Method Article
Multiple Intravenous Bolus Dosing and Invasive Hemodynamic Assessment in a Hypoxia-Induced Mouse Pulmonary Artery Hypertension Model
In This Article
Summary
This protocol provides a step-by-step procedure for executing multiple intravenous bolus dose administration and invasive hemodynamic monitoring in mice. Investigators can use this protocol for future therapeutic compound screening for pulmonary artery hypertension.
Abstract
Pulmonary arterial hypertension (PAH) is a progressive life-threatening disease, primarily affecting small pulmonary arterioles of the lung. Currently, there is no cure for PAH. It is important to discover new compounds that can be used to treat PAH. The mouse hypoxia-induced PAH model is a widely used model for PAH research. This model recapitulates human clinical manifestations of PAH Group 3 disease and is an important research tool to evaluate the effectiveness of new experimental therapies for PAH. Research using this model often requires the administration of compounds in mice. For a compound that needs to be given directly into the bloodstream, optimizing intravenous (IV) administration is a key part of the experimental procedures. Ideally, the IV injection system should permit multiple injections over a set time course. Although the mouse hypoxia-induced PAH model is very popular in many laboratories, it is technically challenging to perform multiple IV bolus dosing and invasive hemodynamic assessment in this model. In this protocol, we present step-by-step instructions on how to carry out multiple IV bolus dosing via mouse jugular vein and perform arterial and right ventricle catheterization for hemodynamic assessment in mouse hypoxia-induced PAH model.
Introduction
Pulmonary artery hypertension (PAH) is defined by a mean pulmonary artery systolic pressure greater than 20 mmHg at rest1,2. It is a progressive and fatal disease characterized by a sustained elevation in pulmonary arterial pressure, leading to right ventricle overload and ultimately death due to right ventricular failure1. Currently, there is no cure for PAH.
The use of animal models of pulmonary hypertension is important for testing the effectiveness of experimental PAH therapies. Among those models, the mouse hypoxia-induced PAH model has provided key insights into human PAH group 3 disease development3,4. Research using this model often requires the administration of compounds in mice to evaluate the novel compound's effectiveness and safety. Therefore, investigators need a detailed experimental procedure for compound dosing and hemodynamic measurements to ensure injection consistency and blood pressure measurement reproducibility from the beginning to the end.
Methods for intravenous (IV) injection and blood pressure measuring have been reported in the literature5,6. However, the methodology lacks visual illustration and detailed description. Here we illustrate the key steps for a successful IV bolus injection and accurate measurement and recording of systemic and right ventricle blood pressure. The procedures presented here are an important resource for investigators interested in the IV route of compound administration platform to develop a treatment for PAH.
Protocol
All animal procedures were performed under protocols approved by Yale University Institutional Animal Care and Use Committees.
1. Preparation of animals, tools, blood pressure measuring equipment, and hypoxia chamber
- Animal acclimation.
NOTE: Experimental animals used for this study were male, 8-week-old C57BL/6 mice weighing 25-27 g. Several factors should be considered when estimating the number of animals required for the experiment, including surgery-associated mortality, unexpected surgical complications, and sudden unexpected death. Use at least 10 mice per group to reach statistical power and avoid underpowered studies.- Upon reception, house the animals in ventilated rodent cages (groups of five animals per cage) provided with appropriate bedding, rodent chow, and water. Let the animals acclimate to the new environment (12 h light-dark cycle at 18-20 °C) for at least 3 days.
- Randomly assign them to the following groups: Normoxia (Group 1), hypoxia (Group 2), and hypoxia + 7C1/let-7 miRNA (Group 3).
- Surgical tools and blood pressure measuring equipment preparation.
- Sterilize all surgical tools by autoclaving (Figure 1A).
- Prepare a makeshift injection platform with a homemade anesthesia nose cone (Figure 1B), suture packs (Figure 1C), and equipment for the PAH procedure (Figure 1D-F).
- Experimental setting for pulmonary artery hypertension (PAH) induction.
- Set the N2 tank, the oxygen sensor, and the semisealable hypoxia chamber (Figure 2A).
- Establish a set point of 10% O2 in the oxygen sensor and let the system reach the steady-state (Figure 2B, C).
- Keep hypoxia (Group 2) and hypoxia + 7C1/let-7 miRNA (Group 3) animals in hypoxia (10% O2) for 3 weeks. After 3 weeks of hypoxia, place the animals under normoxic conditions for 1 week (Figure 2D). The normoxic group (Group 1) stays in normoxia for 4 weeks.
NOTE: (1) 3 weeks of hypoxia followed by 1 week of normoxia is a well-established method for developing PAH and right ventricle heart failure7. The oxygen sensor detects O2 concentration inside the semisealable hypoxia chamber and corrects it by infusing N2 gas through the gas infusion tube. - Inspect the animals daily for the entire duration of the experiment (3 weeks). Consult a veterinarian if the animals exhibit signs of distress such as dramatic weight loss and difficulty breathing. If euthanasia is necessary for the animals in severe distress, exclude the animal from the study.
NOTE: Hypoxia exposure causes mouse body weight loss. A loss of 10% body weight is typically used as a reliable indication of PAH development. - Avoid extensive opening of the hypoxia chamber. For cage cleaning, replenishing food, changing water bottles, and compound administration, open the chambers for not more than 1 hour per week.
2. Intravenous bolus injection via the jugular vein
- Mouse preparation and anesthesia.
- Take out hypoxia (Group 2) and hypoxia + 7C1/let-7 miRNA (Group 3) mouse cages from the hypoxia chamber and gently remove the animal from the cage.
NOTE: The dosage regimen for 7C1/let-7 miRNA (1.5 mg/kg IV/dose) is twice per week for 4 weeks of treatment. It is recommended that investigators take both hypoxia and hypoxia + compound treatment cages out of the hypoxia chamber during IV injection to make sure all the animals receive the same magnitude of hypoxia exposure per time interval. - Weigh the mouse using a precision scale and record its weight (Figure 3A).
- Place the mouse into an anesthesia induction chamber connected to the anesthetic vaporizer and close it (Figure 3B). Provide thermal support and apply eye lubricant to both eyes to prevent drying while anesthetizing. Expose the mouse to 3% isoflurane until unconscious (Figure 3C-D).
- Remove the mouse from the chamber and shave the fur from the jaw cranially to the middle of the sternum caudally. Laterally, shave the fur from the jaw angles, through the sides of the neck, and toward the shoulders (Figure 3E).
- Place the isoflurane-anesthetized mouse in a supine position (belly side face-up) on an injection platform underneath a dissection microscope. Maintain the anesthesia via a nose cone with 1.5% isoflurane and gently restrain the four legs with adhesive tape to immobilize the body (Figure 3F).
- Apply a noxious stimulus (i.e., toe pinch) with straight forceps to ensure an adequate level of anesthesia. The anesthetized mouse should not respond to the stimulation prior to and throughout the surgical procedure.
- Take out hypoxia (Group 2) and hypoxia + 7C1/let-7 miRNA (Group 3) mouse cages from the hypoxia chamber and gently remove the animal from the cage.
- Injection agent preparation.
- Prepare a single-dose injection compound at a dose of 1.5 mg/kg under sterile conditions.
NOTE: Warm the injection compound to room temperature (RT) since injection of cold substances can cause discomfort and a drop in mouse body temperature (if this does not damage the compound). The optimal dose and duration of compound 7C1/let-7 miRNA used in this study are based on previous publications8,9. - Load the sterile single-use syringe with the volume to be injected. Hold the syringe upright and advance the plunger to expel the air from the syringe. Do not reuse the syringe.
- Limit the injection volume to 200 μL in a 25 g mouse to reduce the incidence of hemodilution and abnormal cardiac effects on the animals. If a larger volume is required, divide the injection compound into two injections with an interval of 10 min.
- Prepare a single-dose injection compound at a dose of 1.5 mg/kg under sterile conditions.
- Prepare mouse for IV injection.
- Gently scrub the surgical area three times with three alternating rounds of povidone-iodine solution and 70% ethanol. Administer buprenorphine (0.05 mg/kg, SQ) 30 min prior to the surgical procedure.
- Make a 0.5 cm longitudinal cut slightly to the right of the midline of the neck using a scalpel blade (Figure 3G).
- Use forceps to separate the muscle and the fat tissues to locate the right external jugular vein (Figure 3H).
NOTE: Rotate injection sites each time to avoid scar formation. - Use a high-power objective lens to allow easy visualization of the injection area (Figure 3I).
- IV injection
- Insert a 28 G sterile needle into the jugular vein with the needle bevel facing up (Figure 3J, K).
NOTE: Tail vein injection is an alternative to jugular vein injection. However, this technique is difficult to carry out repeat-dosing due to the variability in vein depth, mice tail skin color, and skin hardness. - Slowly press the syringe plunger to inject the compound into the vein. Allow the needle to remain within the vein for an additional 10 s to prevent backflow of the injectant (Figure 3L).
NOTE: The bluish dye allows for easy visualization of the injection. Do not include the dye when injecting test materials. An inaccurate injection will result in the accumulation of bluish dye around the IV injection site. - Remove the needle and use a cotton swab to apply pressure to the injection site to prevent bleeding (Figure 3M).
- Suture the skin with 5-0 suture (Figure 3N). After surgery, move the animal to a warm, clean, dry area and provide Meloxicam (1 mg/kg, SQ, q24h). Place the animal in a clean recovery cage with no bedding but the bottom covered in a paper towel.
NOTE: The mouse should be awake from anesthesia and regain consciousness within 5 min once back to the recovery cage. Monitor the mouse for signs of distress. - Return the animals to their home cage and put the mouse cage back in the hypoxia chamber.
NOTE: The entire procedure, from anesthetizing a mouse to finishing jugular vein injection, takes about 10-15 min by a single experimenter. To shorten the normoxia exposure in mice, it is recommended that at least two investigators collaborate to accomplish a jugular vein injection procedure.
- Insert a 28 G sterile needle into the jugular vein with the needle bevel facing up (Figure 3J, K).
3. Blood pressure measurement
- Prepare instruments for blood pressure measuring.
- Soak the tip of 1.0 F catheter in 37 °C pre-heated PBS at least 30 min before hemodynamic measurement (Figure 4A).
- Measure the distance from the catheter insertion site to the desired catheter tip location. For example, the distance between the mouse ascending aorta to the mid of the neck is approximately 1-1.2 cm. The distance between the right ventricle of the heart to the mid of the neck is approximately 2.3-2.8 cm.
- Mark two catheter distance markings to provide a visual indication of insertion depth (Figure 4B).
- Attach the catheter to the pressure transducer, connect the pressure transducer to input channel 1 on the data acquisition device, turn on the pressure-volume control unit, and initiate data acquisition blood pressure analysis software. Create a new blood pressure analysis document and set channel 1 for pressure.
- Perform a pressure calibration according to the manufacturer's protocol. Allow the whole setup to stabilize for at least 5 min (Figure 4C).
- In blood pressure analysis software, select Units Conversion from the Channel 1 dropdown menu (Figure 4D, red arrow).
- Set the default Units Conversion Values (Figure 4E).
NOTE: Blood pressure is represented as millimeters of mercury (mmHg). The standardized pressure output from Pressure Control Unit is 1 V per 100 mmHg. 25 mmHg corresponds to 0.25 V output, and 100 mm Hg corresponds to 1 V output.
- Prepare mouse for blood pressure measurement procedure.
- Anesthetize the mouse with 3% isoflurane inhalation through a nose cone.
- Apply the veterinary ointment directly onto the ocular surface of the mouse eyes to prevent dryness, as the mouse cannot close its eyes under anesthesia. Shave the fur from the mouse's neck while under anesthesia.
- Scrub the shaved region with three alternating rounds of povidone-iodine solution and 70% ethanol swab. Place the anesthetized mouse in a supine position on an injection platform underneath a dissection microscope. Place the mouse's nose into the nose cone to maintain anesthesia (1.5% isoflurane) throughout the surgical procedure.
- Test the anesthetized mouse's motor response to the noxious stimulus. The anesthetized mouse should not respond to a noxious stimulus before and during the surgery.
NOTE: Inhalable (isoflurane) and injectable (ketamine/xylazine) anesthetics can decrease blood pressure. In general, isoflurane inhalation anesthesia has a slight effect on lowering the blood pressure than ketamine/xylazine. Therefore, isoflurane is the preferred inhalant anesthetic over ketamine/xylazine. Achieving the appropriate depth of anesthesia is critical for accurate and reproducible hemodynamic measurements. The investigator needs to keep the depth of anesthesia constant for each mouse.
- Ascending aorta catheterization
- Apply a noxious stimulus (i.e., toe pinch) with straight forceps to ensure an adequate level of anesthesia. Make a midline incision of the skin from the mandible to the sternum (Figure 5A).
- Separate the salivary glands and expose the trachea (Figure 5B).
- Use forceps to clear the soft tissue along the vessels to expose the right carotid artery and right external jugular vein (Figure 5C).
- Put 0.5 mL of PBS in the cavity to slow the development of vasospasm while manipulating the carotid artery.
- Carefully isolate a 5 mm section of the right carotid artery. Place a piece of sterile white paper underneath the vessel as a background to make the artery more visible (Figure 5D).
NOTE: Carefully separate the vagus nerve (white) from the artery and ensure not to cut or damage the nerve or the artery. - Using an 8-0 suture tie a permanent knot (#1) to close off the cranial end of the vessel (Figure 5E).
- Tie a first loose knot (#2) to temporarily occlude blood flow from the aorta. Then, tie a second loose knot (#3) between the first two sutures (Figure 5F). The second loose knot (#3) will be used to quickly secure the catheter after placement.
- Using a 25 G needle, make a small hole, large enough to pass the catheter, in line with the vessel between #3 and #1 ligatures (Figure 5G).
NOTE: Carotid arteries carry oxygenated blood from the heart and have very high pressure. If the carotid artery is cut, that pressure will cause the blood to spurt out (Figure 5H). - Hold the catheter 1.5 inches from the tip and gently insert the tip of the catheter through the hole of the artery (X-mark). Tighten the middle suture node (#3) around the catheter and vessel that still allows passage of the catheter (Figure 5I-J).
NOTE: This step requires practice. The potential complications with this step include bleeding at the catheter insertion site and vasospasm. When bleeding occurs, blood loss from the bleeding artery reduces blood volume, leading to a severe drop in systemic blood pressure. Due to the severity, the animal has reached a humane endpoint and must be euthanized. For mechanically induced vasospasm, it usually occurs during catheter insertion caused by a persistent contraction of the blood vessels. This makes the blood vessel opening smaller and prevents catheter advancement to the carotid artery. Do not use excessive force against resistance to advance the catheter. When moderate or severe vasospasm resistance is encountered, try again in a little while or use a smaller catheter (e.g., 1.0 F). Experienced microsurgeons can achieve 100% success rates for ascending aorta catheterization. - After the catheter passes the first loose knot (#2) with the sensor tip, fasten the second loose knot (#3) more tightly to secure the catheter and gently release the first loose knot (#2) (Figure 5K, L).
- Continue to insert the catheter toward the ascending aorta according to the mark on the catheter (Figure 4B) until pressure analysis shows an arterial blood pressure profile (Figure 5M). Record systemic blood pressure (SBP) data using the data acquisition system and software.
- Loosen the middle suture node (#3) to allow the catheter to be pulled out (Figure 5N).
- Tie the middle suture node (#3) around the vessel before pulling the catheter out of the carotid artery (Figure 5O-P).
- Place the catheter in PBS.
- Right heart catheterization.
- Carefully isolate the right external jugular vein from the surrounding connective tissue and ligate all the small branches with 8-0 suture (blue arrowheads) (Figure 6A).
NOTE: For right heart catheterization, the heart is commonly accessed via the right jugular vein. - Using an 8-0 suture, tie a permanent knot (#1) to close off the cranial end of the vessel (Figure 6B). Then, tie a loose knot (#2) on the caudal end of the vessel (Figure 6C).
- Use a 25 G needle to make a small hole proximal to the permanent knot (#1) (Figure 6D).
NOTE: Jugular veins carry deoxygenated blood to the heart and have low pressure. If the jugular vein is cut, the blood will not spurt out (Figure 6D, E). - Hold the catheter and insert the catheter into the cut of the vein (X-mark) (Figure 6E) and tighten the caudal knot (#2) around the catheter and the vessel (Figure 6F).
- Slowly and gently push the catheter into the right heart. Monitor the catheter tip depth based on the catheter mark (Figure 4B).
NOTE: Assessment of the right ventricular systolic pressure (RVSP) in closed-chest mice is a challenge because of the complex RV anatomy and structure. This step requires a high level of expertise and lots of practice. In the hands of an experienced microsurgeon, the successful rate for right ventricle catheterization can approach 90%. - Assess the catheter tip position according to the pressure wave tracing in the software. When the tip of the catheter is in the right ventricle, the monitor will show a typical RVSP tracing (Figure 6G, H).
NOTE: When the shape of pulmonary pressure curves looks atypical (e.g., spiky curves), this implies incorrect positioning of the catheter. Adjust the catheter position by gently pulling the catheter a little bit back, and then slowly advancing the catheter to a more central position within the right ventricle. To avoid the generation of artifacts in research data, the investigator should avoid prolonged (not more than 1 min) or repeated attempts (not more than two attempts) at right ventricle catheterization. - Keep the catheter immobile and collect the data for 5 min.
- After the recording is complete, carefully pull the catheter out and tie the caudal knot (#2) around the vessel (Figure 6I). Place the catheter back in PBS solution.
NOTE: After completion of the experiment, clean the catheter with 1% digestive enzyme solution according to the manufacturer's instructions. In addition to assessing hemodynamic status, investigators may harvest the hearts and the lungs for PAH histopathological examination. To ensure the effectiveness of multiple IV bolus dosing, investigators can isolate lung endothelial cells and measure let-7 miRNA levels.
- Carefully isolate the right external jugular vein from the surrounding connective tissue and ligate all the small branches with 8-0 suture (blue arrowheads) (Figure 6A).
4. Blood pressure data analysis
- Examine the blood pressure recording.
- Open the blood pressure analysis software data file (PAH JOVE.adicht).
- In channel 1, select an area representing the pressure signal and place the Waveform Cursor on the Peak (X-mark) to measure pressure amplitude (Figure 7A).
- Determine the maximum amplitude of the pressure wave. This represents the systolic pressure (Figure 7A, red arrow).
- Extract the region of interest (Figure 7B gray area) from the image by pressing the Shift + Command + 3 (for Mac) or Windows + Shift + S (for Windows PC) and paste it into a graphics file.
- Statistical analysis of blood pressure data.
- Enter the individual mouse blood pressure data in statistical analysis software.
- Perform an unpaired Student's t-test for statistical analysis of two study groups (normoxia vs. hypoxia; hypoxia vs. hypoxia + 7C1/let-7 miRNA). Consider the differences in mean values as significant as p < 0.05.
Results
Anesthesia often reduces blood pressure. Therefore, a minimum dose of anesthesia was used to abolish the movements in response to a noxious stimulus. Successful right ventricular chamber access can be visualized as the hemodynamic waveform changes in different regions of venous systems (Figure 8).
In this study, mice were randomly assigned to the normoxic (21% O2) group (n = 10), hypoxia (10% O2) group (n = 10), or hypoxia + 7C1/let-7 treatment group (n = 10). To examine the effect of let-7 miRNA in the suppression of hypoxia-induced PAH development, formulated 7C1/let-7 miRNA was administered to the C57BL/6 mice intravenously at a dose of 1.5 mg/kg twice per week for 4 weeks (Figure 2D).
4 weeks after exposure to hypoxia or normoxia, SBP and RVSP were measured in a closed-chest mouse. Figure 9A shows the representative blood pressure curve from the normoxic, hypoxia, or hypoxia + 7C1/let-7 miRNA treatment groups. Compared to those in the normoxia control group, RVSP was significantly increased in the hypoxia group. In addition, compared with the hypoxia group, treatment with 7C1/let-7 miRNA compound in mice resulted in significantly decreased RVSP (Figure 9B). SBP did not change in any groups, which is consistent with the previous reports7. 7C1/let-7 miRNA targets endothelial cells and decreases TGFβ signaling cascade8. The data show that 7C1/let-7 miRNA 1.5 mg/kg is highly effective in lowering blood pressure in the right ventricle, demonstrating the effectiveness of the multiple IV bolus dosing.

Figure 1: Surgical instruments and blood pressure measurement equipment required for pulmonary artery hypertension procedures. (A) Surgical tools used for PAH procedure. (B) A makeshift injection platform made from an absorbent pad wrapped around a Styrofoam rack from a 50 mL conical package. Attaching a 10 cm length anesthesia tube to the injection platform as nose cone with type. (C) Suture packs. 5-0 suture for incision closure and 8-0 suture for ligation. (D-F) Blood pressure measure equipment used for the PAH procedure. Please click here to view a larger version of this figure.

Figure 2: Experimental setting for PAH induction. (A) Photograph of setting up BioSpherix hypoxic system. Different parts of the induction system are indicated. (B-C) Oxygen sensor monitoring the hypoxia chamber O2 concentration. (D) Experimental timeline for 7C1/let-7 miRNA compound treatment and oxygen level exposure for all animal groups during PAH induction. Please click here to view a larger version of this figure.
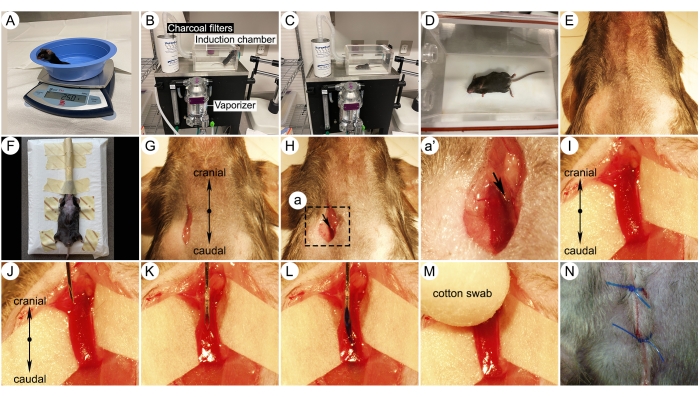
Figure 3: Photographs of key surgical steps for jugular vein injection. (A) Mouse on a weight scale. (B) Rodent anesthesia induction system setup. Different parts of the induction system are indicated. (C-D) Pictures of an isoflurane-anesthetized mouse in an induction chamber. (E) Fur removed surgical zone. (F) A mouse placed on an injection platform and breathed 1.5% isoflurane through a nose cone from a vaporizer. (G) Skin incision for jugular vein approach. (H) Surgical dissection of the right external jugular vein. (I) Higher magnification imaging showing the isolated right jugular vein. Note a white paper underneath the vessel, making the vein more visible. (J-K) Right jugular vein needle insertion with the bevel up. (L) Injection of a compound with bluish dye into the jugular vein. (M) Applying pressure to the injection site using a cotton swab after withdrawing the needle. (N) Suturing the wound with a 5-0 suture. Please click here to view a larger version of this figure.
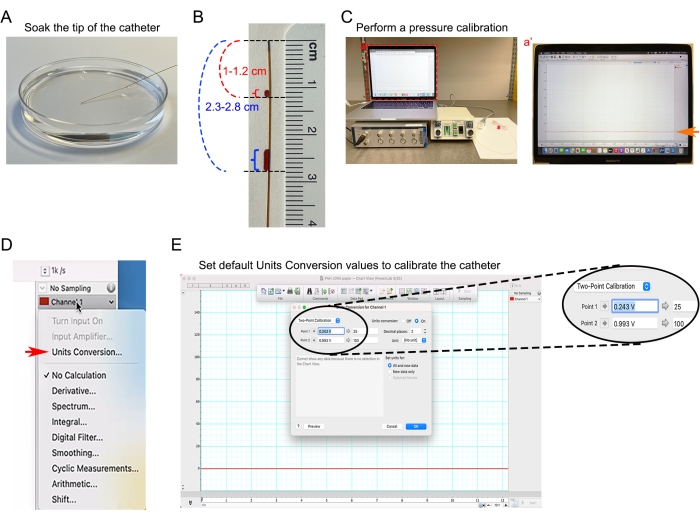
Figure 4: Catheter calibration. (A) Soaking 1.0 F catheter tip in 37 °C pre-heated PBS. (B) Distance markings on the catheter to help estimate the depth of insertion of the catheter in the ascending aorta and right ventricle. (C) Blood pressure measuring equipment undergoing a zero baseline calibration. (Ca') Screenshot of blood pressure analysis software-based catheter baseline analysis. (D) Under Channel 1 dropdown menu, selecting the Units Conversion dialog in the blood pressure analysis software. (E) Setting the default Units Conversion values to convert the input voltage signal to mmHg unit. Please click here to view a larger version of this figure.
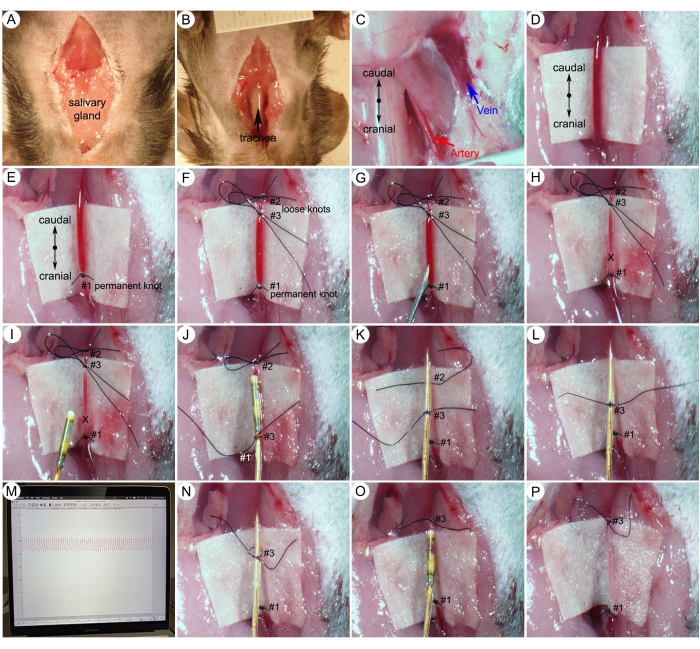
Figure 5: Surgical procedures for systemic blood pressure (SBP) measurements. (A) A midline incision from the mandible to the sternum on the skin of the neck. (B) Separation of the salivary gland to expose the trachea. (C) Exposed right carotid artery and right external jugular vein after tissue dissection. (D) An isolated 5 mm section of the carotid artery. (E,F) Suture permanent knot at the cranial extremity and two loose knots at the caudal extremity. (G,H) Making a small hole (X-mark) on the carotid artery just caudal to the permanent knot (#1). (I) Insertion of the catheter into the carotid artery. (J) Securing the catheter with a middle suture knot (#3). (K,L) Gently releasing the first loose knot (#2). (M) Representative arterial pressure waves. (N) Loosening the middle suture node (#3). (O,P) Tightening the middle suture node (#3) around the vessel. Please click here to view a larger version of this figure.
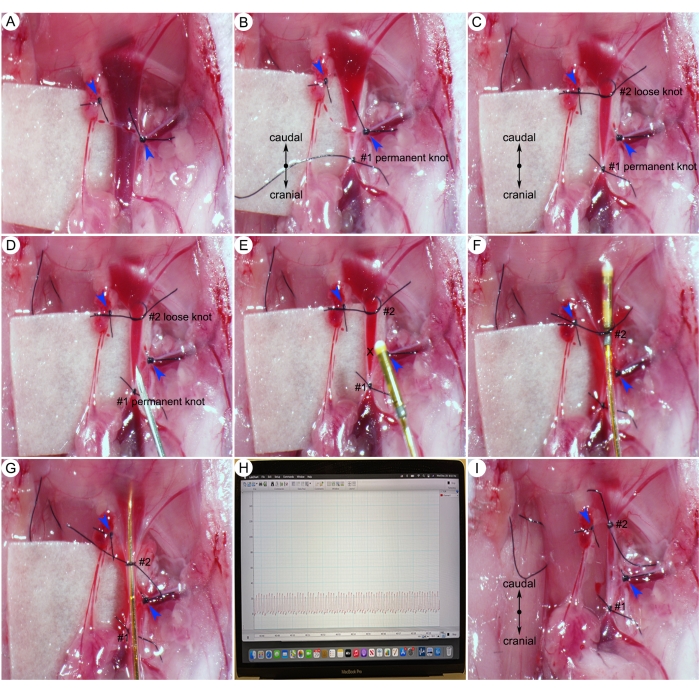
Figure 6: Surgical procedures for right ventricle systolic pressure (RVSP) measurements. (A) Ligation of the small branches of the right jugular vein (blue arrowheads). (B) A permanent knot (#1) on the cranial end of the jugular vein. (C) A loose knot (#2) on the caudal end of the jugular vein. (D) Making a small hole on the right jugular vein caudal to the permanent knot (#1). (E) Insertion of a catheter in the jugular vein through a small hole (X-mark). (F) Tightening the caudal knot (#2) around the catheter and the vessel. (G) Pushing the catheter into the right ventricle of the heart. (H) Representative RVSP. (I) Tightening the caudal node (#2) around the vessel. Please click here to view a larger version of this figure.

Figure 7: Blood pressure analysis software data analysis after recording. (A) Using Waveform Cursor to measure pressure amplitude from the raw blood pressure analysis software data in Channel 1. (B) Extracting the region of interest from the raw blood pressure analysis software data image. Please click here to view a larger version of this figure.
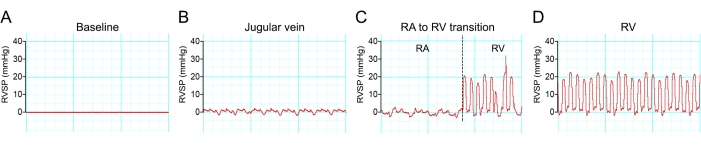
Figure 8: Hemodynamic waveform transition during right ventricle catheterization. (A-D) Representative traces of pressure changes during mouse right ventricle catheterization of a C57BL/6 mouse. Please click here to view a larger version of this figure.

Figure 9: Blood pressure analysis representation figures and data analysis. (A) Representative SBP and RVSP curves in normoxia, hypoxia, and hypoxia + 7C1/let-7 miRNA treated mice. (B) Summary plots of SBP and RVSP in normoxia, hypoxia, and hypoxia + 7C1/let-7 miRNA treated mice (NS: not significant; **p < 0.01; ***p < 0.001; unpaired two-tailed Student's t-test). N = 10 per group. Please click here to view a larger version of this figure.
Discussion
Several pulmonary hypertension animal models have been established to mimic the elevated pulmonary vascular resistance events in human subjects. Among them, the mouse hypoxia-induced PAH model has been widely used for evaluating the effectiveness of new experimental therapies for PAH. Research using this model often requires the administration of compounds to the mice. In comparison with other published intravenous (IV) injection and invasive hemodynamic assessment protocols, this method provides both visual illustration and detailed description.
There are three critical steps for the successful execution of the procedure and for obtaining accurate and reproducible blood pressure measurements. First, ensure the syringe needle is correctly positioned in the jugular vein. Incorrect jugular vein injection may result in subcutaneous injection. Second, ensure the sufficient depth of anesthesia. Consistent anesthetic depth in each mouse is important for the generation of data that are comparable between groups. Too deep anesthesia can cause a significant decrease in blood pressure levels. In addition to isoflurane inhalation anesthesia, intraperitoneal injection of ketamine/xylazine is another widely used anesthetic method for mouse surgery. Both methods have advantages and disadvantages. The isoflurane inhalation anesthesia has several advantages over injectable ketamine/xylazine, including quick onset, no controlled drugs, rapid recovery, and is much easier to control the depth of anesthesia. The disadvantages are the cost of the equipment, unpleasant smell, and human exposure to waste anesthetic gases. Third, ensure the catheter is inside the right ventricle of the heart. Prolonged or multiple failed attempts at right ventricle catheterization can cause false blood pressure readings.
IV injection in mice is predominantly administered via the lateral tail veins. While this route is easy to reach with needles, this technique is sometimes difficult to carry out multiple IV bolus dosing. The two major challenges in performing this technique are the variability in vein depth and the difficulty of needle visualization due to mice tail skin color and skin hardness. More importantly, there is no way to confirm whether the entire contents of the injection have successfully entered the bloodstream and not the surrounding tissues. The jugular vein is a preferred access site because (1) it is clinically relevant, (2) it provides visual confirmation of the delivery of injectate to the vein, (3) it allows for multiple injections of a group of animals during the course of the experiment, and (4) this injection technique is safe, and the procedure does not cause any side effects.
There are three ways to record blood pressure in mice: (1) Non-invasive tail-cuff plethysmography10. The systems enable repeated measurements over the course of a longitudinal study. (2) Radio telemetry11. The systems enable the monitoring of real-time blood pressures in awake and freely moving laboratory animals. (3) Invasive intra-arterial catheters12. The systems enable acute SBP and RVSP measurements. In this protocol, we chose a pressure catheter for high-fidelity systemic and right ventricle pressure measurements. However, this method has some limitations. First, the pressure catheter and blood pressure measuring equipment are expensive (Figure 1E-F). Second, it requires anesthetizing the animals, this causes decrease in blood pressure. Third, right heart catheterization is a terminal procedure that does not allow for serial measurements. Fourth, the procedure is not easy to learn even by a well-trained microsurgeon.
Once the blood pressure is recorded, the investigator can isolate the hearts and the lungs from the animals for histological PAH characterization. For example, right ventricular wall thickness measurements for right ventricular hypertrophy and muscularized pulmonary distal vessel analysis for muscular pulmonary artery remodeling. The data show that 7C1/let-7 miRNA is highly effective in lowering pulmonary blood pressure demonstrating the effectiveness of our multiple IV bolus dosing. Additionally, investigators can isolate lung endothelial cells from the freshly isolated whole lung to evaluate the effectiveness of injected materials.
In summary, this protocol provides a step-by-step procedure for executing multiple IV bolus dosing and invasive hemodynamic monitoring in a mouse hypoxia-induced PAH model. Investigators can use jugular vein injection and arterial/right ventricle catheterization techniques described here for wide variety of rodent models requiring IV injection and hemodynamic monitoring.
Disclosures
K Zsebo, M Simons, and P-Y Chen are scientific founders and shareholders of VasoRx, Inc. M Simons is a member of the Scientific Advisory Board of VasoRx, Inc. HJ Duckers is an employee and shareholder of VasoRx. The other authors declare no competing interests.
Acknowledgements
This work was supported, in part, by a Joint Biology Consortium Microgrant provided under NIH grant P30AR070253 (PYC), Cardiovascular Medical Research Education Fund (PYC), VasoRx, Inc. Fund (MS) and NIH grants HL135582 (MS), HL152197 (MS).
Materials
| Name | Company | Catalog Number | Comments |
| 5-0 prolene suture pack | Ethicon | 8698G | for incision closure |
| 8-0 nylon suture pack | AROSurgical Instruments | T06A08N14-13 | for ligation |
| Anesthesia induction chamber | VETEQUIP | #941444 | Holds the animal during anesthesia exposure |
| Catheter Interface Cable PEC-4D | Millar | for connecting Millar Mikro-Tip catheter to PCU-2000 | |
| Charcoal canister filters | VETEQUIP | #931401 | to help remove waste anesthetic gases |
| Cotton swabs | McKesson | 24-106 | for applying pressure to the injection site to prevent bleeding |
| Fine scissors | Fine Science Tools | 14059-11 | Surgical tools |
| Insulin syringe 28 G | EXEL | 26027 | for jugular vein IV injection |
| Isoflurane | COVETRUS | #029405 | for mouse anesthesia |
| LabChart 8 Software | ADInstruments | for data analysis | |
| Mikro-Tip Pressure Catheter SPR-1000 (1.0 F) | Millar | for invasive blood pressure measurement | |
| Needle-25 G | BD | 305124 | for making a samll hole in a vessel |
| Oxygen controller ProOx Oxygen Sensor | BioSpherix | E702 | for oxygen concentration monitoring |
| PCU-2000 Pressure Control Unit | Millar | for connecting Millar Mikro-Tip catheter to PowerLab 4/35 | |
| PowerLab 4/35 | ADInstruments | for Data Acquisition. Investigator needs to connect the PowerLab 4/35 to a personal laptop containing LabChart 8 software for operation. | |
| Prism 8 | GraphPad | for statistics and scientific graphing | |
| Semisealable hypoxia chamber | BioSpherix | an artificial environment that simulates high-altitude conditions for animals | |
| Spring Scissors | Fine Science Tools | 15021-15 | Surgical tools |
| Tweezer Style 4 | Electron Microscopy Sciences | 0302-4-PO | Surgical tools |
| VasoRx compound 7C1/let-7 miRNA | VasoRx, Inc. | Lot# B2-L-16Apr | IV injection compound |
| VIP 3000 Veterinary Vaporizer | COLONIAL MEDICAL SUPPLY CO., INC. | for accurate anesthesia delivery |
References
- McLaughlin, V. V., McGoon, M. D. Pulmonary arterial hypertension. Circulation. 114 (1), 1417-1431 (2006).
- Hoeper, M. M., Humbert, M. The new haemodynamic definition of pulmonary hypertension: evidence prevails, finally. European Respiratory Journal. 53 (3), 1900038(2019).
- Chen, Y., et al. A novel rat model of pulmonary hypertension induced by mono treatment with SU5416. Hypertension Research. 43 (8), 754-764 (2020).
- Xiong, M., et al. Mouse model of experimental pulmonary hypertension: Lung angiogram and right heart catheterization. Pulmonary Circulation. 11 (4), 20458940211041512(2021).
- Kmiotek, E. K., Baimel, C., Gill, K. J. Methods for intravenous self administration in a mouse model. Journal of Visualized Experiments. (70), e3739(2012).
- Potus, F., Martin, A. Y., Snetsinger, B., Archer, S. L. Biventricular assessment of cardiac function and pressure-volume loops by closed-chest catheterization in mice. Journal of Visualized Experiments. (160), e61088(2020).
- Bueno-Beti, C., Hadri, L., Hajjar, R. J., Sassi, Y. The Sugen 5416/hypoxia mouse model of pulmonary arterial hypertension. Experimental Models of Cardiovascular Diseases. 1816, 243-252 (2018).
- Chen, P. Y., et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Reports. 2 (6), 1684-1696 (2012).
- Chen, P. Y., et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nature Metabolism. 1 (9), 912-926 (2019).
- Daugherty, A., Rateri, D., Hong, L., Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. Journal of Visualized Experiments. (27), e1291(2009).
- Alam, M. A., Parks, C., Mancarella, S. Long-term blood pressure measurement in freely moving mice using telemetry. Journal of Visualized Experiments. (111), e53991(2016).
- Luo, F., et al. Invasive hemodynamic assessment for the right ventricular system and hypoxia-induced pulmonary arterial hypertension in mice. Journal of Visualized Experiments. (152), e60090(2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved