Method Article
Local Anesthetic Thoracoscopy for Undiagnosed Pleural Effusion
In This Article
Erratum Notice
Summary
Local anesthetic thoracoscopy (LAT) is essential for diagnosing recurrent, undiagnosed pleural effusion when a guideline-based workup fails to provide a specific cause. LAT can be performed as a day-case procedure by chest physicians. Here, we present a step-by-step approach for a successful and safe procedure.
Abstract
Local anesthetic thoracoscopy (LAT) is a minimally invasive diagnostic procedure gaining recognition among chest physicians for managing undiagnosed pleural effusions. This single-port procedure is conducted with the patient under mild sedation and involves a contralateral decubitus position. It is performed in a sterile setting, typically a bronchoscopy suite or surgical theater, by a single operator with support from a procedure-focused nurse and a patient-focused nurse.
The procedure begins with a thoracic ultrasound to determine the optimal entry point, usually in the IV-V intercostal space along the midaxillary line. Lidocaine/mepivacaine, with or without adrenaline, is used to anesthetize the skin, thoracic wall layers, and parietal pleura. A designated trocar and cannula are inserted through a 10 mm incision, reaching the pleural cavity with gentle rotation. The thoracoscope is introduced through the cannula for systematic inspection of the pleural cavity from the apex to the diaphragm. Biopsies (typically six to ten) of suspicious parietal pleura lesions are obtained for histopathological evaluation and, when necessary, microbiological analysis. Biopsies of the visceral pleura are generally avoided due to the risk of bleeding or air leaks. Talc poudrage may be performed before inserting a chest tube or indwelling pleural catheter through the cannula. The skin incision is sutured, and intrapleural air is removed using a three-compartment or digital chest drainage system. The chest tube is removed once there is no airflow, and the lung has satisfactorily re-expanded. Patients are usually discharged after 2-4 h of observation and followed up on an outpatient basis. Successful LAT relies on careful patient selection, preparation, and management, as well as operator education, to ensure safety and a high diagnostic yield.
Introduction
The incidence and prevalence of pleural diseases are increasing worldwide, especially pleural effusions, which have more than 50 recognized causes1,2. Pleural malignancy is the leading cause of recurrent pleural effusion, mostly due to metastases from extrapleural lung, breast, or lymphoma malignancies3. Existing guidelines recommend pleural biopsies if the medical history, physical examination, radiology, and pleural tap for cytology, culture, and biochemistry fail to provide a diagnosis4. Pleural biopsies can be obtained either image-guided or under direct vision. Ultrasound (US)- or CT-guided percutaneous core biopsies allow sampling from most lesions in the costal part of the parietal pleura, with a diagnostic yield of 84% and 93%, respectively, in a recent systematic review5. US-guided pleural biopsy is superior in terms of a lower complication rate (4% vs. 7%), no irradiation, and availability as a bedside procedure5. Case reports show that lesions in the mediastinal pleura can be sampled using echoendoscopes6.
Thoracoscopy allows direct visual inspection and sampling of lesions in both the mediastinal, diaphragmatic, or costal pleura, making it the gold standard in the diagnosis of recurrent pleural effusion of an unknown cause4,7. Thoracoscopy is performed as either a local anesthetic thoracoscopy (LAT) or video-assisted thoracoscopic surgery (VATS)8. LAT (also known as medical thoracoscopy or pleuroscopy) is a single-port procedure, routinely performed by pulmonologists in the bronchoscopy suite, and it carries a diagnostic yield for malignancy of 93%9,10. LAT can be performed with either a semirigid or rigid endoscope, usually connected to a video-imaging source. A few publications discuss the benefits and disadvantages of these approaches8,11,12,13. Briefly, the semirigid thoracoscope resembles a bronchoscope, and biopsies are obtained using similar forceps. The rigid endoscope has a larger diameter, is cheaper, and allows for larger biopsies, although this does not readily translate into marked differences in diagnostic yield10,14,15. The safety of LAT is high, with a mortality rate of less than 0.5%, with this strongly related to pre-existing medical conditions3,4. Contraindications of LAT are fewer than for VATS and include complete obliteration of the pleural space due to adhesions, skin infection at the site of entry, respiratory failure, cardiac instability, and uncorrectable coagulopathy7,8,12. VATS is an in-hospital, 2 or 3-port procedure performed by thoracic surgeons, which must take place in a surgical theatre, requiring general anesthesia, intubation, single-lung ventilation, and post-operative admission. VATS has a diagnostic yield and complication rate similar to that of LAT but allows for more complex procedures to be undertaken10,16.
Guidelines recommend LAT as the first-choice thoracoscopy due to its high diagnostic yield, low risk, lower costs, and the possibility for daycase management, reserving VATS for selected cases4. The choice of a rigid or semirigid thoracoscope is usually determined by local preference. LAT is not a new procedure, as it was described as early as the mid-19th century, popularized by Jacobaeus in 1910, increasingly used to treat pleural tuberculosis until the 1950s, and "rediscovered" in the 1980s as an important tool to diagnose recurrent pleural effusions8,17.
Protocol
The following protocol will describe how to perform LAT in a clinical setting. The protocol is in accordance with the clinical practice and guidelines of the authors' hospitals (Odense, Næstved, Lleida, Bristol, and Preston). Written informed consent is obtained from the patient prior to the procedure. The main inclusion criteria for the procedure is recurrent pleural effusion of unknown cause, despite guideline-based work-up. Exclusion criteria include complete obliteration of the pleural space due to adhesions, skin infection at the site of entry, respiratory failure, cardiac instability, and uncorrectable coagulopathy.
1. Preparing for the procedure
- Examine the available image and perform thoracic ultrasound (US) to confirm the presence of pleural effusion and a safe distance (>2 cm) from the parietal to visceral pleura at the expected entry site, preferably in the middle of the intercostal spaces 4 or 5 in the midaxillary line12.
- Mark the expected entry site with a designated pen.
- In case of small-volume effusions, induce an artificial pneumothorax by inserting a blunt needle (e.g., 'Boutin needle') or a small-bore chest tube under direct ultrasound guidance. After fluid removal, air is introduced via the needle or drain, and ultrasound confirms the disappearance of lung sliding8,12.
- If in doubt, perform a lateral decubitus chest X-ray to confirm the presence and depth of the pneumothorax.
- Place the patient in the lateral decubitus position on a pillow or roll, facing the operator, with the effusion side up. The pillow/roll spreads the contralateral ribs, making it easier to insert the trocar and cannula, and minimizing the patient's discomfort11.
- Prepare for the sterile procedure according to local standards:
- For operator and any assistant: surgical hand scrub, sterile gown and gloves, mask, cap.
- For patient: cleanse the skin with an alcohol-based sterilizing solution, use sterile draping for coverage while leaving the expected entry site accessible.
- For tools: sterile table with a knife, trocar, cannula, endoscope, forceps, suction, and skin suture tools11(see Table of Materials).
- Commence sedation with a benzodiazepine (e.g., midazolam 1 mg/mL) IV in incremental doses, combined with an opiate (e.g., fentanyl 25 microgram/mL) IV in incremental doses to control pain and cough. In select centers, LAT may be performed with thoracic nerve blocks or with remifentanil sedation12.
- Ensure sufficient additional sedation is available during the procedure in case of pain, cough, or agitation.
2. Patient preparation
- Anesthetize all layers step by step with 20 mL of lidocaine 1% (or mepivacaine 2%) plus adrenaline: the skin, subcutaneous tissue, intercostal muscle, parietal pleura, intercostal nerve, and rib periosteum (the caudal rim of the upper and cranial rim of the lower rib)7,11,18.
- End the local anesthesia by checking the correctness of the entry site through the aspiration of pleural fluid (or air if an artificial pneumothorax is present), and note the distance from the skin to the pleural space11,12.
- Have additional doses of local anesthesia available during the procedure in case of pain or agitation.
- Perform skin incision and insertion of trocar and cannula.
- Make an approximately 10 mm incision in the skin and subcutaneous tissue parallel to the intercostal space to allow for the trocar and cannula to pass easily.
NOTE: A longer incision does not add maneuverability and needs to be closed with more stitches, which is associated with the risk of infections19. - Ensure with the tip of the scalpel that adequate parietal pleural anesthesia has been achieved before trocar insertion.
- Make an approximately 10 mm incision in the skin and subcutaneous tissue parallel to the intercostal space to allow for the trocar and cannula to pass easily.
- Perform a careful blunt dissection of the various layers between the skin and the pleura with either a narrow-tip straight or curved forceps until the pleural cavity is entered, and a tract of sufficient width to allow the trocar and cannula to pass has been created.
NOTE: The release of fluid or air (the latter often heard as a 'hiss') confirms the location. Optional: Blunt dissection might not be necessary for semirigid procedures given the smaller diameter of the working channel. - Consider inserting a closing ("mattress") suture (see Table of Materials), which is used later to seal the skin once the post-LAT chest drain is removed.
- Guided by the distance from the skin to the pleural space, insert the trocar and cannula with a cork-screw movement until a sudden release of resistance is felt. Then, remove the trocar and push the cannula forward to lie 1-3 cm within the pleural cavity11,12.
3. Inspection of the pleural cavity
- Ensure that the thoracoscope image is optimized for focus and color correction, and that the lens has been warmed or has demisting liquid applied.
- For semi-rigid LAT, insert the thoracoscope via the cannula and apply suction to remove the remaining fluid. For rigid LAT, insert a separate suction tube via the cannula to remove fluid before inserting the camera (Figure 1 and Figure 2).
- Inspect the pleural cavity systematically: the apex, mediastinal surface, diaphragm, and costal pleura back to the apex. As with other endoscopic techniques, experts use deliberate and minimal movements to achieve full inspection20,21.
NOTE: If using a rigid setup, initial inspection is best carried out using a 50-degree lens before switching to a 0-degree lens for intervention. - Full visibility of the pleural space may be limited by fibrous pleuro-pulmonary adhesions12. Gently swipe away fine adhesions with the thoracoscope, but avoid those with a vascular supply.
4. Tissue biopsy
- Sample suspicious lesions on the parietal pleura with six to ten biopsies using appropriate forceps with a 'grip and strip' approach (Figure 3A,B). Ensure that there is sufficient penetration of the parietal pleural membrane, as the invasion of subpleural fat can affect mesothelioma T classification8,12.
- Take care to avoid the visceral pleura due to the risk of lung injury and subsequent persistent air leak.
- Exercise caution to avoid the intercostal neurovascular bundle due to the risk of bleeding and neuralgia.
NOTE: Safety tip: Feeling the rib with the forceps and obtaining biopsies from the parietal pleura 'on' the rib is believed to reduce the risk of bleeding and damage to underlying vessels. - For semirigid thoracoscope procedures, introduce the forceps via the endoscope working channel. Switching to narrow-band imaging may improve the visualization of areas with abnormal vascular patterns13,22.
- For rigid thoracoscope procedures, introduce over-scope forceps.
- In the absence of clear malignant infiltration but with strong clinical suspicion of malignancy, perform random biopsies11.
- Send 1-2 biopsies for culture and tuberculosis analysis, especially in endemic areas, or in patients with risk factors23.
- If pleural infection is suspected during LAT, remove infectious debris (Figure 3C) and adhesions as far as possible12,24. Note that LAT is not an optimal procedure for the clearance of complex or widespread pleural infection9.
5. Termination and insertion of chest tube
- Remove the thoracoscope and cannula.
- Insert the post-LAT chest tube (typically 16-24 F, see Table of Materials) into the pleural space via the port tract.
- Close the incision with the "mattress" suture (as described in 2.6) or use another closing suture.
NOTE: Talc pleurodesis and/or placement of an indwelling pleural catheter (IPC) can be optionally performed in combination with the LAT procedure and are now standard practice in many centers8. The description of talc pleurodesis or IPC procedures is beyond the scope of the current review.
6. Follow-up procedures
- Remove the chest tube when air and fluid production ceases, typically within 1-2 h.
NOTE: While a pre-removal chest X-ray or thoracic ultrasound is not mandatory, it may be helpful for assessing lung re-expansion. - Close the incision with appropriate sutures or adhesive glue (see Table of Materials).
- Observe the patient for stability over 2-4 h.
NOTE: Although a post-removal chest X-ray or thoracic ultrasound is not mandatory, it can be helpful in assessing lung re-expansion. Patients who are well and stable after observation are discharged with a written action plan in case of serious symptoms25. - Admit frail patients to the ward for further observation and treatment.
Results
The described LAT technique using either the rigid or semirigid thoracoscopes (Figure 1 and Figure 2) enables the operator to perform conclusive biopsy sampling from the visceral pleural (Figure 3) with a diagnostic yield of 93% in a large systematic review on more than 5000 procedures (Table 1 and Supplementary File 1) (rigid: 93% [95% confidence interval 91%-95%]; semirigid: 93% [89%-97%])10. The described LAT procedure is safe with an overall complication rate of 4% (rigid: 4.2% [2.3%-6.6%]; semirigid: 4.0% [2.0%-6.4%]10 (Table 1). Fatalities are few and most often related to underlying disease10,26.
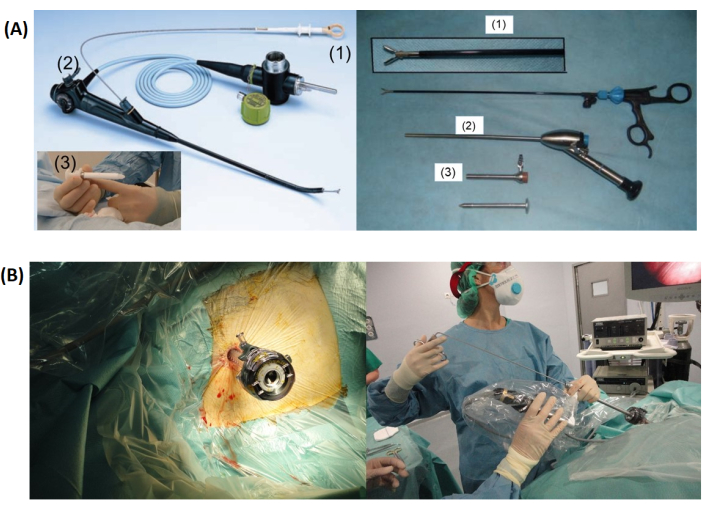
Figure 1: Thoracoscopy setup. (A) Shows a comparison between the semirigid (left) and rigid thoracoscopy setups, including the forceps (1), thoracoscope (2), and trocar with cannula (3). (B) Depicts the trocar inserted into the chest wall (left) before LAT (Local Anaesthetic Thoracoscopy) with a rigid thoracoscope. Please click here to view a larger version of this figure.

Figure 2: Semirigid thoracoscope placement. This drawing illustrates the placement of a semirigid thoracoscope in the pleural cavity above the collapsed lung. Please click here to view a larger version of this figure.

Figure 3: Thoracoscopic images. (A) Displays nodules on the parietal pleura, which are indicative of lung adenocarcinoma. (B) Shows a forceps biopsy being performed during thoracoscopy. (C) Depicts thin non-vascular septations observed in malignant pleural effusion. Please click here to view a larger version of this figure.
Table 1: Meta-analyses of LAT diagnostic sensitivity and complications. This table presents a summary of meta-analyses regarding the diagnostic sensitivity and complications associated with LAT. Adapted from Martinez-Zaya et al.10. Please click here to download this Table.
Supplementary File 1: The Local Anaesthetic Thoracoscopy assessment tool. This includes eight procedural steps of semirigid LAT, each scored from 1 to 5, with anchor descriptors provided for scores 1, 3, and 5. Adapted from Nayahangan et al.27. Please click here to download this File.
Discussion
This article provides a practical approach for performing LAT.
A single randomized trial compared rigid with semirigid LAT and found no differences concerning diagnostic yield or safety15. Evidence on how to optimize the critical steps is sparse. A group of one of the authors suggested eight critical steps for semirigid LAT and presented a competence checklist for learning LAT in a phantom based on their own experiences, narrative reviews8,10,12,15,26, trials, and systematic reviews27 (Supplementary File 1). The checklist clearly separated novices from experienced LAT operators and is, so far, the only publication suggesting a standardized assessment of critical LAT steps. However, the checklist is not exhaustive, as it focuses strictly on procedural aspects to safely obtain tissue samples and does not cover generic key aspects of patient selection, informed consent, or follow-up. Furthermore, the checklist needs validation in other centers, besides adaptation for clinical use, and inclusion of blunt dissection, which is a critical step when using the rigid thoracoscope7. Another critical step is the management of adverse events.
Major complications include bleeding, persistent air leak, pleural infection, and adverse events due to the medication used, while minor complications are transient pain, dyspnea, and the need for oxygen supplementation8,14. However, during the specialization period, training pulmonologists become competent in managing these adverse events, as these also occur after bronchoscopy and chest tube insertion, which are standard procedures in adult respiratory medicine28.
In 2018, the European Respiratory Society (ERS) revised the 2006 HERMES syllabus for adult respiratory medicine, and LAT was considered a key procedure for all respiratory physicians on a "knows how" level, though stating that the ERS "recognizes and supports the further development of medical thoracoscopy performed by pulmonologists"28. The principles of LAT are almost unchanged since the first case-series was presented more than 100 years ago, and traditional apprenticeship with training on patients is still the most common method to achieve LAT competences8. LAT training is not evidence-based despite courses on LAT with both types of thoracoscopes being offered by the ERS and the British Thoracic Society (BTS), involving basic physiology, and hands-on procedural training on pigs, since publications on content and learning efficacy are sparse29. Unlike, for example, bronchoscopy and endobronchial ultrasound, there are no commercially available LAT simulation devices27,30,31. As with all technical procedures, there is a learning curve before full LAT competence is achieved12, but very few studies report on LAT learning curves. A congress abstract presented that diagnostic yield increased over a period as long as 2 years after the ERS LAT course32, suggesting that false-negative LAT procedures potentially could be reduced markedly by access to simulation-based LAT training31.
The present protocol has some important limitations. Its focus has been on LAT as a diagnostic procedure and not on its therapeutic applications. Therefore, the protocol does not cover LAT talc pleurodesis for recurrent pleural effusion or LAT in the treatment of multiloculated or fibrinopurulent pleural infections8,12,24,33,34. Additionally, we did not address the health-economic aspects of LAT compared to VATS in a pleural service. In summary, LAT allows for day case management and for thoracoscopy in patients with contraindications for VATS or general anesthesia29,35.
In summary, LAT is an important tool in the diagnosis of recurrent or undiagnosed pleural effusions, with a diagnostic yield of 93%, and its establishment is based on meta-analyses and systematic reviews of more than 5,000 procedures10. Further research is encouraged, including the development of evidence-based LAT simulation training, to further optimize the LAT protocol.
Disclosures
The authors have no real or perceived disclosures to report.
Acknowledgements
No funding was received. JP and MM captured the photographs shown in Figure 1A. RB and MM captured the ones shown in Figure 3.
Materials
| Name | Company | Catalog Number | Comments |
| Chest tube | 16-24F | ||
| Forceps | narrow tip, straight or curved | ||
| Indwelling pleural catheter | Rocket Medical plc. | R55400-16-MT | or PleurX (from Becton Dickinson) or similar |
| Local anaesthetics | 20 mL of lidocaine 1% (or mepivacaine 2%) ± adrenaline | ||
| Non-absorbable suture | Eg. Dafilon 2/0 | ||
| Rigid thoracoscope | Karl Storz GmbH | Hopkins-II | with forceps 26072A and cannula+trocar 30120 NOL (or similar from eg. Richard Wolf GmbH) |
| Scalpel | triangular | ||
| Semirigid thoracoscope | Olympus | LTF-160 | with forceps FB-420K and cannula+trocar N1002130 |
References
- Bodtger, U., Hallifax, R. J., Maskell NA, L. C., Lee, Y. C. G., Rahman, N. M. . Pleural disease. Ers monograph 2020;87:1-12. 87, (2020).
- Froudarakis, M. E. Diagnostic work-up of pleural effusions. Respiration. 75 (1), 4-13 (2008).
- Holling, N., Patole, S., Medford, A. R. L., Maskell, N. A., Bibby, A. C. Is systemic anticancer therapy associated with higher rates of malignant pleural effusion control in people with pharmacologically sensitive tumors?: A retrospective analysis of prospectively collected data. Chest. 160 (5), 1915-1924 (2021).
- Roberts, M. E. R., et al. British thoracic society guideline for pleural disease. Thorax. 78, s1-s42 (2023).
- Mei, F., et al. Diagnostic yield and safety of image-guided pleural biopsy: A systematic review and meta-analysis. Respiration. 100 (1), 77-87 (2021).
- Bibi, R., et al. Endoscopic ultrasound-guided pleural biopsy in the hands of the pulmonologist. Respirol Case Rep. 8 (2), e00517 (2020).
- Asciak, R., et al. British thoracic society clinical statement on pleural procedures. Thorax. 78 (Suppl 3), s43-s68 (2023).
- Bhatnagar, R., Maskell, N. A. Medical pleuroscopy. Clin Chest Med. 34 (3), 487-500 (2013).
- Wei, Y., et al. Comparison between closed pleural biopsy and medical thoracoscopy for the diagnosis of undiagnosed exudative pleural effusions: A systematic review and meta-analysis. Transl Lung Cancer Res. 9 (3), 446-458 (2020).
- Martinez-Zayas, G., Molina, S., Ost, D. E. Sensitivity and complications of thoracentesis and thoracoscopy: A meta-analysis. Eur Respir Rev. 31 (166), (2022).
- Alraiyes, A. H., Dhillon, S. S. H., Kaseem, U., Kaphle, F., Kheir, Medical thoracoscopy: Technique and application. Pleura. 3 (1), 1-11 (2016).
- Loddenkemper, R., Lee, P., Noppen, M., Mathur, P. N. Medical thoracoscopy/pleuroscopy: Step by step. Breathe. 8 (2), 156-167 (2011).
- Li, D., Jackson, K., Panchal, R., Aujayeb, A. Local anaesthetic thoracoscopy for pleural effusion-a narrative review. Healthcare (Basel). 10 (10), (2022).
- Agarwal, R., Aggarwal, A. N., Gupta, D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: A meta-analysis. Chest. 144 (6), 1857-1867 (2013).
- Dhooria, S., Singh, N., Aggarwal, A. N., Gupta, D., Agarwal, R. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care. 59 (5), 756-764 (2014).
- Ali, M. S., Light, R. W., Maldonado, F. Pleuroscopy or video-assisted thoracoscopic surgery for exudative pleural effusion: A comparative overview. J Thorac Dis. 11 (7), 3207-3216 (2019).
- Loddenkemper, R., Mathur, P. N., Lee, P., Noppen, M. History and clinical use of thoracoscopy/pleuroscopy in respiratory medicine. Breathe. 8 (2), 144-155 (2011).
- Kostroglou, A., Kapetanakis, E. I., Rougeris, L., Froudarakis, M. E., Sidiropoulou, T. Review of the physiology and anesthetic considerations for pleuroscopy/medical thoracoscopy. Respiration. 101 (2), 195-209 (2022).
- Millbourn, D., Cengiz, Y., Israelsson, L. A. Effect of stitch length on wound complications after closure of midline incisions: A randomized controlled trial. Arch Surg. 144 (11), 1056-1059 (2009).
- Cold, K. M., et al. Automatic and objective assessment of motor skills performance in flexible bronchoscopy. Respiration. 100 (4), 347-355 (2021).
- Colella, S., et al. Assessment of competence in simulated flexible bronchoscopy using motion analysis. Respiration. 89 (2), 155-161 (2015).
- Zhang, X., Wang, F., Tong, Z. Application of narrow-band imaging thoracoscopy in diagnosis of pleural diseases. Postgrad Med. 132 (5), 406-411 (2020).
- Zhao, T., et al. Medical thoracoscopy for tuberculous pleurisy: A retrospective analysis of 575 cases. Ann Thorac Med. 14 (2), 134-140 (2019).
- Sumalani, K. K., Rizvi, N. A., Asghar, A. Role of medical thoracoscopy in the management of multiloculated empyema. BMC Pulm Med. 18 (1), 179 (2018).
- Kern, R. M., Depew, Z. S., Maldonado, F. Outpatient thoracoscopy: Safety and practical considerations. Curr Opin Pulm Med. 21 (4), 357-362 (2015).
- Rahman, N. M., et al. Local anaesthetic thoracoscopy: British thoracic society pleural disease guideline 2010. Thorax. 65 Suppl 2, ii54-ii60 (2010).
- Nayahangan, L. J., et al. Assessment of competence in local anaesthetic thoracoscopy: Development and validity investigation of a new assessment tool. J Thorac Dis. 13 (7), 3998-4007 (2021).
- Tabin, N., Mitchell, S., O'connell, E., Stolz, D., Rohde, G. Update of the ers international adult respiratory medicine syllabus for postgraduate training. Breathe (Sheff). 14 (1), 19-28 (2018).
- Froudarakis, M. E., Anevlavis, S., Marquette, C. H., Astoul, P. Medical thoracoscopy implementation after a european respiratory society course held from 2003 to 2016: A survey. Respiration. 100 (6), 523-529 (2021).
- Konge, L., et al. Simulator training for endobronchial ultrasound: A randomised controlled trial. Eur Respir J. 46 (4), 1140-1149 (2015).
- Naur, T. M. H., Nilsson, P. M., Pietersen, P. I., Clementsen, P. F., Konge, L. Simulation-based training in flexible bronchoscopy and endobronchial ultrasound-guided transbronchial needle aspiration (ebus-tbna): A systematic review. Respiration. 93 (5), 355-362 (2017).
- Khanna, A., et al. Medical thoracoscopy: Learning curve of a new service. European Respiratory Journal. 40, P3362 (2012).
- Murthy, V., Bessich, J. L. Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease. J Thorac Dis. 9 (Suppl 10), S1011-S1021 (2017).
- Mondoni, M., et al. Medical thoracoscopy treatment for pleural infections: A systematic review and meta-analysis. BMC Pulm Med. 21 (1), 127 (2021).
- Depew, Z. S., et al. Feasibility and safety of outpatient medical thoracoscopy at a large tertiary medical center: A collaborative medical-surgical initiative. Chest. 146 (2), 398-405 (2014).
Erratum
Formal Correction: Erratum: Local Anesthetic Thoracoscopy for Undiagnosed Pleural Effusion
Posted by JoVE Editors on 1/05/2024. Citeable Link.
An erratum was issued for: Local Anesthetic Thoracoscopy for Undiagnosed Pleural Effusion. The Authors section was updated from:
Uffe Bodtger1,2
José M. Porcel3
Rahul Bhatnagar4,5
Mohammed Munavvar6,7
Casper Jensen1
Paul Frost Clementsen1,8
Daniel Bech Rasmussen1,2
1Respiratory Research Unit PLUZ, Department of Respiratory Medicine, Zealand University Hospital
2Institute of Regional Health Research, University of Southern Denmark
3Pleural Medicine Unit, Department of Internal Medicine, Hospital Universitari Arnau de Vilanova, IRBLleida
4Respiratory Department, Southmead Hospital, North Bristol NHS Trust
5Academic Respiratory Unit, University of Bristol
6Lancashire Teaching Hospitals
7University of Central Lancashire
8Centre for HR and Education, Copenhagen Academy for Medical Education and Simulation
to:
Uffe Bodtger1,2
José M. Porcel3
Rahul Bhatnagar4,5
Nick Maskell4,5
Mohammed Munavvar6,7
Casper Jensen1
Paul Frost Clementsen1,8
Daniel Bech Rasmussen1,2
1Respiratory Research Unit PLUZ, Department of Respiratory Medicine, Zealand University Hospital
2Institute of Regional Health Research, University of Southern Denmark
3Pleural Medicine Unit, Department of Internal Medicine, Hospital Universitari Arnau de Vilanova, IRBLleida
4Respiratory Department, Southmead Hospital, North Bristol NHS Trust
5Academic Respiratory Unit, University of Bristol
6Lancashire Teaching Hospitals
7University of Central Lancashire
8Centre for HR and Education, Copenhagen Academy for Medical Education and Simulation
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved