Method Article
Agrobacterium-Mediated Genetic Transformation, Transgenic Production, and Its Application for the Study of Male Reproductive Development in Rice
* These authors contributed equally
In This Article
Summary
This work describes the use of CRISPR-Cas9 genome editing technology to knockout endogenous gene OsABCG15 followed by a modified Agrobacterium-mediated transformation protocol to produce a stable male-sterile line in rice.
Abstract
Male sterility is an important agronomic trait for hybrid seed production that is usually characterized by functional defects in male reproductive organs/gametes. Recent advances in CRISPR-Cas9 genome editing technology allow for high editing efficacy and timesaving knockout mutations of endogenous candidate genes at specific sites. Additionally, Agrobacterium-mediated genetic transformation of rice is also a key method for gene modification, which has been widely adopted by many public and private laboratories. In this study, we applied CRISPR-Cas9 genome editing tools and successfully generated three male sterile mutant lines by targeted genome editing of OsABCG15 in a japonica cultivar. We used a modified Agrobacterium-mediated rice transformation method that could provide excellent means of genetic emasculation for hybrid seed production in rice. Transgenic plants can be obtained within 2–3 months and homozygous transformants were screened by genotyping using PCR amplification and Sanger sequencing. Basic phenotypic characterization of the male sterile homozygous line was performed by microscopic observation of the rice male reproductive organs, pollen viability analysis by iodine potassium iodide (I2-KI) staining semi-thin cross-sectioning of developing anthers.
Introduction
Rice is the most important food crop, particularly in developing countries, and represents a staple food for over half of the world’s population. Overall, the demand for rice grain is growing and is projected to increase 50% by 2030 and 100% by 20501,2. Future improvements in rice yield will need to capitalize on diverse molecular and genetic resources that make rice an excellent model for monocotyledonous plant research. These include an efficient transformation system, advanced molecular map, and publicly accessible database of expressed sequence tags, which have been generated over many years3,4. One strategy to improve crop yield is hybrid seed production5, a central element of which is the ability to manipulate male fertility. Understanding the molecular control of male fertility in cereal crops can help to translate key knowledge into practical techniques to improve hybrid seed production and enhance crop productivity6,7.
Genetic transformation is a key tool for basic research and commercial agriculture since it enables introduction of foreign genes or manipulation of endogenous genes in crop plants, and results in the generation of genetically modified lines. An appropriate transformation protocol can help to accelerate genetic and molecular biology studies for fundamental understanding of gene regulation8. In bacteria, genetic transformation takes place naturally; however, in plants, it is performed artificially using molecular biology techniques9,10. Agrobacterium tumefaciens is a soil-borne, Gram-negative bacterium that causes crown gall disease in plants by transferring T-DNA, a region of its Ti plasmid, into the plant cell via a type IV secretion system11,12. In plants, A. tumefaciens-mediated transformation is considered a widespread method for gene modification because it leads to stable and low copy number integration of T-DNA into the host genome13. Transgenic rice was first generated through Agrobacterium-mediated gene transformation in the mid-1990s in the japonica cultivar14. Using this protocol, several transgenic lines were obtained within a period of 4 months with a transformation efficiency of 10%–30%. The study indicated that there are two critical steps for the successful transformation: one is the induction of embryogenic callus from mature seeds and another is the addition of acetosyringone, a phenolic compound, to the bacterial culture during co-cultivation, which allows for higher transformation efficiency in plants14,15. This protocol has been extensively used with minor alterations in japonica16,17,18,19 as well as other cultivars such as indica20,21,22,23 and tropical japonica24,25. Indeed, over 80% of the articles describing rice transformation use Agrobacterium-mediated gene transformation as a tool13. To date, several genetic transformation protocols have been developed using rice seed as a starting material for callus induction16,17,18,19. However, very little is known about young inflorescence as explants for callus production. Overall, it is important to establish a rapid, reproducible, and efficient gene transformation and regeneration protocol for functional genomics and studies on crop improvement.
In recent years, the advancement of CRISPR-Cas9 technology has resulted in a precise genome editing mechanism to understand gene function and deliver agronomically important improvements for plant breeding26,27. CRISPR also offers considerable promise for the manipulation of male reproductive development and hybrid production. In this study, we utilized a gene knockout system using CRISPR-Cas9 technology and coupled it to an efficient rice gene transformation protocol using young inflorescences as explants, thereby creating stable male sterile lines for the study of reproductive development.
Protocol
1. sgRNA-CAS9 plant expression vector construction and Agrobacterium-mediated transformation
- Target a male sterile gene OsABCG15 in rice according to the published literature28.
- Design sgRNA for the targeted site located between 106–125 bp in the second exon of OsABCG15 (Figure 1).
- Use T4 polynucleotide kinase to synthesize the sgRNA oligos (sgR-OsABCG15-F: 5’TGGCAAGCACATCCTCAAGGGGAT3’ and 5’sgR-OsABCG15-R: AAACATCCCCTTGAGGATGTGCTT’).
- Use endonuclease BbsI to digest the psgR-Cas9 backbone vector29.
- Ligate the synthesized sgRNA oligos with linearized psgR-Cas9 backbone using T4 ligase following the manufacturer’s protocol.
- Using HindIII/EcoRI, digest the sgRNA cassette from step 1.5 and subclone into the HindIII/EcoRI site of the pCAMBIA1300 binary vector for stable transformation29.
- Confirm the constructed plasmid by enzyme digestion and sequencing. Later, transform the binary construct into the Agrobacterium tumefaciens strain EHA105 and grow them on Kan/Rif selection plates at 28 °C for 2–3 days.
2. Rice genetic transformation and plant tissue culture
- Callus induction and regeneration
NOTE: Use fresh young rice inflorescences as the starting material to induce callus (Figure 2).- Collect the young inflorescences from the paddy field or green house at meiosis stage, determined by floret length of 1.6-4.8 mm (Figure 2A)29. Ensure that the rice inflorescences are covered with leaf sheath. Wipe each inflorescence with a 70% alcohol swab and let it dry before cutting.
- Bring the inflorescence to a clean sterile bench. Cut it into small pieces (the smaller the better) with sterilized scissors and then transfer cuttings to a Petri plate containing NBD2 medium (Figure 2B, Table 1).
- Incubate the plate in the dark at 26 °C for about 10-14 days to induce callus (Figure 3A).
NOTE: Use the newly formed callus for Agrobacterium infiltration (step 2.2.7) directly or recondition one more time in fresh NBD2 medium to obtain more callus. Transfer the callus into the new NBD2 medium every 8–10 days.
- Transformation and co-cultivation
- Use A. tumefaciens bacteria containing the binary plasmid from step 1.6 for transformation. Store the A. tumefaciens strains in YEB medium (Table 1) with 50% glycerol at -80 °C for further use.
- Streak A. tumefaciens from a -80 °C glycerol stock to YEB agar medium containing selective antibiotics (Table 1) and grow at 25–28 ˚C for 48–72 h, to allow colonies to appear.
- Inoculate a single colony from the YEB plate with selective antibiotics to 5 mL of liquid YEB medium containing the same selective antibiotics (Table 1), in a 50 mL conical sterile test tube. Shake on an orbital shaker at 250 x g, at 25–28 °C until bacteria grow to an OD600 of 0.5.
- Add 1 mL of bacterial suspension to 100 mL of YEB medium (Table 1) with the same selective antibiotics in a 250 mL conical flask and shake on an orbital shaker at 250 x g, at 28 °C for 4 h.
- Centrifuge at 4,000 x g for 10 min at room temperature to collect bacteria. Discard the supernatant.
- Resuspend the bacterial pellet with AAM-AS medium (Table 1) and dilute the suspension to an OD600 = 0.4.
NOTE: The perfect OD of bacterial suspension is critical for efficient transformation. It helps in eliminating excess bacterial growth on the callus. - Collect around 150 healthy growing light-yellow fragile embryogenic calli from step 2.1.3 into a 150 mL sterile flask. Add 50–75 mL bacterial cell suspension from step 2.2.6 into the sterile flask, and then add some 10–25 mL fresh AAM-AS medium to immerse the calli for 10–20 min, shaking occasionally.
- Pour out the bacterial suspension from the flask carefully and dry the excess bacterial suspension from the callus using sterile filter paper #1 or tissue paper. Then place them on a Petri dish with NBD-AS medium (Table 1) covered with a sterile filter paper #1. Incubate at 25–28 °C in the dark for 3 days and check for bacterial overgrowth.
- Selection for resistant callus
- After 3 days of co-cultivation, transfer the calli to a sterile Petri dish with a sterile filter paper.
- Air-dry the calli for 2 h on a clean bench. Ensure that the callus is not adhered to the filter paper.
- Transfer the calli evenly using sterile tweezer to the primary selection medium NBD2 (with 40 mg/L hygromycin and 250 mg/L timentin) (Table 1). Culture calli for 2 weeks at 25–28 °C in the dark.
- After 2 weeks, transfer calli evenly to a new plate containing fresh selection medium NBD2 (with 40 mg/L hygromycin and 250 mg/L timentin) (Table 1). Culture the calli for another 2 weeks at 25–28 °C in the dark.
- Callus differentiation
- Transfer callus to fresh MS Medium (with 25 mg/L hygromycin and 150 mg/L timentin) (Table 1). Culture under the light at 25–28 °C for around 2 weeks.
- Repeat step 2.4.1 one more time.
- Transfer the shoot buds to the new MS medium (with 10 mg/L hygromycin) to proliferate more shoots.
- Root induction
- Transfer the new shoots into ½ MS medium (Table 1). Culture under the light at 25 °C - 28 °C. The transformed plants produce roots within 2 weeks.
- After 1 week, transfer the plants to clean pots covering the plant to prevent dehydration.
3. Genotype identification
- Collect young leaves from newly generated plants for total DNA extraction using the cetyltrimethyl ammonium bromide (CTAB) method31.
- Using the genomic DNA as template, choose the primers (Hygromycin-F: 5’ GATGTTGGCGACCTCGTATT 3’ and Hygromycin-R: 5’ CGAAGAATCTCGTGCTTTCA 3’ located in the Hygromycin region) to perform polymerase chain reaction (PCR) to validate the transgene integration and frequencies (Figure 4). The following PCR conditions: 94 °C for 5 min; 36 cycles (94 °C for 30 s; 56 °C for 30 s; 72 °C for 1 min ); 72 °C for 7 min. Successful PCR reactions yield a 604 bp amplicon.
- Perform another PCR to amplify the genomic region surrounding the CRISPR target sites using specific primers (OsABCG15-ID-F: 5’ GTCTCATTGCTCAACAGTTTCT 3’ and OsABCG15-ID-R: 5’ TTGGTGTTTAAAACATTGCTAT 3’) to identify the genotype of the transformants. The PCR conditions are the same as in step 3.2.
- Use PCR fragments from step 3.3 directly for Sanger sequencing to identify mutations.
4. Observe the basic phenotype of the mutant
- Prepare the I2-KI solution: dissolve 2 g of KI (potassium iodide) in 10 mL of distilled water and then add 0.2 g of I2 (resublimed iodine) in it. After mixing, bring the total volume to 100 mL with distilled water.
- Prepare the FAA solution (63% anhydrous ethanol, 5% glacial acetic acid, 5% formaldehyde and 27% water) and mix before using.
- Prepare the 0.5% Toluidine blue staining solution: dissolve 0.5 g of Toluidine blue in 100 mL of distilled water.
- Perform vegetative phenotypic observation, by imaging the whole plants with a digital camera.
- Perform reproductive phenotype observation, remove the palea and lemma from the floret. Take photos of whole anthers under a stereomicroscope.
- Observe the pollen viability by iodine staining.
- Pick mature rice spikelets before the flower anthesis, remove the palea and lemma to release the anthers.
- Take 6 anthers and place them on a glass slide. Add 1 drop of distilled water, crush the anther well with tweezers to release the pollen grains, and then add 2–3 drops of I2-KI solution. Cover with the coverslip.
- Observe under a low magnification microscope. Pollen grains dyed black show more vigorous viability, no color or yellow-brown dyed are stunted or degenerated.
- Perform a semi-thin section of rice anther
- Fix the rice flowers from different developmental stages into an FAA solution. Vacuum the materials at 4 °C (generally 15 min) to allow the fixative to penetrate to the tissues until the material sinks to the bottom of the collection bottle.
- Replace with a new FAA solution the next day. Once the material sinks to the bottom, replace with 70% ethanol and store at 4 °C for long-term use.
- Dehydrate the materials with different gradients of ethanol (70%, 80%, 95% each time for 30 min, 100% ethanol for 2 h, 100% ethanol containing a little of Safranin dye for 2 h).
NOTE: Add a little of Safranin dye into the second 100% ethanol to color the anthers for easier sectioning afterwards. - Perform embedding by following the manufacturer’s instruction (Table of Materials). Then, bake in an oven at 60 °C for 48 h before sectioning.
- Section the sample to a thickness of 2 μm using a microtome. Add a drop of water on the glass slide. Then, pick up the thin sections containing anther blocks by forceps and put them onto the surface of the water drop on the slides.
- Place the slides on the water bath at 50 °C to completely spread the sections to let it firmly adhere to the slide.
- Use 0.5% toluidine blue solution stain the slides for 30 min. Then, rinse with water and dry in the fume hood. Seal with neutral tree glue. Gently place a coverslip on the slide and dry in the fume hood.
- Observe and photograph the sample under a microscope.
Results
Demonstrated here is the use of gene editing technology to create a male sterile line for future research by Agrobacterium-mediated genetic transformation in rice. To create the male sterile line of osabcg15, CRISPR-CAS9-mediated mutagenesis was used for binary vector construction. The sgRNA was driven by the OsU3 promoter, whereas the expression cassette of hSpCas9 was driven by the double 35S promoter, and the middle vector was assembled in a single binary vector pCAMBIA1300 designed for Agrobacterium-mediated stable transformation of rice. A graphical representation of the construct that was used for CRISPR/Cas9-mediated mutagenesis of rice is provided in Figure 1A.
Figure 2 and Figure 3 represent the entire procedure of rice transgene production. The young inflorescences were chosen as explants (Figure 2) and induced the friable embryogenic callus (FEC) within 14 days of inoculation on the NBD2 medium (Figure 3A). The embryogenic calli were directly used for Agrobacterium-mediated transformation (Figure 3C) or subculture for proliferation to obtain more calli for further infection (Figure 3B). After co-cultivating for 48 h on NBD2-AS medium in dark conditions (Figure 3D), the calli were shifted to a second round of selection medium. After 10–14 days, transformed calli showed some small newly formed microcalli on the periphery of the mother calli, while the color of untransformed calli turned into brown and died eventually (Figure 3E,F). Later, healthy and creamy colored calli were transferred into the regeneration medium twice. At this stage, transformed calli gradually showed green spots (shoot buds) (Figure 3G,H). These newly generated green-spots were cultured in a sterile plastic bottle containing regeneration medium to allow shoot growth (Figure 3I), and then shifted (as fresh shoots) into a 1/2 MS medium to induce roots. Later, healthy and vigorous growing shoots with well-developed roots were harvested (Figure 3J).
In total, 21 regenerated seedlings were obtained. PCR amplification for the hygromycin region showed that 18 out of 21 can amplify the corresponding band, indicating these lines are transgenic (Figure 4), and the corresponding transformation frequency is about 85.71% (Table 2). The transformants were genotyped to identify the mutations at the sgRNA target site(s) using primers spanning the target region by PCR amplicon and Sanger sequencing, Among 18 T0 transgenic plants, eight heterozygous lines and three homozygous lines were CRISPR-Cas9 positive, with corresponding mutation frequencies of 61.11% (Figure 1B, Table 2).
The homozygous knockout mutants were validated by basic male reproductive organ observation (Figure 5). The anthers of osabcg15 were smaller and paler than those of the wild-type (Figure 5A,D), and lacked mature pollen grains (Figure 5B,E). Transverse sectioning microscopy was performed to investigate the anther morphological defects in osabcg15 compared to the wild type. At stage 10, wild-type microspores became round shaped and vacuolated with dark blue-stained exine (Figure 5C). By contrast, osabcg15 microspores collapsed and degraded, only debris of degenerated microspore exist in the anther locule (Figure 5F).

Figure 1: Construct information for the CRISPR/Cas9 system and targeted mutagenesis of the OsABCG15. (A) The sgRNA was driven by the OsU3 promoter, whereas the expression cassette of hSpCas9 was driven by the double 35S promoter; the middle vector was assembled in a single binary vector designed for Agrobacterium-mediated stable transformation of rice. (B) The sequence (5’-AAGCACATCCTCAAGGGGAT-3’) located in the second exon of the OsABCG15 gene was selected as the target site of sgRNA. Three types of homozygous mutation events were generated by CRISPR-Cas9 in japonica cultivar 9522 background, and Sanger sequencing chromatograms of CRISPR-Cas9 induced mutation sites are shown. Line 9522osabcg15-1 has a single-nucleotide (1-nt) ‘A’ insertion; line 9522osabcg15-2 has a single-nucleotide (1-nt) ‘G’ insertion; and line 9522osabcg15-3 has a single-nucleotide (1-nt) ‘G’ deletion. Please click here to view a larger version of this figure.

Figure 2: Starting materials for inducing callus. (A) Young rice inflorescences at meiosis stage. (B) Dissected young inflorescences growing on the NBD2 medium. Bar = 2 cm in (A). Please click here to view a larger version of this figure.
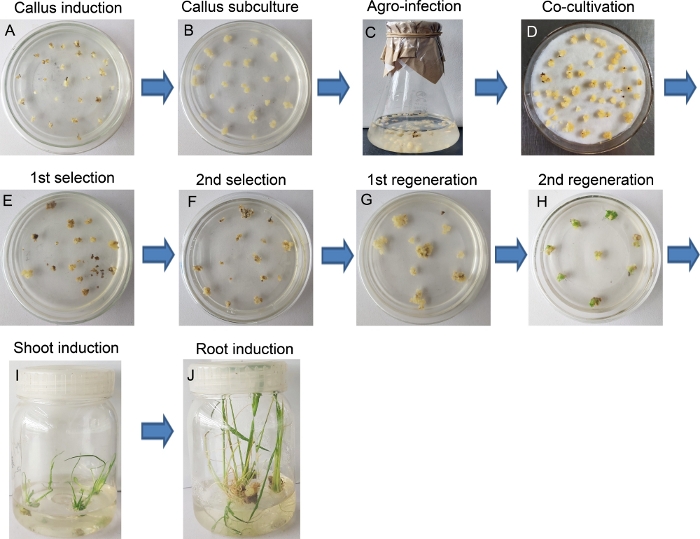
Figure 3: Procedure for producing transgenic lines by Agrobacterium-mediated transformation. (A) Rice calli generated from the young inflorescences. (B) Subculture of calli. (C) Incubation of calli with A. tumefaciens. (D) Co-cultivation of calli with A. tumefaciens. (E) First selection cycle of transformed calli in the presence of Hygromycin (40 mg/L). (F) Second selection cycle of transformed calli in the presence of Hygromycin (40 mg/L). (G) First regeneration of transformed calli. (H) The second regeneration of transformed calli, noting the green shoot point in the culture plate. (I) Shoot induction culture. (J) Root induction. Please click here to view a larger version of this figure.

Figure 4: Analysis of transformation frequencies of regenerated rice seedlings. Amplified PCR products that show a 604 bp fragment (1–8, 10–14, 16–20) after 1.5% agarose gel electrophoresis indicate transgenic positive lines. Lines that lack the fragment are untransformed (9, 15, 21), similar to the wild-type (WT) control. Please click here to view a larger version of this figure.

Figure 5: Basic phenotypic observation of the wild-type (9522) and mutant 9522osabcg15-1. (A) Wild-type (9522) flower and (D) 9522osabcg15-1 mutant flower after removal of the lemma and palea. gl, glume; fi, filament; lo, lodicule; pi, pistil; st, stamen. (B) I2-KI staining of mature pollen grains from wild-type (9522) and (E) 9522osabcg15-1 line. (C) Analysis of anther development in the wild-type (9522) and (F) 9522osabcg15-1 mutant by transverse section observation. DMsp, Degenerated microspores; E, epidermis; En, endothecium; Msp, microspore; T, tapetum. Bars = 2 mm in (A) and (D), 100 μm in (B) and (E), 50 μm in (C) and (F). Please click here to view a larger version of this figure.
Table 1: Media compositions for rice transformation. Please click here to download this table.
Table 2: Transformation and gene editing efficiency of the mutants of OsABCG15 generated by CRISPR-Cas9 in the T0 generation. Please click here to download this table.
Discussion
Artificial genic male sterile mutants are traditionally generated by random physical, chemical, or biological mutagenesis. Although these are powerful techniques, their random nature fails to capitalize on the vast amount of modern genomic knowledge that has the potential to deliver tailored improvements in molecular breeding32. The CRISPR-Cas9 system has been widely used in plants due to its simple and affordable means to manipulate and edit DNA29,33; it has potential to save time compared to traditional mutagenesis methods.
Here, we developed a convenient Agrobacterium-mediated transformation system using young inflorescences as explants for callus induction. There are some steps in the transgene procedure that can be omitted to save time. Using young inflorescences as starting material is a good choice to minimize the overall duration of the callus induction compared with using seeds as explants. In addition, the cell differentiation and regeneration capacity are very strong in the calli obtained from young inflorescences. In this process, subculture of rice calli can be avoided, which eventually saves time as well as reduces the risk of bacterial and fungal contamination. Moreover, timentin was used as an antibiotic instead of carbenicillin, which is more effective in inhibiting bacteria.
The study and the introduced protocol for basic phenotypic observation of male reproductive development in rice is useful for undergraduate and graduate students who are interested in plant reproductive biology. This convenient protocol should be quick to grasp and reproducible for use in subsequent research studies.
Disclosures
None.
Acknowledgements
The authors would like to acknowledge Xiaofei Chen for providing the young rice inflorescences and assistance in making the rice tissue culture medium. This work was supported by the National Natural Science Foundation of China (31900611).
Materials
| Name | Company | Catalog Number | Comments |
| 1-Naphthaleneacetic acid | Sigma-Aldrich | N0640 | |
| 2, 4-Dichlorophenoxyacetic Acid | Sigma-Aldrich | D7299 | |
| 6-Benzylaminopurine (6-BA) | Sigma-Aldrich | B3408 | |
| Acetosyringone | Sigma-Aldrich | D134406 | |
| Agar | Sinopharm Chemical Reagent Co., Ltd | 10000561 | |
| Ammonium sulfate | Sinopharm Chemical Reagent Co., Ltd | 10002918 | |
| Aneurine hydrochloride | Sigma-Aldrich | T4625 | |
| Anhydrous ethanol | Sinopharm Chemical Reagent Co., Ltd | 10009218 | |
| Bacteriological peptone | Sangon Biotech | A100636 | |
| Beef extract | Sangon Biotech | A600114 | |
| Boric acid | Sinopharm Chemical Reagent Co., Ltd | 10004808 | |
| Calcium chloride dihydrate | Sinopharm Chemical Reagent Co., Ltd | 20011160 | |
| Casein acid hydrolysate | Beijing XMJ Scientific Co., Ltd | C184 | |
| Cobalt(II) chloride hexahydrate | Sinopharm Chemical Reagent Co., Ltd | 10007216 | |
| Copper(II) sulfate pentahydrate | Sinopharm Chemical Reagent Co., Ltd | 10008218 | |
| D(+)-Glucose anhydrous | Sinopharm Chemical Reagent Co., Ltd | 63005518 | |
| D-sorbitol | Sinopharm Chemical Reagent Co., Ltd | 63011037 | |
| EDTA, Disodium Salt, Dihydrate | Sigma-Aldrich | E5134 | |
| EOS Digital SLR and Compact System Cameras | Canon | EOS 700D | |
| Formaldehyde | Sinopharm Chemical Reagent Co., Ltd | 10010018 | |
| Fully Automated Rotary Microtome | Leica Biosystems | Leica RM 2265 | |
| Glacial acetic acid | Sinopharm Chemical Reagent Co., Ltd | 10000208 | |
| Glycine | Sinopharm Chemical Reagent Co., Ltd | 62011516 | |
| Hygromycin | Beijing XMJ Scientific Co., Ltd | H370 | |
| Inositol | Sinopharm Chemical Reagent Co., Ltd | 63007738 | |
| Iodine | Sinopharm Chemical Reagent Co., Ltd | 10011517 | |
| Iron(II) sulfate heptahydrate | Sinopharm Chemical Reagent Co., Ltd | 10012116 | |
| Kanamycine | Beijing XMJ Scientific Co., Ltd | K378 | |
| Kinetin | Sigma-Aldrich | K0753 | |
| L-Arginine | Sinopharm Chemical Reagent Co., Ltd | 62004034 | |
| L-Aspartic acid | Sinopharm Chemical Reagent Co., Ltd | 62004736 | |
| L-Glutamine | Beijing XMJ Scientific Co., Ltd | G229 | |
| L-proline | Beijing XMJ Scientific Co., Ltd | P698 | |
| Magnesium sulfate heptahydrate | Sinopharm Chemical Reagent Co., Ltd | 10013018 | |
| Manganese sulfate monohydrate | Sinopharm Chemical Reagent Co., Ltd | 10013418 | |
| Microscopes | NIKON | Eclipse 80i | |
| MS | Phytotech | M519 | |
| Nicotinic acid | Sigma-Aldrich | N0765 | |
| Phytagel | Sigma-Aldrich | P8169 | |
| Potassium chloride | Sinopharm Chemical Reagent Co., Ltd | 10016308 | |
| Potassium dihydrogen phosphate | Sinopharm Chemical Reagent Co., Ltd | 10017608 | |
| Potassium iodide | Sinopharm Chemical Reagent Co., Ltd | 10017160 | |
| Potassium nitrate | Sinopharm Chemical Reagent Co., Ltd | 1001721933 | |
| Pyridoxine Hydrochloride (B6) | Sigma-Aldrich | 47862 | |
| Rifampicin | Beijing XMJ Scientific Co., Ltd | R501 | |
| Sodium hydroxide | Sinopharm Chemical Reagent Co., Ltd | 10019718 | |
| Sodium molybdate dihydrate | Sinopharm Chemical Reagent Co., Ltd | 10019816 | |
| Stereo microscopes | Leica Microsystems | Leica M205 A | |
| Sucrose | Sinopharm Chemical Reagent Co., Ltd | 10021418 | |
| Technovit embedding Kits 7100 | Heraeus Teknovi, Germany | 14653 | |
| Timentin | Beijing XMJ Scientific Co., Ltd | T869 | |
| Toluidine Blue O | Sigma-Aldrich | T3260 | |
| Water bath for paraffin sections | Leica Biosystems | Leica HI1210 | |
| Yeast extract | Sangon Biotech | A515245 | |
| Zinc sulfate heptahydrate | Sinopharm Chemical Reagent Co., Ltd | 10024018 |
References
- Izawa, T., Shimamoto, K. Becoming a model plant: The importance of rice to plant science. Trends in Plant Science. 1 (3), 95-99 (1996).
- Shimamoto, K., Kyozuka, J. Rice as a model for comparative genomics of plants. Annual Review of Plant Biology. 53 (1), 399-419 (2002).
- Selva, C., et al. Hybrid breeding in wheat: how shaping floral biology can offer new perspectives. Functional Plant Biology. 47 (8), 675-694 (2020).
- Lippman, Z. B., Zamir, D. Heterosis: revisiting the magic. Trends in Genetics. 23 (2), 60-66 (2007).
- Zhang, D., Liang, W. Improving food security: using male fertility for hybrid seed breeding. Science. , 45-48 (2016).
- Masters, A., et al. Agrobacterium-Mediated Immature Embryo Transformation of Recalcitrant Maize Inbred Lines Using Morphogenic Genes. Journal of Visualized Experiments. (156), e60782 (2020).
- Laurenceau, R., et al. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathogens. 9 (6), 1003473 (2013).
- Tzfira, T., Citovsky, V. Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Current Opinion in Biotechnology. 17 (2), 147-154 (2006).
- Gelvin, S. B. Agrobacterium in the genomics age. Plant Physiology. 150 (4), 1665-1676 (2009).
- Lacroix, B., Citovsky, V. The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. International Journal of Developmental Biology. 57, 467-481 (2013).
- Hiei, Y., Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nature Protocols. 3 (5), 824 (2008).
- Hiei, Y., Ohta, S., Komari, T., Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal. 6 (2), 271-282 (1994).
- Hiei, Y., Komari, T., Kubo, T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Molecular Biology. 35 (1-2), 205-218 (1997).
- Nishimura, A., Aichi, I., Matsuoka, M. A protocol for Agrobacterium-mediated transformation in rice. Nature Protocols. 1 (6), 2796 (2006).
- Yara, A., et al. Production of transgenic japonica rice (Oryza sativa) cultivar, Taichung 65, by the Agrobacterium-mediated method. Plant Biotechnology. 18 (4), 305-310 (2001).
- Cho, S. K., et al. Efficient transformation of Korean rice cultivars (Oryza sativa L.) mediated by Agrobacterium tumefaciens. Journal of Plant Biology. 41 (4), 262-268 (1998).
- Toki, S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Molecular Biology Reporter. 15, 16-21 (1997).
- Zhang, J., Xu, R. j., Elliott, M. C., Chen, D. F. Agrobacterium-mediated transformation of elite indica and japonica rice cultivars. Molecular Biotechnology. 8 (3), 223-231 (1997).
- Aldemita, R. R., Hodges, T. K. Agrobacterium tumefaciens-mediated transformation of japonica and indica rice varieties. Planta. 199 (4), 612-617 (1996).
- Rashid, H., Yokoi, S., Toriyama, K., Hinata, K. Transgenic plant production mediated by Agrobacterium in indica rice. Plant Cell Reports. 15 (10), 727-730 (1996).
- Sahoo, K. K., Tripathi, A. K., Pareek, A., Sopory, S. K., Singla-Pareek, S. L. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods. 7 (1), 49 (2011).
- Rachmawati, D., Hosaka, T., Inoue, E., Anzai, H. Agrobacterium-mediated transformation of Javanica rice cv. Rojolele. Bioscience, Biotechnology, and Biochemistry. 68 (6), 1193-1200 (2004).
- Dong, J., Teng, W., Buchholz, W. G., Hall, T. C. Agrobacterium-mediated transformation of Javanica rice. Molecular Breeding. 2 (3), 267-276 (1996).
- Bortesi, L., Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnology Advances. 33 (1), 41-52 (2015).
- Li, Q., et al. Development of japonica photo-sensitive genic male sterile rice lines by editing carbon starved anther using CRISPR/Cas9. Journal of Genetics and Genomics. 43 (6), 415 (2016).
- Qin, P., et al. ABCG15 encodes an ABC transporter protein, and is essential for Post-Meiotic anther and pollen exine development in rice. Plant and Cell Physiology. 54, (2013).
- Mao, Y., et al. Application of the CRISPR-Cas system for efficient genome engineering in plants. Molecular Plant. 6 (6), 2008-2011 (2013).
- Itoh, J. I., et al. Rice plant development: from zygote to spikelet. Plant and Cell Physiology. 46 (1), 23-47 (2005).
- Gawel, N. J., Jarret, R. L. A modified CTAB DNA extraction procedure forMusa andIpomoea. Plant Molecular Biology Reporter. 9 (3), 262-266 (1991).
- Wei, F. J., Droc, G., Guiderdoni, E., Hsing, Y. i. C. International Consortium of Rice Mutagenesis: resources and beyond. Rice. 6 (1), 39 (2013).
- Feng, Z., et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Research. 23 (10), 1229 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved