | Disposable tips used with pipettes for transferring liquids accurately. |
| Serological pipettes | Falcon | 357551 | Graduated pipettes used for transferring liquids in larger volumes. |
| SPSS software version 16 | IBM | N/A (software, not a physical product) | A statistical software package used for data analysis. |
| Standard laboratory cage (Super Mouse 750) | Lab Products, Inc. | 10021 | A cage designed to house laboratory mice, providing a controlled environment. |
References
- Da Costa, G. F., et al. The beneficial effect of anthocyanidin-rich Vitis vinifera l. Grape skin extract on metabolic changes induced by a high-fat diet in mice involves anti-inflammatory and antioxidant actions. Phytother Res. 31 (10), 1621-1632 (2017).
- Andersen, M. M., Varga, S., Folker, A. P. On the definition of stigma. J Evaluat Clin Pract. 28 (5), 847-853 (2022).
- Archer, E., Hill, J. O. Body, and fat mass are not regulated, controlled, or defended: An introduction to the invisible hand and 'competition ' models of metabolism. Progr Cardiovasc Dis. 79, 56-64 (2023).
- Imhagen, A., Karlsson, J., Jansson, S., Anderzén-Carlsson, A. A lifelong struggle for a lighter tomorrow: A qualitative study on experiences of obesity in primary healthcare patients. J Clin Nurs. 32 (5-6), 834-846 (2023).
- Alonso-Castro, A. J., et al. Self-treatment with herbal products for weight loss among overweight and obese subjects from central Mexico. J Ethnopharmacol. 234, 21-26 (2019).
- Alsareii, S. A., et al. Iot framework for a decision-making system of obesity and overweight extrapolation among children, youths, and adults. Life. 12 (9), 1414 (2022).
- Bautista, R. J. H., Mahmoud, A. M., Königsberg, M., Guerrero, N. E. L. D. Obesity: Pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed Pharmacother. 111, 503-516 (2019).
- Radin, R. M., et al. Do stress eating or compulsive eating influence metabolic health in a mindfulness-based weight loss intervention. Health Psychol. 39 (2), 147 (2020).
- De Freitas Junior, L. M., De Almeida Jr, E. B. Medicinal plants for the treatment of obesity: Ethnopharmacological approach and chemical and biological studies. Am J Transl Res. 9 (5), 2050 (2017).
- Awuchi, C. G. Medicinal plants: The medical, food, and nutritional biochemistry and uses. Int J Adv Acad Res. 5 (11), 220-241 (2019).
- Ozioma, E. O. J., Chinwe, O. a. N. Herbal medicines in african traditional medicine. Herbal Med. 10, 191-214 (2019).
- Al-Snafi, A. E., Singh, S., Bhatt, P., Kumar, V. A review on prescription and non-prescription appetite suppressants and evidence-based method to treat overweight and obesity. GSC Biol Pharmaceut Sci. 19 (3), 148-155 (2022).
- Saad, B., Zaid, H., Shanak, S., Kadan, S. . Anti-diabetes and Anti-obesity Medicinal Plants and Phytochemicals: Safety, Efficacy, and Action Mechanisms. , (2017).
- Ardeshirlarijani, E., et al. Potential anti-obesity effects of some medicinal herbs: In vitro α-amylase, α-glucosidase, and lipase inhibitory activity. Int Biol Biomed J. 5 (2), 1-8 (2019).
- Haber, I., Pergolizzi, J., Lequang, J. A. Poppy seed tea: A short review and case study. Pain Ther. 8, 151-155 (2019).
- Liu, R., et al. Omega-3 polyunsaturated fatty acids prevent obesity by improving tricarboxylic acid cycle homeostasis. J Nutri Biochem. 88, 108503 (2021).
- Basak, S., Duttaroy, A. K. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients. 12 (7), 1913 (2020).
- Mcclatchey, K. . Clinical laboratory medicine. , (2002).
- Padmaja, T. K., Naidu, P. B., Kumar, G. E. N. H., Ganapathy, S., Balaji, M. Antiobesity activity of bauhinia purpurea extract: Effect on hormones and lipid profile in high-calorie diet-induced obese rats. Adv Biosci Biotechnol. 5 (11), 861 (2014).
- Bülbül, T., Gür, E., Bozkurt, F., Eryavuz, A., Bülbül, A. Biochemical, hematological and histopathological evaluation of the food-safety of the leaf extract of Papaver somniferum in rats. J Trad Compl Med. 10 (6), 544-554 (2021).
- Precious, I. O., Ayoka, T. O., Nnadi, C. O. In-vivo sub-chronic toxicological evaluation of extract of vernonia glaberrima leaves in experimental rats. Notulae Sci Biol. 14 (2), 11181-11181 (2022).
- Bonizzi, A., Piuri, G., Corsi, F., Cazzola, R., Mazzucchelli, S. Hdl dysfunctionality: Clinical relevance of quality rather than quantity. Biomedicines. 9 (7), 729 (2021).
- Cabot, S. Hepatitis and aids: A plan to recover with complementary and modern treatments. SCB International. , (2015).
- Mohamed, S. S., Fayed, A. H. M. Anti-obesity synergistic effect of pomegranate seed oil (pso) and arabic gum (ag) in albino rats. Int J Vet Sci. 9 (1), 84-89 (2020).
- Czaja, A. J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 20 (10), 2515 (2014).
- Barrett, A. H., Farhadi, N. F., Smith, T. J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins-a review of efficacy and mechanisms. Lwt. 87, 394-399 (2018).
- Nakajima, K., Muneyuki, T., Munakata, H., Kakei, M. Revisiting the cardiometabolic relevance of serum amylase. BMC Res Notes. 4, 1-5 (2011).
- Lesmana, R., et al. Nutmeg extract potentially alters the characteristics of white adipose tissue in rats. Vet Med Sci. 7 (2), 512-520 (2021).
- Azman, K. F., et al. Antiobesity effect of tamarindus indica l. Pulp aqueous extract in high-fat diet-induced obese rats. J Natural Med. 66, 333-342 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved