Method Article
A Porcine Model of Acute Autologous Pulmonary Embolism
In This Article
Summary
This study presents a porcine model of pulmonary embolism (PE) using large autologous emboli that replicate acute intermediate-risk PE. The model is well-suited for the evaluation of both pathophysiology and treatment responses.
Abstract
Acute pulmonary embolism (PE) is a potentially life-threatening condition that causes abrupt obstruction of the pulmonary arteries, leading to acute right heart failure. Novel diagnostic methods and catheter-directed therapies are being developed rapidly, and there is an obvious need for a realistic PE animal model that can be used for pathophysiological evaluation and preclinical testing.
This protocol introduces a porcine model employing large autologous pulmonary emboli. Instrumentations are performed with minimally invasive techniques, creating a close-chest model that enables the investigation of various treatment options with high reproducibility. Three hours after drawing blood to create autologous emboli ex vivo, the induction of PE caused an immediate increase in the mean pulmonary arterial pressure (17 ± 3 mmHg to 33 ± 6 mmHg, p < 0.0001) and heart rate (50 ± 9 beats·min-1 to 63 ± 6 beats·min-1, p < 0.0003) accompanied by a decreased cardiac output (5.0 ± 0.8 L/min to 4.5 ± 0.9 L/min, p < 0.037) compared to baseline. The CT pulmonary angiography revealed multiple emboli, and the pulmonary obstruction percentage was increased compared to baseline (0% [0-0] to 57.1% [38.8-63.3], p < 0.0001). In the acute phase, the phenotype is comparable to intermediate-risk PE.
The model represents a realistic and well-characterized phenotype of intermediate-risk PE and creates an opportunity to test novel diagnostic methods, interventional and pharmaceutical treatments, and hands-on training for healthcare workers in interventional procedures.
Introduction
Acute pulmonary embolism (PE) is the third most common cause of cardiovascular death and is a manifestation of venous thromboembolism (VTE)1. The incidence of VTE ranges between 75 to 269 per 100,000 population per year and is increasing with age2. Initial survivors face a 30-day risk of death ranging from 0.5 % for low-risk patients and up to 22 % for high-risk patients3. The cause of death is right ventricular (RV) failure, which predominantly happens within hours4,5. Even if patients survive, there is still a risk of significant morbidity and chronic disease.
Treatment options in the acute phase of the disease include surgical embolectomy, catheter-based or systemic thrombolysis, low-molecular-weight heparin, and oral anticoagulants1. The number and variety of treatment options are expanding, and new techniques and methods for diagnosis and severity assessment are continuously being developed. Before clinical studies can be performed, the feasibility and safety must be determined in a reproducible and consistent setup, as can be achieved in an animal model. Furthermore, investigating the acute pathophysiology of PE requires an animal model with near-human cardiovascular- and pulmonary physiology. Models in both rodents and larger animals, i.e., pigs, have been developed6. The advantage of a large animal model is the possibility to use clinical techniques and to evaluate equipment and surgical interventions used in clinical practice. However, most of these models use artificial materials, such as plastic spheres or occlusive ballons, or require large invasive procedures for pulmonary arterial banding to mimic acute right heart failure7,8,9. One study used an inferior vena cava filter to create thrombosis in situ10. However, this is time-consuming, and the clot burden is difficult to control. Other studies have created autologous emboli ex vivo, but the PE has been smaller in size11,12. Hence, these models might not be suitable for testing interventional procedures.
There is a need for an animal model that can replicate the human pathology of PE. Based on previous studies conducted by our group13,14,15,16, we aim to present a porcine model of acute PE.
Protocol
This study was conducted with approval from the Danish Animal Inspectorate (license no. 2021-15-0201-00944) and in compliance with the Danish and university guidelines on laboratory animal welfare and ethics.
NOTE: This study followed the ARRIVE guidelines 2.017. The principles of the 3Rs (Replacement, Reduction, and Refinement) were respected by evaluating each animal repeatedly to serve as its own control, thereby reducing the number of animals needed and to maximize the information gathered. The pigs used in this animal model were female Danish slaughter pigs of  60 kg (a crossbreed of Yorkshire, Duroc, and Danish Landrace). All pigs followed the Danish Specific Pathogen Free (SPF) program. The pigs were acclimatized at the research farm one week before the study to train human contact. The pigs were housed in pens with solid concrete floors and straw bedding. Each pen measured 2.35 m x 2.9 m with adjoining pens to allow snout contact. The pigs had free access to water and were fed twice a day with a conventional pig diet, adding shredded beet to decrease weight gain. The stable had a 12:12 h light-dark cycle (lights on from 6 am to 6 pm).
60 kg (a crossbreed of Yorkshire, Duroc, and Danish Landrace). All pigs followed the Danish Specific Pathogen Free (SPF) program. The pigs were acclimatized at the research farm one week before the study to train human contact. The pigs were housed in pens with solid concrete floors and straw bedding. Each pen measured 2.35 m x 2.9 m with adjoining pens to allow snout contact. The pigs had free access to water and were fed twice a day with a conventional pig diet, adding shredded beet to decrease weight gain. The stable had a 12:12 h light-dark cycle (lights on from 6 am to 6 pm).
1. Anesthetization, intubation, and ventilation
- Pre-anesthetize the pig with an intramuscular injection (0.1 mL/kg) consisting of 2.5 mL of tiletamin (25 mg/mL), 2.5 mL of zolazepam (25 mg/mL), 2.5 mL of burophanol (10 mg/mL), 1.25 mL of ketaminol (100 mg/mL), and 6.25 mL of xylazin (20 mg/mL) to reduce potential pain, stress, and anxiety of the animal before transportation from the animal housing facility.
- Transport the animal in an approved transport box with wheat straw bedding.
- Establish intravenous access upon arrival.
- Place a tourniquet at the proximal part of the ear and tighten it lightly to obtain venous blood stasis. Disinfect the skin over a vein twice with an ethanol swab.
- Use a 20 G venous catheter to puncture the vein. Release the tourniquet. Carefully fixate the access properly to avoid displacement.
- Check for correct placement by flushing the access with isotonic saline water.
NOTE: A subcutaneous bulge will appear if the catheter is no longer in the vein. Establishing a second intravenous access in the opposing ear may be considered as a contingency.
- Move the animal to an operation table and place it supine.
- Intubate the pig using direct laryngoscopy with a size 7.5 tracheal tube and inflate the tracheal cuff. Fixate the tube to the snout/head of the animal. This will prevent unintentional extubation. Check for correct tube positioning by observing the expiratory carbon dioxide value on the ventilator screen.
- Connect the tube to a pre-tested ventilator and begin mechanical ventilation.
- Choose the pressure-controlled, volume-gated ventilation setting and set the tidal volume (TV) to 8 mL/kg with low-flow ventilation. Set the positive end-expiratory pressure (PEEP) to 5 cmH2O.
- Set the fraction of inspired oxygen (FiO2) to normoxia (0.21) or higher, depending on the experimental protocol. The target end-tidal carbon dioxide (EtCO2) value is approximately 5.0-5.5 kPa. Adjust the respiratory rate (RR) to achieve this.
- Initiate and maintain general anesthesia through intravenous access in the ear using propofol at 4.0 mg/kg/h and fentanyl at 12.5 µg/kg/h. Check for lack of corneal reflexes and responses to painful stimuli to ensure that sufficient anesthesia is being administered. Increase infusion rate if reflexes or responses are present and check for reflexes at regular intervals.
CAUTION: Do not leave the animal unattended at any point during the protocol. Refrain from using neuromuscular blocking agents, as they may obscure the signs of inadequate anesthesia. - Attach 3-lead electrocardiogram (ECG) wires and a pulse oximetry sensor to monitor heart rate, heart rhythm, and oxygen saturation.
- Monitor core temperature with a rectal thermometer. Target a normal porcine temperature of 38-39 °C. If necessary, heat the animal using a forced-air warming blanket.
- Insert a urinary bladder catheter and connect the external end to a urine sample bag.
- Apply veterinarian eye ointment to prevent dryness.
2. Ultrasound-guided intravascular accesses
NOTE: Intravascular accesses are established as previously described18.
- Establish intravascular access in the right external jugular vein, the right femoral vein, and the left femoral artery at a minimum.
NOTE: Further access can be obtained depending on the experimental protocol.- Shave and disinfect the skin with chlorhexidine.
- Under sterile procedure, use an ultrasound device to guide a 17 G venous catheter into the intravascular position.
- Remove the needle from the venous catheter and use the Seldinger technique to insert a guidewire. Remove the venous catheter and leave the guidewire in place.
- At the access point, cut a small incision in the skin and insert the sheath over the guidewire.
- To ensure correct placement of sheaths, draw blood from each sheath using a 10 mL or 20 mL syringe. A correctly placed sheath will have no resistance when blood is aspirated or the access is flushed with saline.
- Suture the sheaths to the skin (size 4.0).
- Connect the sheath in the femoral artery to a pressure transducer. Calibrate to atmospheric pressure and observe the screen for a correct arterial pressure curve.
- Connect infusion pumps with isotonic saline to the venous sheaths. This prevents intraluminal blood clotting.
- To counteract hypovolemia from fasting prior to the experiment and draw blood to create emboli, start a bolus infusion of 800 mL over 30-60 min on the pump connected to the right external jugular vein.
- To correct hourly fluid loss from perspiration and urination, start an infusion of 4 mL/kg/h on the pump connected to the femoral vein.
3. Clot formation
- Unpack a cardiopulmonary oxygenation system and locate the non-heparin-coated polyvinyl chloride (PVC) tubes with an external and internal diameter of 1/2 inches and 3/32 inches, respectively. Cut into pieces of ~30 cm in length. Fabricate seven tubes in total.
NOTE: Any smaller diameter PVC tubes can be used if thinner emboli are preferred. - Close of one end of the tubes with large hemostat forceps.
- Pause the isotonic saline infusion on one of the venous sheaths and draw a total of 180 mL of blood.
- Partition the blood into six PVC tubes (30 mL x 6) and close the top of the PVC tube with another hemostat forceps. Hang the tubes vertically for a minimum of 3 h at room temperature (RT)(Figure 1A).
- Flush saline in the sheath and restart the saline infusion.
4. Fluoroscopy-guided insertion of 26 F sheath
CAUTION: Protective gear, such as lead aprons and thyroid collars, against ionizing radiation, should be worn whenever fluoroscopy is in use.
- Pause the infusion pump connected to the sheath in the right external jugular vein.
- Insert a long extra-stiff guidewire through the sheath. Use fluoroscopy to observe the wire leaving the sheath. Advance the wire, guided by fluoroscopy, caudal through the upper central veins, the superior vena cava (SVC), the right atrium (RA), and into the inferior vena cava (IVC).
NOTE: Premature systolic events may occur as the wire passes through the RA. No resistance should be felt at any point while advancing the wire. - Extract the sheath slowly while observing by fluoroscopy that the guidewire stays in the IVC. Compress the entry point with a sterile napkin when retracting the sheath.
- Use the Seldinger technique to exchange the sheath with a 16 F dilator. Extend the skin incision if there is too much resistance. Advance the sheath into the venous circulation guided by fluoroscopy. Pre-soak with saline to minimize resistance (Figure 2B).
NOTE: It is extremely important to follow the course of the guidewire with the dilator and make sure that the dilator is not deviating from the wire and, hence, the lumen of the vessel. - Use the Seldinger technique to exchange the 16 F dilator for the 26 F sheath. Extend the skin incision by at least 10 mm. Advance the 26 F sheath slowly, guided by fluoroscopy, through the large veins until the tip of the sheath, indicated by a radiopaque marker (not the dilator), reaches the SVC (Figure 2D). Expect some resistance when advancing through layers of muscle.
NOTE: If the resistance is too great, the sheath can be retracted, and a larger and deeper incision made which encompasses the muscle tissue close to the entry point. - Under the guidance of fluoroscopy, carefully retract the dilator and guidewire out of the pig while ensuring that the sheath stays in place.
- Draw blood to ensure that the sheath is still in place. Flush with 90 mL of saline to ensure that the full length of the sheath is flushed.
- Place a stack of sterile napkins under the external end of the sheath (and under the sterile drape) to elevate it above heart level and avoid refilling blood into the sheath (Figure 2C).
- Reconnect the infusion pump and resume the saline infusion.
5. Right heart catheterization
- Flush both ports of a Swan-Ganz (SG) catheter with saline water. Check if the balloon inflates correctly.
- Connect each of the SG catheter ports to a 3- or 4-way stopcock. Connect an unused port of the stopcock to pressure transducers. The remaining port of each stopcock may later be used for central venous and pulmonary arterial blood gas sampling.
- Reset the transducers to atmospheric pressure by holding the distal ports of the SG catheter at the mid-axillary level of the pig.
- Insert the SG catheter through the 26 F sheath (Figure 2C).
- Use fluoroscopy to observe when the distal end of the SG catheter leaves the sheath. Observe that the balloon inflates properly. Inflation should be resistance-free.
NOTE: The balloon can be damaged if inflated inside the sheath. An anterior-posterior view is used for all procedures. Never retract the catheter while the balloon is inflated. This may cause the balloon to dislodge or damage the valves and chordae. - With the balloon inflated, slowly advance the balloon through the central veins, the RA, the right ventricle (RV), and into the main pulmonary artery (MPA) (Figure 2E).
- Observe that the pressure signal and pressure curve shape changes as the distal port moves into the RV and again into the MPA.
- Ensure that the pressure signal changes from 2-8 mmHg in the central venous circulation to a systolic and diastolic RV pressure of 20-30 mmHg and 0-5 mmHg, respectively. When advancing into the MPA, ensure that the systolic pressure is 25-35 mmHg and diastolic 10-15 mmHg.
- Deflate the balloon. Ensure that the SG catheter is still in place by using fluoroscopy and overserving the pressure signals and curves.
NOTE: The experiment can be paused at this point.
6. Assembling the embolus delivery device (Figure 3)
NOTE: The embolus device consists of two parts, which are referred to as part A and part B from here on (Figure 3).
- Unpack the rest of the cardiopulmonary oxygenation system with an integrated cardioplegia line and the aortic perfusion cannula under sterile conditions.
- From the autotransfusion set, locate the 10 cm long silicone tube (3/8 inch external and 3/32 inch internal diameter) attached to the bottom of the cardiotomy container and the 3/8 to ¼ inch connector piece attached to the silicone tube (Figure 3A).
- Cut the silicone tube into two equally sized tubes. Put the half without the connector aside for now.
- Locate the quick prime line. Cut the line approximately 20 cm from the Luer lock end and attach the open end of the quick prime line to the 1/4 inch end of the connector piece (Figure 3A).
- Locate a 3/8 inch to 1/2 inch connector piece. Connect it to the open end of the silicone tube. Part A is now complete (Figure 3A, C).
- Attach the remaining half of the silicone tube to the distal end of the aortic perfusion cannula. Locate and attach any 3/8 inch to 1/2 inch connector to the open end of the silicone tube. Part B is now complete (Figure 3B,C).
7. Baseline evaluation
NOTE: It is important to achieve hemodynamic stabilization after instrumentation and before baseline evaluation. The following measures are recommended. The scope of baseline measurement can be adjusted according to the specific protocol.
- Record the values of the systemic- and pulmonary arterial pressures, as well as central venous pressure.
NOTE: Acceptable pulmonary arterial pressures are a systolic pressure < 40 mmHg and mean pulmonary arterial pressure (mPAP) ≤ 20 mmHg. - Record central body temperature, peripheral saturation, and heart rate.
- On the respirator, record the values for FiO2, EtCO2, TV, minute volume (MV), RR, and peak pressure (Ppeak).
- Draw 1 mL blood samples from the arterial sheath and the distal (yellow) port of the SG catheter (mixed venous) for blood gas analysis.
- If present, correct any electrolyte imbalance and/or low blood glucose to achieve values within the normal range.
- Depending on the experimental protocol, draw venous blood samples in containers appropriate for further analysis.
- Obtain cardiac output (CO) by thermodilution through the SG catheter. Ensure that an average of three measurements within a 10% margin is obtained.
- Obtain pulmonary capillary wedge pressure (PAWP) through the SG catheter.
8. Clot evaluation
NOTE: After a minimum of 3 h, the emboli are ready to be induced. The PVC tube will contain the formed embolus and the liquid supernatant. If the blood has not coagulated, wait for another 30 min before retrieving another embolus.
- Retrieve one of the PVC tubes containing a fully formed embolus and gently place the embolus on a surgical napkin, discarding the supernatant. Ensure that the embolus feels rigid and stable for injection (Figure 1B).
9. Inducing acute pulmonary embolism (Figure 4)
- Place a 1000 mL bag of isotonic saline into a pressure infusion bag. Insert an infusion set and inflate the pressure bag to at least 200 mmHg (but not beyond the recommended pressure).
- Take part A of the embolus delivery device and connect it to the side port of the 3-way stopcock (Figure 4A).
- Connect the last PVC tube to the open end of part A.
- Place one embolus in the tube and fill the system with saline (Figure 4B).
- Attach part B to the other end of the PVC tube (Figure 4C).
NOTE: Make sure the whole system is filled with saline. - Insert the embolus device into the 26 F sheath and infuse the embolus by opening the pressurized saline flow for about 5 s (Figure 4D).
CAUTION: Carefully observe the vital parameters before and after injection of an embolus. If no response is observed, the embolism may still be in transit, and flush saline for an additional 3 s.
10. Acute PE model (Figure 5 and Figure 6)
- Induce emboli until mPAP has doubled from baseline or until all six emboli are induced. Monitor the hemodynamic response and wait for stabilization before inducing another embolus.
CAUTION: The pig can get hemodynamically unstable during the induction of an embolus. If mean systemic arterial pressure decreases to 50 mmHg, a bolus of 0.02 mg norepinephrine can be necessary. Repeat the bolus if needed. - After injecting the suited number of emboli, the pig is stable for 30 min.
NOTE: The manifestation of PE is a hyperdynamic condition. Therefore, mPAP should be at a plateau before proceeding to section 11.
11. Hemodynamics
- After 30 min of stabilization, perform an acute PE evaluation in recording to the baseline evaluation performed in section 7.
- Depending on the protocol, interventions can now begin.
12. Computed tomography pulmonary angiography (CTPA) (Figure 7)
NOTE: This part of the protocol can be excluded depending on the scientific scope.
- While still intubated and anesthetized, connect the pig to a transportable mechanical ventilator and transport the pig to the CTPA facilities.
- Perform CTPA during inspiratory breath-hold before the PE induction as a part of the baseline evaluation.
- Use automatic exposure control at 120 KV with a collimation set to 0.5 x 80 mm.
- Via an ear vein by an automated injection pump, inject 75 mL of contrast solution of Iomeron (350 mg/mL) with a flow of 0.5 mL/s followed by 30 mL of saline solution injected at 3.0 mL/s.
- Transport the pig back to the operator room and continue with the protocol.
- After PE induction, repeat steps 12.1-12.2 for a PE evaluation.
13. Other methods
- Depending on the scope of the scientific work, evaluate the pig accordingly.
NOTE: Numerous methods of evaluation, not described in detail in this protocol, can be applied in the model: magnetic resonance imaging, transesophageal echocardiography, bi-ventricular pressure-volume loop recordings, biochemical, ex vivo-physiology and histological analysis have been used in previous work13,14,15,16,18,19,20.
14. Euthanasia and necropsy
- Euthanize the pig with a lethal dose of pentobarbital (1.5 mL/kg, 400 mg/mL) at the end of the protocol.
NOTE: Depending on the protocol, a necropsy can be performed, and histological sampling can be obtained (Figure 8).
Results
In a pooled analysis of pigs included in previous studies, we present the results characterizing the acute PE model described in this protocol15,16. Two pigs died from acute right heart failure following PE. In total, we included 24 pigs.
Hemodynamics
The response after each embolus is evident in Figure 5. The induction of PE (5 ± 1) caused an immediate increase in mPAP (17 ± 3 mmHg to 33±6 mmHg, p < 0.0001) and HR (50 ± 9 beats·min-1 to 63 ± 6 beats·min-1, p < 0.0003) accompanied by a decrease in CO (5.0 ± 0.8 L/min to 4.5 ± 0.9 L/min, p < 0.037) and EtCO2 (Figure 5 and Figure 6). The MAP was unaltered (79 ± 9 mmHg to 77 ± 11 mmHg, p = 0.1955). (Figure 6). The PE induction resulted in elevated troponin T (TnT)13, increased RV afterload, RV ventriculo-arterial uncoupling, and RV dilatation, making it compatible with intermediate-risk PE (data not shown)1,14.
Imaging
To evaluate the clot burden, a pulmonary obstruction percentage was calculated as previously described (Figure 7)15. In short, the percentage was calculated as 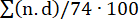 , where n is the presence of segmental embolus, and d is the degree of obstruction on a scale from 0-2, computing with a maximum of 74 points. The CTPA performed at baseline showed no signs of PE (Figure 7A), but after PE induction, multiple emboli in the pulmonary arteries were evident on the CTPA, yielding an increase in the CT obstruction percentage (0 [0 - 0] to 57.1% [IQR 38.8-63.3]) (Figure 7C-E).
, where n is the presence of segmental embolus, and d is the degree of obstruction on a scale from 0-2, computing with a maximum of 74 points. The CTPA performed at baseline showed no signs of PE (Figure 7A), but after PE induction, multiple emboli in the pulmonary arteries were evident on the CTPA, yielding an increase in the CT obstruction percentage (0 [0 - 0] to 57.1% [IQR 38.8-63.3]) (Figure 7C-E).

Figure 1: Embolus formation. (A) PVC tubes filled with 30 mL blood, each hanging vertically for a minimum 3 h. (B) One formed embolus. Please click here to view a larger version of this figure.
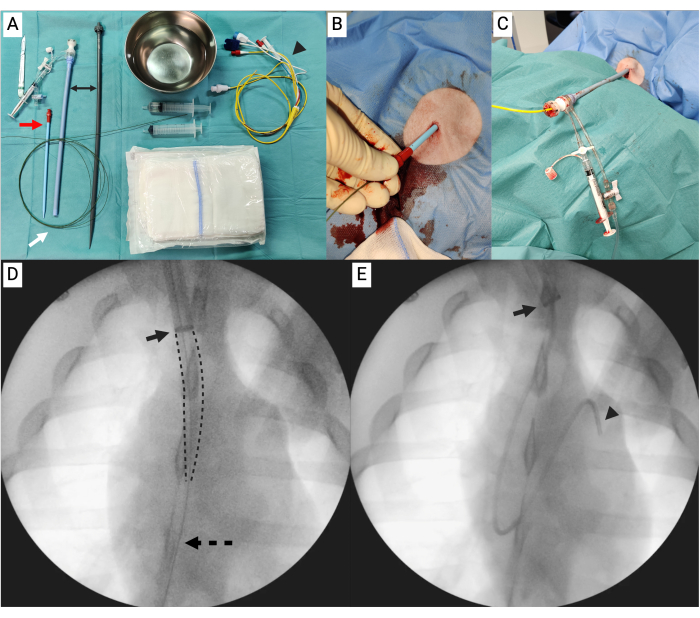
Figure 2: Insertion of 26 F sheath and right heart catheterization. (A) Table with equipment needed for replacing the 8 F sheath, using a 16 F dilator (red arrow) and long extra-stiff guidewire (white arrow), into a 26 F sheath (black double arrow), including the Swan-Ganz (SG) catheter (arrowhead). (B) The wire with the 16 F dilator. (C) The 26 F sheath in place with napkins under the sterile drape to elevate the end. The SG is inserted into the 26 F sheath. (D) Fluoroscopy showing the 26 F (arrow indicating the radiopaque ring of the sheath) with the dilator (marked by dotted lines) and the wire (dotted arrow). (E) Fluoroscopy showing the 26 F sheath (arrow) with the SG catheter (arrowhead). Please click here to view a larger version of this figure.
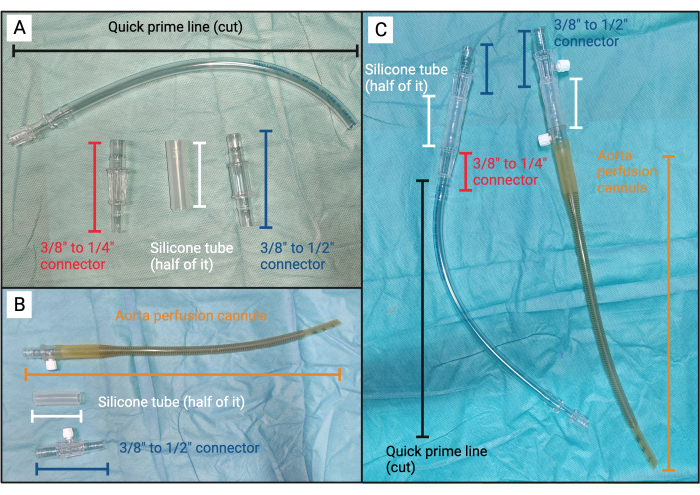
Figure 3: Embolus device. (A) Unassembled components for part A of the embolus device. (B) Unassembled components for part B of the embolus device. (C) From left to right: assembled part A of the embolus device and part B of the embolus device. Please click here to view a larger version of this figure.

Figure 4: Induction of embolus. (A) From left to right: assembled part B of the embolus device, PVC tube, and part A of the embolus device connected to the infusion set by the 3-way side-port. (B) The PVC tube is attached to part A of the embolus device, and an embolus is placed in the tube. (C) Fully assembled embolus device with an embolus. (D) The embolus device (black dotted arrow) with an embolus (black arrow) is inserted into the 26 F sheath (white arrow) with the Swan Ganz catheter (white dotted arrow) in place. Please click here to view a larger version of this figure.
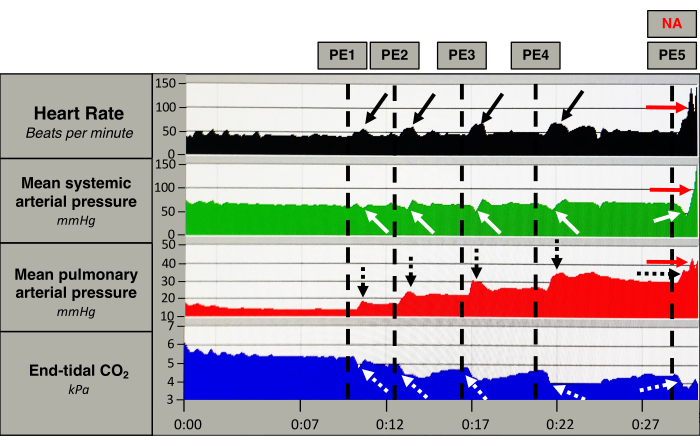
Figure 5: Acute hemodynamic response. Measurements of heart rate (black), mean systemic arterial pressure (green), mean pulmonary arterial pressure (red), and end-tidal CO2 (blue) from one pig. The vertical dotted lines mark the induction of an embolus (PE). After PE induction, the heart rate increases momentarily (black arrows), together with a persistent increase in the mean pulmonary arterial pressure (black dotted arrow). The mean systemic arterial pressure decreases slightly after each PE, and the decrease becomes bigger after each embolus (white arrows). The end-tidal CO2 decreases abruptly after each PE (white dotted arrows). After the fifth PE the pig receives a bolus of norepinephrine (NA) that causes an increase in heart rate and mean systemic- and pulmonary arterial pressure (red arrows). Please click here to view a larger version of this figure.

Figure 6: Acute physiological response. Comparison of (A) mean pulmonary arterial pressure, (B) mean arterial pressure, (C) heart rate, and (D) cardiac output at baseline and after induction of pulmonary emboli (PE). Timepoints are compared with paired sample t-tests. Please click here to view a larger version of this figure.
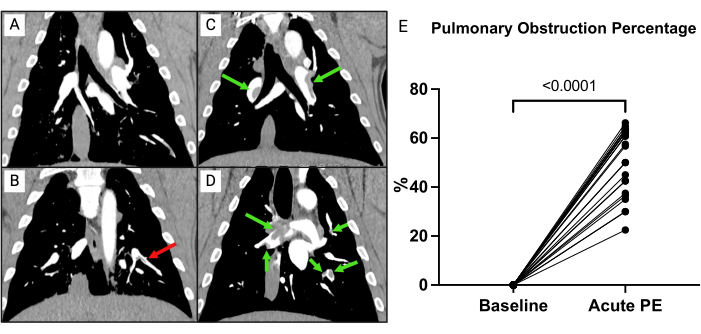
Figure 7: CT pulmonary angiography (CTPA). (A) Pig after full instrumentation, receiving only saline. No signs of embolic material. (B) CTPA of a pig after full instrumentation receiving only saline. A non-indented embolus (red arrow) is present. (C,D) Immediately after the induction of emboli (green arrows) from two different pigs. (E) Pulmonary obstruction percentage at baseline compared to after induction of pulmonary emboli (PE). Timepoints are compared with paired sample t-tests. Please click here to view a larger version of this figure.
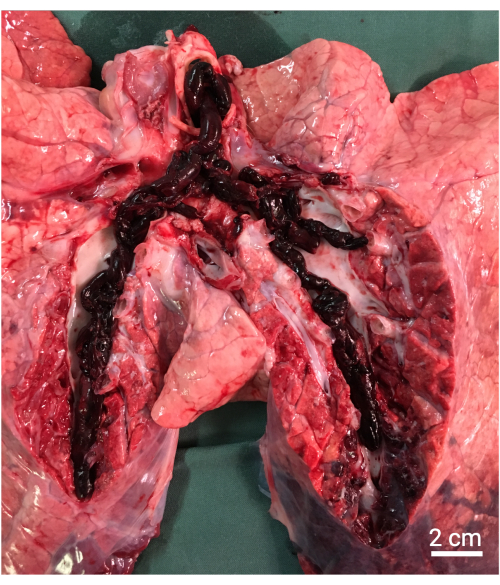
Figure 8: Necropsy. Macroscopically findings showing the large autologous emboli in the pulmonary arteries. Please click here to view a larger version of this figure.
Discussion
This paper describes a porcine model of acute, intermediate-risk PE using autologous emboli that is minimally invasive and reproducible.
There are some critical steps in this protocol. First, the dilation of the access in the right external jugular vein is crucial for the model as it serves as the access point for the emboli. When advancing the large sheath, it is essential to adhere to the guidance of the stiff wire under continuous fluoroscopy to prevent rupture or dissection of major vessels or right heart chambers. If resistance is encountered, do not apply excessive pressure but instead replace the wire and ensure optimal dilatation of the access. Furthermore, pigs are arrhythmogenic, and the instrumentation can cause atrial fibrillation. It is, therefore, crucial to observe the pig closely.
Second, the model has a hemodynamic phenotype of intermediate-risk PE in the acute phase. The careful titration of the optimal number of emboli can pose a challenge. If the emboli volume surpasses the heart's capability to withstand high pressure, the pig may experience acute right heart failure and death. The closed-chest approach to PE induction relies on the investigator closely monitoring the hemodynamic response, such as increased mPAP, decreased EtCO2, elevated HR, or reduced systemic arterial pressure, during the embolus induction. If systemic hypotension is severe and prolonged, it suggests that maximal thrombus burden has been reached. By allowing for sufficient time between emboli the risk of sudden collapse is decreased. If there is no apparent response, the embolus may be in transit within the sheath or right-sided chambers of the heart. Then we recommend re-flushing and waiting as an immediate induction of a novel embolus, which could lead to two simultaneous emboli, which can be fatal.
Third, the model is established without the use of heparin. The investigator should pay close attention to always having saline connected to the accesses and remember to draw spill and flush with saline before use. If not done so, embolic material will form and can dislodge, causing smaller emboli to be induced throughout the protocol that can interfere with the results (Figure 4B).
The model contributes to a realistic physiological response, owing to the closed-chest setup but also due to the utilization of autologous embolic material. In the pursuit of developing an acute thromboembolic phenotype in animals, various models have been developed, most requiring the use of inorganic material, pharmacological treatments and/or ligation of the pulmonary artery to induce acute pulmonary hypertension and RV heart failure in a large animal model6,7,8,21,22. Consequently, these models do not mimic the clinical presentation of a patient with PE. One study has created the thromboembolic material in vivo by occlusion of the inferior vena cave to create a deep vein thrombosis (DVT)10. However, the creation of the DVT is time-consuming, and the induction of the DVT is insufficient to create a model with RV dysfunction.
In the present model, the goal was to create a hemodynamic phenotype that is comparable with intermediate-risk PE, which was achieved in the model after 5 ± 1 emboli. The clot burden can be changed depending on the scope of the model to achieve a desired hemodynamic phenotype, or one could use a fixed thrombus load. However, a study from our group showed that the hemodynamic changes in pulmonary pressure and RV dysfunction are not directly related to the clot burden alone23. The study found that induction of the first embolus caused the largest increase in mPAP compared to following embolic inductions. However, the RV afterload increased at the induction of the third embolus.
The experimental setup used in this study further provides opportunities to investigate the pathophysiological response in relation to imaging and hemodynamic changes13,14,15. Studies have investigated the acute response of the RV in the first 12 hours after an event of intermediate-risk PE14. Another study has used the model to look at the prolonged response one month after induction of embolic material15. Furthermore, the model has been used to test pharmacological treatments in the acute and subacute phase16,21,22,24,25,26,27. One of the studies found that the use of oxygen therapy at 40% FiO2 decreased the afterload and mechanical work of RV. The model has proven to be versatile.
There are some limitations to the protocol. First, the emboli are formed shortly before induction and created ex vivo, which can be a limitation as the in vitro created thrombus has been found to consist of less fibrin28. However, the benefit is that the clot burden can be controlled while still using autologous material. Second, the animals are fully anesthetized during the protocol, and the anesthesia can affect the hemodynamic response. However, doing repeated measurements equalizes any effect.
The model is established in slaughter pigs and not genetically modified or bred in isolation. The pigs can be prone to diseases that can affect the cardiovascular system and pulmonary pressure29. Therefore, it is essential to perform a baseline evaluation of the hemodynamic status.
In conclusion, we present a pig model using autologous emboli. The model presents a phenotype that is comparable with intermediate-risk PE. This model serves as a valuable tool for testing novel interventional and pharmaceutical treatments, as well as for training medical doctors and professional healthcare workers in interventional procedures.
Disclosures
AA has received speaker honoraria (ABBOTT, Gore Medical, Angiodynamics, EPS Vascular, and Jannsen), and he is a consultant for Inari Medical.
Acknowledgements
We wish to express our sincere gratitude for the tremendous dedication and hard work exhibited by the staff at the Department of Clinical Medicine, Aarhus University, in completing the experiments. Furthermore, we want to thank our collaborators at the Department of Forensic Medicine, Aarhus University, and the Department of Radiology, Massachusetts General Hospital, for the invaluable assistance in conducting and analyzing the CT pulmonary angiography. The work has been supported by Aarhus University Graduate School, Karen Elise Jensen's Foundation, Danish Heart Foundation, NIH-grant no. 1R01HL168040-01, Novo Nordisk Foundation [NNF17OC0024868], Holger og Ruth Hesse's Mindefond, Laerdal Foundation [3374], Alfred Benzons Foundation, A.P. Møller Fonden, Direktør Emil C. Hertz og hustru Inger Hertz Fond, P.A. Messerschmidt og Hustrus fond, and Helga og Peter Kornings Fond.
Materials
| Name | Company | Catalog Number | Comments |
| 12L-RS | GE Healthcare Japan | 5141337 | Ultrasound probe |
| 50 mL BD Luer-Lock | BD Plastipak | 300865 | |
| Adhesive Aperature Drape (OneMed) | evercare | 1515-01 | 75 cm x 90 cm (hole: 6 cm x 8 cm) |
| Alaris GP Guardrails plus | CareFusion | 9002TIG01-G | Infusion pump |
| Alaris Infusion set | BD Plastipak | 60593 | |
| Alcohol swap | MEDIQ Danmark | 3340012 | 82% ethanol, 0.5% chlorhexidin, skin disinfection |
| Amplatz Support Wire Guide Extra-Stiff | Cook Medical | THSF-25-260-AES | diameter: 0.025 inches, length: 260 cm |
| Aortic Perfusion Cannula | Edwards Lifesciences | AA024TFTA | Size: 24F. Length: 30 cm. |
| BD Connecta | BD | 394601 | Luer-Lock |
| BD Emerald | BD | 307736 | 10 mL syringe |
| BD Platipak | BD | 300613 | 20 mL syringe |
| BD Venflon Pro | Becton Dickinson Infusion Therapy | 393204 | 20 G |
| BD Venflon Pro | Becton Dickinson Infusion Therapy | 393208 | 17 G |
| Butomidor Vet | Richter Pharma AG | 531943 | 10 mg/mL |
| Chlorhexidine 0.5% | Meda AB | N/A | |
| Cios Connect S/N 20015 | Siemens Healthineers | N/A | C-arm |
| CP Oxygenation System Adult With Fusion and Cardioplegia 1/B | Medtronic | M450311W | Custom cardiopulmonary oxygenation system including a cardioplegia line. |
| D-LCC12A-01 | GE Healthcare Finland | N/A | Pressure measurement monitor |
| Durapore | 3M | N/A | Adhesive tape |
| E-PRESTIN-00 | GE Healthcare Finland | 6152932 | Respirator tubes |
| Euthanimal | Alfasan | 136278 | Pentobarbitalnatrium 400 mg/mL (0.5 mL/kg for euthanasia) |
| Favorita II | Aesculap | GT104 | |
| Fentanyl | B. Braun | 71036 | 50 µg/mL |
| Glucose isotonic | SAD | 419358 | 55 mg/mL Isotonic glucose (500 mL bag) |
| Gore DrySeal Flex Introducer Sheath | GORE | DSF2633 | Size: 26 French. Working length: 33 cm. |
| Ketaminol Vet | MSD/Intervet International B.V. | 511519 | 100 mg/mL |
| Lawton 85-0010 ZK1 | Lawton | N/A | Laryngoscope |
| Lectospiral | VYGON | 1159.90 | 400 cm (Luer-LOCK) |
| MBH qufora | MBH-International A/S | 13853401 | Urine bag |
| Natriumchlorid | Fresenius Kabi | 7340022100528 | 9 mg/mL Isotonic saline |
| Noradrenalin | Macure Pharma | 425318 | 1 mg/mL |
| PICO50 Aterial Blood Sampler | Radiometer | 956-552 | 2 mL |
| Portex Tracheal Tube | Smiths Medical | 100/150/075 | Cuffed Clear Oral/Nasal Murphy Eye |
| Pressure Extension set | CODAN | 7,14,020 | Tube for anesthetics, 150 cm long, inner diameter 0.9 mm |
| Propolipid | Fresenius Kabi | 21636 | Propofol, 10 mg/mL |
| Radiofocus Introducer II | Radiofocus/Terumo | RS+B80N10MQ | 7 + 8F sheaths |
| Rompun Vet | Beyer | 86450917 | Xylazin, 20 mg/mL |
| Rüsch Brilliant AquaFlate Glycerine | Teleflex | 178000 | Bladder catheter, size 14 |
| S/5 Avance | Datex-Ohmeda | N/A | Mechanical ventilator |
| Safersonic Conti Plus & Safergel | SECMA medical innovation | SAF.612.18120.WG.SEC | 18 cm x 120 cm (Safersonic Sterile Transducer Cover with Adhesive Area and Safergel) |
| Standard Dilator | Cook Medical | G01212 | Size: 16 French. Length: 20 cm. |
| Swan-Ganz CCOmbo | Edwards Lifesciences | 744F75 | 110 cm |
| TruWave Pressure Monitoring Set | Edwards Lifesciences | T434303A | 210 cm |
| Vigilance VGS Patient Monitor | Edwards Lifesciences | N/A | |
| Vivid iq | GE Medical Systems China | Vivid iq | |
| Zoletil 50 Vet (tiletamin 125 mg and zolazepam 125 mg) | Virbac | 83046805 | Zoletil Mix for pigs: 1 vial of Zoletil 50 Vet (dry matter); add 6.25 mL Xylozin (20 mg/mL), 1.25 mL ketamin (100 mg/mL) and 2.5 mL Butorphanol (10 mg/mL). Dose for pre-anesthesia: 0.1 mL/kg as intramuscular injection |
References
- Konstantinides, S. V., et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 41 (4), 543-603 (2019).
- Wendelboe, A. M., Raskob, G. E. Global burden of thrombosis. Circ. Res. 118 (9), 1340-1347 (2016).
- Becattini, C., et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur. Respir. J. 48 (3), 780-786 (2016).
- Wood, K. E. Major Pulmonary embolism: Review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 121 (3), 877-905 (2002).
- Bĕlohlávek, J., Dytrych, V., Linhart, A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis, and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 18 (2), 129-138 (2013).
- Andersen, A., et al. Animal models of right heart failure. Cardiovasc Diagn Ther. 10 (5), 1561-1579 (2020).
- Tsang, J. Y., Lamm, W. J., Starr, I. R., Hlastala, M. P. Spatial pattern of ventilation-perfusion mismatch following acute pulmonary thromboembolism in pigs. J Appl Physiol. 98 (5), 1862-1868 (2005).
- Böttiger, B. W., et al. Inhaled nitric oxide selectively decreases pulmonary artery pressure and pulmonary vascular resistance following acute massive pulmonary microembolism in piglets. Chest. 110 (4), 1041-1047 (1996).
- Kudlička, J., et al. Pig model of pulmonary embolism: where is the hemodynamic break point. Physiol Res. 62 (Suppl 1), S173-S179 (2013).
- Barbash, I. M., et al. Experimental model of large pulmonary embolism employing controlled release of subacute caval thrombus in swine. J Vasc Interv Radiol. 22 (10), 1471-1477 (2011).
- Beam, D. M., et al. Comparison of isoflurane and α-chloralose in an anesthetized swine model of acute pulmonary embolism producing right ventricular dysfunction. Comp Med. 65 (1), 54-61 (2015).
- Pereira, D. J., et al. Near-fatal pulmonary embolism in an experimental model: hemodynamic, gasometric and capnographic variables. Rev Bras Cir Cardiovasc. 26 (3), 462-468 (2011).
- Schultz, J., et al. A porcine in-vivo model of acute pulmonary embolism. Pulm. Circ. 8 (1), 2045893217738217 (2018).
- Lyhne, M. D., et al. Right ventricular adaptation in the critical phase after acute intermediate-risk pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 10 (3), 243-249 (2020).
- Dragsbaek, S. J., et al. A porcine model of human-like chronic thromboembolic pulmonary disease. Thromb. Res. 231, 25-28 (2023).
- Merit, V. T., et al. Changes in pulmonary vascular resistance and obstruction score following acute pulmonary embolism in pigs. Crit Care Explor. 6 (2), e1040 (2024).
- NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Exp Physiol. 95 (8), 842-844 (2010).
- Lyhne, M. D., et al. Closed chest biventricular pressure-volume loop recordings with admittance catheters in a porcine model. J Vis Exp. 171, e62661 (2021).
- Schultz, J., Andersen, A., Gade, I. L., Kjaergaard, B., Nielsen-Kudsk, J. E. Riociguat, sildenafil and inhaled nitric oxide reduces pulmonary vascular resistance and improves right ventricular function in a porcine model of acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 9 (4), 293-301 (2019).
- Schultz, J., et al. Terlipressin increases systemic and lowers pulmonary arterial pressure in experimental acute pulmonary embolism. Crit Care Med. 48 (4), e308-e315 (2020).
- Schmitto, J. D., et al. Progressive right ventricular failure is not explained by myocardial ischemia in a pig model of right ventricular pressure overload. Eur J Cardiothorac Surg. 35 (2), 229-234 (2009).
- Greyson, C., Xu, Y., Lu, L., Schwartz, G. G. Right ventricular pressure and dilation during pressure overload determine dysfunction after pressure overload. Am J Physiol Heart Circ Physiol. 278 (5), H1414-H1420 (2000).
- Lyhne, M. D., et al. Immediate cardiopulmonary responses to consecutive pulmonary embolism: a randomized, controlled, experimental study. BMC Pulm Med. 24 (1), 233 (2024).
- Mortensen, C. S., et al. Impact of preload on right ventricular hemodynamics in acute pulmonary embolism. Crit Care Med. 48 (12), e1306-e1312 (2020).
- Lyhne, M. D., et al. Oxygen therapy lowers right ventricular afterload in experimental acute pulmonary embolism. Crit Care Med. 49 (9), e891-e901 (2021).
- Kramer, A., et al. Inhaled nitric oxide has pulmonary vasodilator efficacy both in the immediate and prolonged phase of acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 10 (3), 265-272 (2020).
- Lyhne, M. D., et al. Levosimendan, milrinone, and dobutamine in experimental acute pulmonary embolism. Pulm Circ. 11 (3), 20458940211022977 (2021).
- Krueger, K., Deissler, P., Coburger, S., Fries, J. W. U., Lackner, K. How thrombus model impacts the in vitro study of interventional thrombectomy procedures. Invest Radiol. 39 (10), 641-648 (2004).
- Brockmeier, S. L., Halbur, P. G., Thacker, E. L. . Porcine Respiratory Disease Complex. Polymicrobial Diseases. , (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved