Method Article
Laparoscopic Pancreatoduodenectomy for Pancreatic Cancer Using In-Situ No-Touch Isolation Technique
In This Article
Summary
No-Touch isolation procedures might prevent the dissemination of cancer cells from the primary tumor. However, these techniques are not widely accepted in laparoscopic pancreatoduodenectomy (LPD) by now. We herein present in-situ No-Touch isolation LPD with partial resection and reconstruction of the superior mesenteric vein (SMV) for pancreatic cancer after neoadjuvant therapy.
Abstract
Laparoscopic pancreatoduodenectomy (LPD) is a standard radical operation for pancreatic head malignant tumors by now. Due to the complex laparoscopic resection and reconstruction techniques, it is difficult to perform LPD for patients with locally advanced pancreatic head cancer after neoadjuvant therapy. Our team initiates LPD using the in-situ No-Touch isolation technique. The innovation and optimization of this modified No-Touch isolation technique emphasize exploring the distal section of superior mesenteric vein (SMV) and the left side of the superior mesenteric artery (SMA) prior to evaluating the resectability by subcolonic mesenteric approach, which is an ideal exploring approach. After that, we use the median-anterior, and left-posterior of SMA approaches to cut off the blood flow of the pancreatic head to make the tumor isolated intact, then move and dissect the tumor. It is a process fitting the surgical principle of tumor-free. This article aims to demonstrate the feasibility and safety of performing LPD using the in-situ No-Touch isolation technique, which might elevate the R0 resection rate. It is an oncological ideal operation process.
Introduction
Pancreaticoduodenectomy (PD) is a standard surgical procedure for cancer in the pancreaticoduodenal region. The Kocher maneuver is widely used for the efficient exposure of the duodenum and pancreatic head during conventional PD. The mobilization and squeezing of the pancreaticoduodenal area during surgery may cause metastasis of the tumor cells before the ligation of surrounding vessels1. A recent study had shown that the tumor cells had the potential possibility of being squeezed into the portal vein (PV) because of the handling and squeezing of the tumor area by the surgeons, which might further increase the risk of liver metastasis after surgery2.
With the development of biomedical technology, a scientist could detect the spread of solid tumor cells, including pancreatic cancer cells, into the vessels as circulating tumor cells (CTC)3,4.
No-Touch isolation procedures, which have been used in colon cancer, might prevent the dissemination of cancer cells, such as circulating tumor cells, from the primary tumor5. Several studies have reported the use of a no-touch isolation technique for pancreatic head cancer during laparotomy pancreaticoduodenectomy6,7. The concept of this procedure is that the surgeon does not touch the duodenum and pancreatic head region (including the tumor) before ligating and dissecting the vessels (arteries and veins) around the pancreatic head.
No-Touch isolation techniques have been reported in LPD for pancreaticoduodenal region neoplasm8. We herein present a modified in-situ No-Touch isolation LPD with partial resection and reconstruction of SMV for pancreatic cancer after neoadjuvant therapy, which dissects all the inflow arteries first, transects the involved vein with sufficient margin, resects the tumor in-situ, and removes the specimen en-bloc.
The goal and advantages of this method are to ensure that all steps follow the oncologic principles of No-Touch in order to decrease the risk of metastasis of the tumor cells. The rationale behind the development and use of this technique is that the tumor should be mobilized at the final stage, including resecting the tumor in situ and removing the specimen en bloc after tumor inflow arteries and outflow veins are occluded. However, as this procedure requires complex resection and reconstruction techniques when surgeons decide whether to use this method, they need to estimate their own situations such as the learning curve, tumor type, vascular condition, and other factors.
Protocol
This study was permitted by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine.
1. Patient selection
- Advise the patients suspected of suffering pancreatic ductal adenocarcinoma (PDAC) in the pancreas head to take a contrast-enhanced computed tomography (CT) scan at first. Then select the following patients: borderline resectable cases after neoadjuvant therapy, tumor regresses significantly and has the possibility of radical resection; meanwhile, SMV reconstruction is potentially needed.
- Exclude the following patients: (1) metastatic PDAC; (2) patients with a poor general condition that cannot tolerate major surgery; (3) vascular involvement of SMA, common hepatic artery (CHA), or celiac artery (CA).
2. Surgical technique
- Operative setting
- Place the anesthetized patient in a completely supine position, with legs spread out.
- During the sterile exposition, ensure that the suprapubic region is exposed adequately for the later specimen removal by a Pfannenstiel incision. Ensure that the operator surgeon stands on the right side, the first assistant is on the left side, and the second assistant holding the laparoscope is positioned between the patient's legs. Use the 5-port technique to perform the procedure (Figure 1).
- After the pneumoperitoneum is created, introduce the rigid laparoscope through the sub-umbilical 12 mm trocar, and place the other four trocars along a semi-circle.
- Exploration phase
- Explore the intraperitoneal organs and peritoneal surfaces meticulously for unexpected extrapancreatic metastases.
- Resect the greater omentum and open the lesser sac by dividing the gastrocolic ligament.
- Ligate Henle's gastrocolic trunk vein. Explore the gap between the pancreatic neck and SMV at the lower edge of the pancreas.
NOTE: The tunnel between the pancreatic neck and SMV can not be created easily if the anterior wall of SMV is involved.
- Dissection phase:
- Subcolonic mesenteric approach: For this approach, follow steps 2.3.2-2.3.5.
- After the transverse colon and its mesentery are elevated cephalad, place the entire small bowel on the left side to facilitate exposure of the pancreaticoduodenal region.
- Expose the second and third parts of the duodenum after the mesentery section.
- In order to reconfirm resectability and mobilize the posterior of the pancreatic head, expose the inferior vena cava (IVC), left renal vein (LRV), celiac trunk, aorta, and SMA (Figure 2).
- Then perform the dissection along the SMV to clear all the tissues on the right side. In order to expose the SMV fully, ligate the root of the middle colonic artery after confirming that there is no ischemia in the transverse colon.
- Left posterior of SMA approach: For this approach, follow steps 2.3.7-2.3.18.
- Place the entire small bowel on the left side to facilitate exposure and the dissection of the distal duodenum proximal to the ligament of Treitz.
- Divide the proximal jejunum with a stapler.
- Expose the SMA by tracing along the jejunal artery.
- Place a Fr8 catheter for hanging for encircling the dorsal aspect of the SMA and SMV.
- Pull the catheter to the upper right side to allow SMA dissection on its periadventitial plane on the anterior-left margin and its separation from the mesopancreas.
- Ligate the first jejunal artery (FJA) involved by the tumor and sacrifice.
- Identify the first jejunal vein (FJV) and the inferior pancreaticoduodenal veins (IPDV), which are the branches from the dorsal side of the SMV.
- Ligate and dissect the IPDV.
- Circumferentially dissect the SMA to identify the inferior pancreaticoduodenal artery (IPDA), which either forms a common trunk with the FJA or arises directly from the SMA (Figure 3).
- Sacrifice the IPDA.
- At the left posterior approach, dissect the SMA, which is identified at its origin above the LRV, free from the mesopancreas.
- In cases that the tumor invades the mesocolon, hang the SMV and SMA through a rubber band followed by coring out of the mesocolon.
- Supracolic median-anterior of SMA approach: For this approach, follow steps 2.3.20-2.3.34.
- Start the SMA approach from the upper colon region.
- Explore the gap between the pancreatic neck and SMV at the lower edge of the pancreas.
- Use a stapler device to divide the stomach 3-5 cm away from the pylorus.
- Remove the gallbladder.
- Divide the common bile duct (CBD) as conventional PD. Take a frozen pathologic examination of the bile duct stump. Apply the laparoscopic bulldog clamps to occlude the CBD temporarily.
- Dissect the hepatoduodenal ligament. Perform a lymphadenectomy along the CHA, the proper hepatic artery (PHA), and the PV.
- Ligate and dissect the right gastric artery.
- Identify the gastroduodenal artery (GDA) at the point where the PHA branches from the CHA.
- Doubly ligate or suture the GDA to minimize the chance of subsequent erosion and bleeding.
- Transect the neck of the pancreas with an ultrasound knife. Take a frozen pathologic examination of the pancreatic stump.
- Suspend the splenic vein (PV) using a rubber band.
- Set up a diamond-shaped window by retracting the transverse mesocolon caudally, the PV cranially, the SMV rightward, and the SMA leftward.
- During the supracolic median-anterior of the SMA approach, dissect the right and dorsal aspects of the SMA within this diamond-shaped window.
- Resect the fat and fibrous tissues around SMA and CA from the caudal side to the cephalic side.
- Ligate and transect the uncinate process artery (UPA) under the PV, leaving the specimen attached to the PV/SMV.
- Combining with median-anterior and left-posterior approaches to the SMA, expose the origin of the IPDA or the common trunk of the IPDA and FJA easily.
NOTE: During this procedure, the GDA, UPA, and IPDA have been completely ligated. Furthermore, all the tumor artery inflows are completely occluded till this moment to reduce bleeding. - Use laparoscopic bulldog clamps to temporarily clamp the PV, the splenic vein, and the SMV. Transect the involved vein with sufficient margin.
- Afterward, cut off the lymphatics and dissect the duodenum from the retroperitoneum.
- Finally, resect the tumor in situ and remove en bloc following the oncologic principles of No-Touch2,6,7.
- Reconstruction phase
- Measure the length of the venous defect with a soft ruler. If the defect exceeds 3 cm, consider using an artificial interposition graft.
- Perform SMV reconstruction from caudal to cephalic by continuous suture using 5-0 prolene sutures (Figure 4).
- Reconstruct the digestive tract by Child's method9.
- An end-to-side, perform single-layer running suturing hepaticojejunostomy with 4-0 absorbable sutures.
- Conduct pancreatic anastomosis by duct-to-mucosal, end-to-side pancreaticojejunostomy within an internal stent9.
- After a side-to-side gastrojejunal anastomosis, place three drainages near the anastomosis.
- Bag the specimen and retrieve it through the Pfannenstiel incision.
Results
A 55-year-old man with upper abdominal pain and marasmus was diagnosed with a 4.2 cm x 3.1 cm tumor in the uncinate process of the pancreas, and the SMV was involved over 180° (Figure 5). The patient was previously healthy and had a relatively normal body mass index (19.47 kg/m2). No distant metastasis was found on the preoperative contrast-enhanced CT scan. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was performed to acquire the pathology diagnosis of adenocarcinoma. Eight cycles of modified-FOLFIRINOX (mFOLFIRINOX) regimen (fluorouracil 2,400 mg/m2, irinotecan 135 mg/m2, oxaliplatin 68 mg/m2) were performed as neoadjuvant chemotherapy on this patient. The medication efficaciously relieved the symptoms, and carbohydrate antigen 199 (CA 199) declined from 857.1 U/mL to 109.4 U/mL, while the maximum diameter of the tumor shrunk from 4.2 cm to 3.5 cm (Figure 6). In addition, a contrast-enhanced CT scan showed that the tumor around SMA regressed.
The total operation time was 450 min with 150 mL of blood loss. The patient had an uneventful postoperative course and was discharged on the 14th day after the operation. The amylase level in the drainage fluid on postoperative day (POD) 3 was 57 U/L. The drain was removed on POD 7. Histopathology revealed a moderately poorly differentiated ductal adenocarcinoma. The resection margins were microscopically radical (R0), and none of the 20 lymph nodes were involved.

Figure 1: Position of the trocars. Use the 5-port technique. The patient is placed in a supine position with legs spread out. Please click here to view a larger version of this figure.
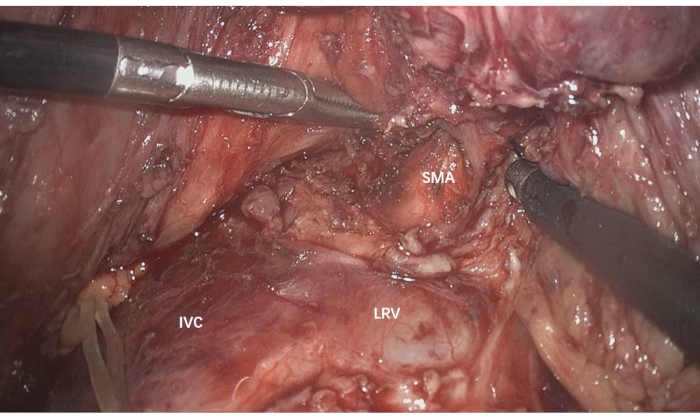
Figure 2: Expose the IVC, LRV, celiac trunk, aorta, and SMA to reconfirm resectability and mobilize the posterior of the pancreatic head. IVC: Inferior vena cava, LRV: left renal vein, SMA: superior mesenteric artery Please click here to view a larger version of this figure.
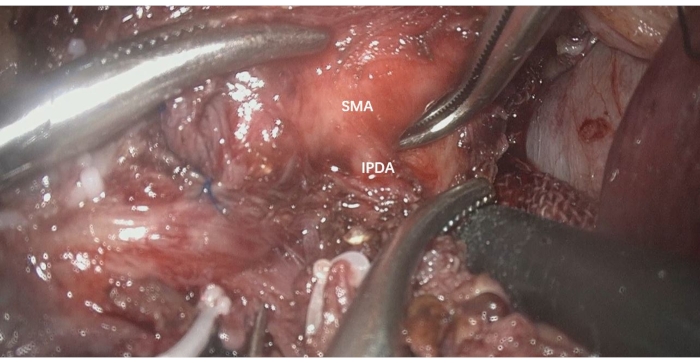
Figure 3: Circumferentially dissect the SMA to identify the IPDA, which arises directly from the SMA. IPDA: inferior pancreaticoduodenal artery, SMA: superior mesenteric artery Please click here to view a larger version of this figure.
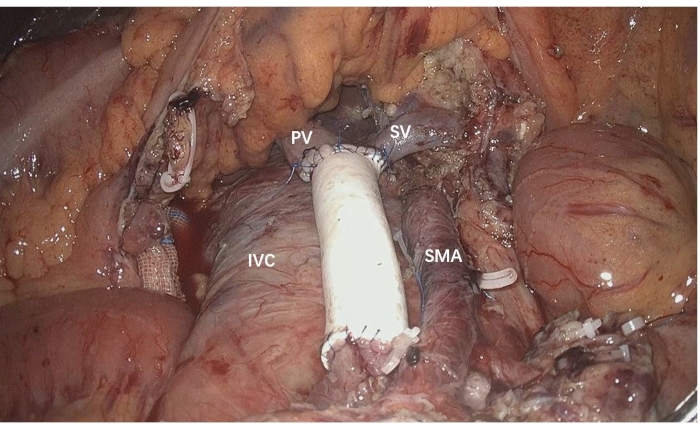
Figure 4: SMV reconstruction performed from caudal to cephalic using an artificial interposition graft. IVC: Inferior vena cava, PV: portal vein, SMA: superior mesenteric artery, SMV: superior mesenteric vein Please click here to view a larger version of this figure.
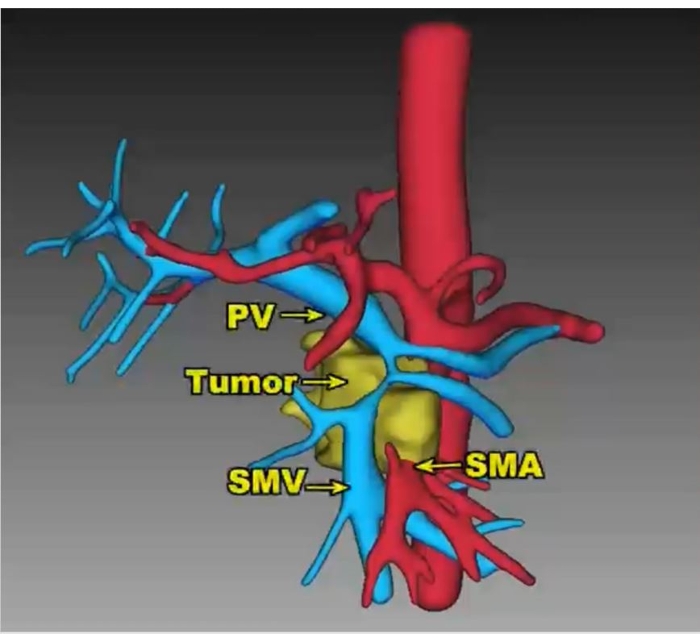
Figure 5: 3D reconstructed vessel images show the mass with over 180° involvement of the SMV. PV: portal vein, SMA: superior mesenteric artery, SMV: superior mesenteric Please click here to view a larger version of this figure.
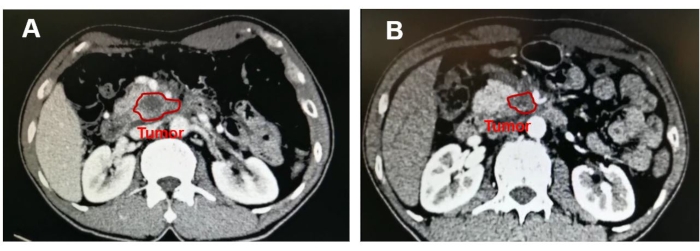
Figure 6: Image showing the mass in the uncinate process of the pancreas. After neoadjuvant chemotherapy, the tumor maximum diameter reduced from (A) 4.2 cm to (B) 3.5 cm. Please click here to view a larger version of this figure.
Discussion
PDAC is one of the most lethal malignant diseases. Despite the fact that the overall 5-year survival rates are still unsatisfactory, surgery remains the only curative therapeutic method till now10. According to the National Comprehensive Cancer Network (NCCN) and International Study Group of Pancreatic Cancer (ISGPS), patients diagnosed with PDAC should be defined as borderline resectable cases while the portal-superior mesenteric vein is suspiciously involved, and in order to improve R0 resection rate, these cohorts are recommended to implement synchronous vein resection11,12. Under this condition, the borderline resectable cases could obtain similar perioperative and survival outcomes compared to resectable ones. Laparoscopic pancreatoduodenectomy, which was considered an extremely complicated and intricate procedure, has been reported to be safe and feasible with the rapid development of laparoscopic techniques and instruments in recent years13,14,15.
Venous resection and reconstruction are even more complex and challenging than the conventional LPD, so LPD with artificial vascular graft reconstruction for the patients after neoadjuvant chemotherapy with PDAC is one of the most complicated radical operations. This study provides a novel in-situ No-Touch isolation LPD with partial resection and reconstruction of SMV, which might potentially develop to a standardized, reproducible, and oncological effective procedure for patients with borderline resectable pancreatic cancer.
This modified No-touch isolation technique emphasizes exploring the distal section of SMV and the left side of SMA below the transverse colon to evaluate the resectability, which is an ideal exploring approach. In order to obey the No-Touch oncologic principles to the maximum extent, the pancreaticoduodenal area, including the tumor, should not be mobilized before the ligation and dissection of the vascular around this region. Combining with the median-anterior and left-posterior approaches to the SMA, the priority is to dissect all the tumor artery inflows to reduce intraoperative bleeding. The following step is transecting the vein outflows, including the involved vein with sufficient margin. Finally, the tumor is resected in situ, and the specimen will be removed en bloc.
The goal and advantages of this method are to ensure that all steps follow the oncologic principles of No-Touch in order to decrease the risk of metastasis of the tumor cells. The rationale behind the development and use of this technique is that the tumor should be mobilized at the final stage, including resecting the tumor in situ and removing the specimen en bloc after tumor inflow arteries and outflow veins are occluded.
Due to the complex resection and reconstruction techniques, this procedure can only be performed by experienced surgical teams in high-volume centers with both open and laparoscopic pancreatic surgical skills. Moreover, the operation procedure has high requirements for the cooperation of the operation team, surgical skills, and anatomical cognition. Neoadjuvant chemotherapy may also increase the operative difficulty at the same time. Beyond that, randomized clinical trials with larger numbers of patients are difficult to design and accomplish for the above reasons. As a result, high-level evidence for the perioperative and survival results of this technique are difficult to establish.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgments.
Materials
| Name | Company | Catalog Number | Comments |
| 3D Laparoscope | STORZ | TC200,TC302 | |
| Absorbable hemostat | ETHICON, LLC | 2 in x 4 in | |
| Artificial Interposition Graft | W.L.Gore & Associates, Inc. | IRTH084040W | |
| Drainage tube | Aiyuan | 424280 | |
| Echelon Flex Powered Plus Articulating Endoscopic Linear Cutter and Endopath Echelon Endoscopic Linear Cutter Reloads with Gripping Surface Technology | Ethicon Endo-Surgery | ECR60G/GST60G | |
| Energy Platform | COVIDIEN ForceTriad Energy Platform | T2131469EX | |
| HARMONIC ACE Ultrasonic Surgical Devices | Ethicon Endo-Surgery | HAR36 | |
| Laparoscopic forceps | Gimmi | ||
| Laparoscopic right angle forceps | KARL STORZ | ||
| Laparoscopic scissors | AESCULAP | ||
| Latex T-shape Catheter | ZHANJIANG STAR ENTERPRISE CO., LTD. | 24Fr | |
| Ligating Clips | Teleflex Medical | 5,44,22,05,44,23,05,44,000 | |
| PDSII | Ethicon, LLC | W9109 | |
| PROLENE | Ethicon, LLC | W8556 | |
| Trocar | Surgaid | NPCS-100-1-12 | |
| Ultrasonic Surgical & Electrosurgical Generator | Ethicon Endo-Surgery | GEN11CN |
References
- Hirota, M., et al. Pancreatectomy using the no-touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: a pilot study. Journal of the Pancreas. 6 (2), 143-151 (2005).
- Gall, T. M., et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surgery. 149 (5), 482-485 (2014).
- Soeth, E., et al. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. Journal of Cancer Research and Clinical Oncology. 131 (10), 669-676 (2005).
- Yap, T. A., Lorente, D., Omlin, A., Olmos, D., de Bono, J. S. Circulating tumor cells: a multifunctional biomarker. Clinical Cancer Research. 20 (10), 2553-2568 (2014).
- Fujita, J., et al. Laparoscopic right hemicolectomy with radical lymph node dissection using the no-touch isolation technique for advanced colon cancer. Surgery Today. 31 (1), 93-96 (2001).
- Hirota, M., et al. Pancreatoduodenectomy using a no-touch isolation technique. The American Journal of Surgery. 199 (5), 65-68 (2010).
- Kuroki, T., Eguchi, S. No-touch isolation techniques for pancreatic cancer. Surgery Today. 47 (1), 8-13 (2017).
- Tan, Z. J., et al. Clinical experience of laparoscopic pancreatoduodenectomy via orthotopic resection. Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery. 58 (10), 782-786 (2020).
- Osada, S., et al. Reconstruction method after pancreaticoduodenectomy. Idea to prevent serious complications. Journal of the pancreas. 13 (1), 1-6 (2012).
- Mizrahi, J. D., Surana, R., Valle, J. W., Shroff, R. T. Pancreatic cancer. Lancet. 395 (10242), 2008-2020 (2020).
- Tempero, M. A., et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network JNCCN. 19 (4), 439-457 (2021).
- Bockhorn, M., et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 155 (6), 977-988 (2014).
- Tee, M. C., et al. Laparoscopic pancreatoduodenectomy does not completely mitigate increased perioperative risks in elderly patients. HPB (Oxford). The Official Journal of the International Hepato Pancreato Biliary Association. 17 (10), 909-918 (2015).
- Palanivelu, C., et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. The British Journal of Surgery. 104 (11), 1443-1450 (2017).
- Wang, M., et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: A multicentre, open-label, randomised controlled trial. The Lancet. Gastroenterology & Hepatology. 6 (6), 438-447 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved