Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Обзор
Source: Deepika Das, Tamara M. Powers, Department of Chemistry, Texas A&M University
Bioinorganic chemistry is the field of study that investigates the role that metals play in biology. Approximately half of all proteins contain metals and it is estimated that up to one third of all proteins rely on metal-containing active sites to function. Proteins that feature metals, called metalloproteins, play a vital role in a variety of cell functions that are necessary for life. Metalloproteins have intrigued and inspired synthetic inorganic chemists for decades, and many research groups have dedicated their programs to modeling the chemistry of metal-containing active sites in proteins through the study of coordination compounds.
The transport of O2 is a vital process for living organisms. O2-transport metalloproteins are responsible for binding, transporting, and releasing oxygen, which can then be used for life processes such as respiration. The oxygen-carrying cobalt coordination complex, [N,N'-bis(salicylaldehyde)ethylenediimino]cobalt(II) [Co(salen)]2 has been studied extensively to gain understanding about how metal complexes reversibly bind O2.1
In this experiment, we will synthesize [Co(salen)]2 and study its reversible reaction with O2 in the presence of dimethylsulfoxide (DMSO). First, we will quantify the amount of O2 consumed upon exposure of [Co(salen)]2 to DMSO. We will then visually observe the release of O2 from the [Co(salen)]2-O2 adduct by exposing the solid to CHCl3.
Принципы
There are two solid polymorphs of [Co(salen)]2 (active and inactive), which can be isolated from different reaction conditions. Active and inactive [Co(salen)]2 vary in their color (brown and red, respectively), structure, and reactivity. Both polymorphs consist of dimeric units. In the case of active [Co(salen)]2, the Co-centers in each of the two Co(salen)2 molecules are in close proximity, forming a very weak van der Waals interaction between the metal centers (Figure 1). While the active form does exhibit a weak Co-Co interaction, the separation between the dimeric units provides space for O2 to react with the Co centers; as a result, the active form of [Co(salen)]2 reacts with O2 in the solid state.
In the so-called inactive form of [Co(salen)]2, there is a dative interaction between the Co center of one molecule and an oxygen atom from the other (Figure 1). The two Co(salen)2 units are closer together compared to the active form and, as a result, the inactive form is stable in air in the solid state and only reacts with O2 in the presence of a coordinating solvent (such as DMSO), which disrupts the dimeric unit and stabilizes the [Co(salen)]2-O2 adduct. Inactive [Co(salen)]2 is easier to handle and study, since the solid can be isolated without using air-free techniques. Therefore, in this experiment we will synthesize inactive [Co(salen)]2 and study its reaction with O2 in the presence of DMSO.
There are several ways that O2, a diatomic molecule, can coordinate to metal center(s) (Figure 2). End-on binding results in a metal-oxygen bond to one of the oxygen atoms in O2. In side-on binding, both oxygen atoms form bonds to the metal center. In some cases, the O2 unit bridges two metal complexes where end-on and side-on binding are also observed.
Inactive [Co(salen)]2 forms a 2:1 cobalt to O2 adduct in the presence of the coordinating solvent, DMSO. The O2 unit bridges the two cobalt centers in an end-on bridging fashion (Figure 3) and coordinated DMSO molecules complete the octahedral coordination sphere of each of the Co centers. If we consider the MO diagram of O2 and d-orbital splitting diagram for [Co(salen)]2, we can understand why the 2:1 O2 adduct is favored (Figure 4). O2 displays a triplet ground state with two unpaired electrons in the π* MOs. [Co(salen)]2 is paramagnetic, with one unpaired electron in its σ*dz2 MO (assuming square planar (D4h), Co2+, 7 de-). The binding of O2 to [Co(salen)]2 is a redox reaction, where two Co(salen) molecules are oxidized by 1 e- each to a final oxidation state of +3 at cobalt, and the O2 molecule is reduced by 2 e-,resulting in the formation of peroxide (O22-). The 1:1 adduct is not favored in this case because Co(III) is d6 and, therefore, does not want to give up another electron (For a review on MO theory/d-orbital splitting, see the video on Group Theory and MO Theory of Transition Metal Complexes).
In this video, we will experimentally determine the Co:O2 ratio upon reaction of inactive [Co(salen)]2 with O2 in the presence of DMSO by measuring the volume of O2 lost in a closed system. We can use the ideal gas law (Equation 1) to calculate the number of moles of O2 consumed.
PV = nRT (Equation 1)
P = pressure = 1 atm
V = volume (L)
R = 0.082 L atm mol-1 K-1
T = temperature (K)
n = moles
We will then study the reversibility of O2 binding by exposing the resulting solid [Co(salen)]2-O2-(DMSO)2 to chloroform (CHCl3). Addition of CHCl3 (a non-coordinating solvent that cannot stabilize the [Co(salen)]2-O2 adduct) leads to a decrease in the concentration of DMSO. Le Châtelier's principle can explain that upon a decrease in concentration of DMSO, the equilibrium shown in Figure 3 will shift towards the reactants, resulting in liberation of O2 gas.
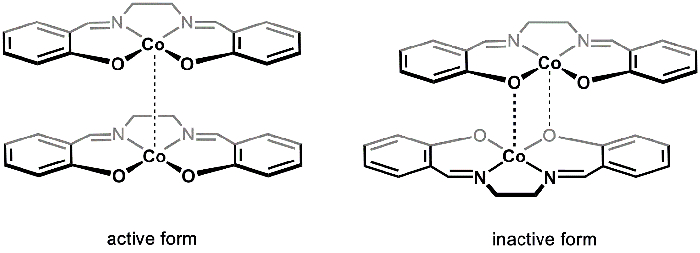
Figure 1. Active and inactive forms of [Co(salen)]2.
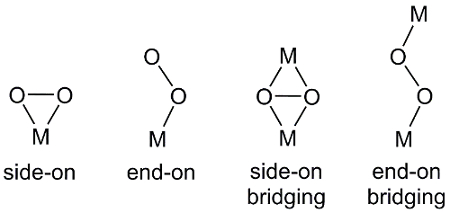
Figure 2. Coordination modes of O2 to metal center, M.
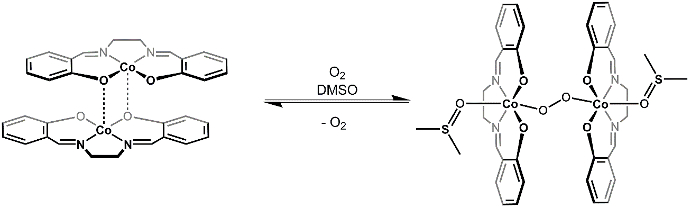
Figure 3. Reversible reaction of O2 with [Co(salen)]2.

Figure 4. MO diagram of O2 and d-orbital splitting diagram of Co(salen) (derived from Group theory, assuming square planar geometry).
Процедура
1. Synthesis of Inactive [Co(salen)]2
- Charge a 250 mL 3-neck round-bottom flask with 120 mL of 95% EtOH and 2.20 g (0.192 mL, 0.018 mol) of salicylaldehyde.
- Fit the center neck with a condenser connected to N2. Fit the other two necks with a rubber septum and an addition funnel fitted with a rubber septum.
- Stir the reaction in a water bath and heat the solution to reflux (80 °C).
- Add ethylene diamine (0.52 g, 0.58 mL, 0.0087 mol) via syringe through the round-bottom flask septum.
- In a 50 mL round-bottom flask, prepare a solution of Co(OAc)2·4H2O (2.17 g, 0.0087 mol) in 15 mL of distilled water. Heat the solution in the same water bath containing the 3-neck flask to ensure that all of the cobalt acetate dissolves.
- Add the cobalt acetate solution to the addition funnel.
- Degas the cobalt acetate solution by bubbling N2 through the liquid in the addition funnel for 10 min (see "Synthesis of a Ti(III) Metallocene Using Schlenk Line Technique" video for a more detailed procedure on purging liquids). The condenser N2 adapter may need to be closed to allow the N2 to bubble through the cobalt acetate solution.
NOTE: Never heat a closed system! Make sure to vent the system during degassing. - Slowly add the cobalt(II) acetate solution (~ 1 drop/s), while vigorously stirring the ethanol mixture. Without sufficient stirring, a chunky precipitate will form that can jam the stir bar.
- Once all of the cobalt acetate has been added, stir the reaction at reflux for 1 h.
- Turn off the hot plate and remove the 3-neck round-bottom flask from the water bath.
- Remove the condenser and addition funnel from the flask. Submerge the flask in an ice bath to facilitate precipitation of the [Co(salen)]2.
- Filter the solution under vacuum to isolate the solid and wash the resulting red solid with cold ethanol.
- Isolate the solid. Calculate the yield of the reaction and collect an IR of the [Co(salen)]2. Make sure that the [Co(salen)]2 is dry before using it in the O2 uptake reaction.
2. Apparatus Setup for O2 Uptake (Figure 5)1
Note: It is very important that the system does not leak. A leak in the system will lead to a lower than expected Co:O2 ratio.
- Connect a needle to an O2 (ultra-high purity) gas cylinder with Tygon tubing. Gently bubble O2 through 5 mL of DMSO for at least 10 min.
- While the DMSO is being saturated with O2, fit the two ends of a graduated 10 mL glass pipette with Tygon tubing (each 1.5 ft in length).
- Attach a glass funnel to one of the Tygon tubing pieces.
- Clamp the glass pipette and the funnel to a ring stand so that the funnel is facing up and the tubing forms a U shape ( Figure 5).
- Fill the pipette and funnel with mineral oil. Add the oil through the funnel, making sure that the oil also fills the tubing connected to the pipette. Continue to add the oil until the funnel is filled about half way up the funnel. Don't let the oil get too close to the top of the funnel, as the O2 bubbling through the funnel can cause splashing if the funnel is too full.
- To the open end of the tubing, attach a side-arm test tube (test tube A).
- Add 50 mg (0.077 mmol) of the inactive [Co(salen)]2 to the side-arm test tube A connected to the glass pipette.
- Add 2 mL of the DMSO saturated with O2 to a 3 mL test tube (test tube B).
- Use a pair of tweezers to gently lower test tube B into test tube A, being careful not to spill any of the DMSO. It is important to not expose the [Co(salen)]2 to the DMSO at this point.
- Seal test tube A with a rubber septum. Wire the septum to prevent leaks.
- Insert the needle connected to the O2 gas tank into the septum and purge the system with O2 for 10 min.
- Remove the O2 needle and grease the top of the rubber septum to prevent leaks.
- Some of the pressure within the setup may need to be released to get oil into the glass pipette. To do this, insert a free needle into the rubber septum on test tube A. Cover the opening with a finger and slowly release the pressure within the setup. Do not forget to cover the new hole with grease to prevent leaks.
- Move the glass pipette and funnel so that the oil levels line up in both pieces of glassware.
- Record the volume level of oil within the glass pipette.

Figure 5. O2 uptake apparatus setup.
3. O2 Uptake Reaction
- Add the DMSO to the solid [Co(salen)]2 by gently tipping the test tubes, making sure that none of the solution enters the side-arm of test tube A.
- Once all of the DMSO has been added, hold the top of the test tube and gently mix the solution by shaking the test tube back and forth.
NOTE: Do not use an up and down shaking motion. Banging the two test tubes together too violently can lead to the breaking of test tube A. - Continue to gently shake the test tubes by hand until the oil level in the pipette stops rising (about 15-20 min).
- Once O2 consumption ceases, move the pipette and funnel so that the oil levels line up.
- Record the new volume level of the oil in the glass pipette. The volume difference is the volume of O2 consumed during the reaction at atmospheric (1 atm) pressure.
- Record the temperature of the room.
4. O2 Liberation from [Co(salen)]2 - O2 Adduct
- Transfer the resulting DMSO solution from step 3 to a 15 mL centrifuge tube.
- Fill a second test tube with an equivalent amount of water.
- Insert the test tubes across from each other into a centrifuge.
- Centrifuge the sample for at least 15 min. The resulting solid pellet quality improves with increasing centrifuge time.
- Gently remove the test tube with the [Co(salen)]2-O2 adduct sample, so not to disturb the pellet.
- Carefully decant the DMSO solution above the pellet.
- Holding the centrifuge tube at a 45 ° angle with the pellet facing up, slowly add 1 mL of CHCl3 with a pipette, by allowing the solution to drip down the side of the centrifuge tube. Take extreme care to not disturb the solid [Co(salen)]2-O2 adduct.
- Observe any physical changes that occur.
Результаты
Characterization of Inactive [Co(salen)]2:

IR (cm-1) collected on ATR attachment: 2357 (w), 1626 (w), 1602 (m), 1542 (w), 1528 (m), 1454 (w), 1448 (m), 1429 (m), 1348 (w), 1327 (w), 1323 (m), 1288 (m), 1248 (w), 1236 (w), 1197 (m), 1140 (m), 1124 (m), 1089 (w), 1053 (m), 1026 (w), 970 (w), 952 (w), 947 (w), 902 (m), 878 (w), 845 (w), 813 (w), 794 (w), 750 (s), 730 (s).
O2 Uptake:
59.2 mg (0.090 mmol) of [Co(salen)]2 consumed 0.002 L of O2. Using standard pressure and the temperature recorded in step 3.6, the number of moles of O2 consumed was:

The calculated moles of Co in 0.090 mmol of [Co(salen)]2:

Therefore the Co:O2 ratio was:
0.180 mmol Co : 0.082 mmol O2
which is equivalent to a 2:0.91 ratio of Co to O2.
Addition of CHCl3 to [Co(salen)]2–O2 Adduct:
Upon addition of CHCl3, the CHCl3 solution turned red and a stream of bubbles was liberated from the solid, indicating release of O2 gas and formation of inactive [Co(salen)]2.
Заявка и Краткое содержание
In this video, we explained the different ways that diatomic oxygen can coordinate to metal center(s). We synthesized the oxygen-carrying cobalt complex [Co(salen)]2 and studied its reversible binding with O2. Experimentally we demonstrated that inactive [Co(salen)]2 reversibly binds O2 and forms a 2:1 Co:O2 adduct in the presence of DMSO.
All vertebrates depend on hemoglobin, a metalloprotein found in red blood cells, to transport oxygen to respiratory organs as well as other tissues. In hemoglobin, oxygen reversibly binds to a heme group that features a single Fe center coordinated to a heterocyclic ring called a porphyrin (Figure 6a). Hemoglobin is not the only oxygen-carrying and storage metalloprotein. For example, mollusks possess a protein called hemocyanin, which features a dicopper active site that is responsible for oxygen transport (Figure 6b).
Using synthetic molecular species to model active sites in metalloproteins is challenging due to the distinct differences in electronic structure of a simple coordination compound compared to that of a metal surrounded by a protein superstructure. As a result, it is often difficult to precisely replicate the structure of the active site in metalloproteins. While there are examples of model complexes that structurally mimic metal active sites, there are fewer examples of structurally similar model complexes that exhibit reactivity inherent to the native metalloenzyme.

Figure 6. (a) The Fe center in hemoglobin binds to O2 in an end-on fashion, while (b) the copper containing active site in hemocyanin binds to O2 in a bridging side-on orientation.
Ссылки
- Niederhoffer, E. C., Timmons, J. H., Martell, A. E. Thermodynamics of Oxygen Binding in Natural and Synthetic Dioxygen Complexes. Chem Rev. 84, 137-203 (1984).
- Appleton, T. G. Oxygen uptake by cobalt(II) complex. An undergraduate experiment. J Chem Educ. 54 (7), 443 (1977).
- Ueno, K., Martell, A. E. Infrared Studies on Synthetic Oxygen Carriers. J Phys Chem.60, 1270–1275 (1956).
Теги
Перейти к...
Видео из этой коллекции:

Now Playing
Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.6K Просмотры

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.6K Просмотры

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Просмотры

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.5K Просмотры

The Evans Method
Inorganic Chemistry
68.3K Просмотры

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.3K Просмотры

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.4K Просмотры

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Просмотры

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.8K Просмотры

Structure Of Ferrocene
Inorganic Chemistry
79.4K Просмотры

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.1K Просмотры

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.3K Просмотры

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Просмотры

Dye-sensitized Solar Cells
Inorganic Chemistry
15.8K Просмотры

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Просмотры
Авторские права © 2025 MyJoVE Corporation. Все права защищены