Method Article
Stab-Wound Mouse Model for Studying Hemorrhage and Inflammation in Traumatic Brain Injury
In This Article
Summary
Simplified traumatic brain injury (TBI) models have facilitated the development of therapeutic approaches. This protocol outlines the creation of a stab-wound mouse cortex using needles, enabling the analysis of hemorrhage and inflammation. The stab-wound TBI mouse model offers the advantage of being performed without requiring specialized equipment.
Abstract
Traumatic brain injury (TBI) results from physical damage, often caused by accidents or sports-related incidents. The causes of TBI are diverse, including concussions, brain contusions, hematomas, and skull fractures. To replicate these different causes, various TBI mouse models have been developed using distinct protocols. Physical brain injury leads to both primary and secondary brain injuries, which exacerbate neuronal loss. Primary injury occurs immediately after the damage, often due to hemorrhage, and subsequently triggers secondary injuries, including inflammation around the lesion. Developing a TBI model suitable for assessing hemorrhage extension and inflammatory severity is therefore crucial. This protocol introduces a method for mimicking penetrating brain injury, referred to as the stab-wound TBI mouse model, to study mechanisms of hemorrhage, inflammation, and neuronal loss associated with TBI pathology. This model is created by puncturing the skull and brain with needles and is simple to execute without the need for specialized experimental equipment. Additionally, the minor injury inflicted on the mouse cerebral cortex using a needle does not affect the animal's behavior post-surgery. This feature allows researchers to study the localized effects of brain injury without concerns about broader behavioral consequences. Sample data from stab-wounded mouse cerebral cortices demonstrate the model's effectiveness in assessing blood leakage into the parenchyma, glial activation, and inflammatory cytokine production. Furthermore, this protocol facilitates the evaluation of blood coagulants and anti-inflammatory compounds, aiding in the development of therapeutic agents for TBI.
Introduction
Traumatic brain injury (TBI) is caused by physical damage, often resulting from accidents, including traffic accidents and fall accidents. TBI is classified into two types: penetrating brain injury, which occurs when a sharp object perforates the skull as well as the brain, and closed brain injury, which is caused by violent shaking of the brain inside without a break in the skull1.
The causes of TBI are very diverse, including concussions, brain contusions, hematomas, and skull fractures; therefore, TBI mouse models have been developed using various protocols to replicate these different causes. For example, a repetitive concussive TBI model involves brain shaking, where mice are stuck several times using an electromagnetically controlled rubber impactor2. Additionally, in the weight-drop TBI model, a strong external force is exerted on the head by a standardized weight-drop device, causing focal blunt injury with an intact skull3. Furthermore, the stab-wound TBI model is prepared by puncturing the skull and brain using a needle4 (Figure 1A). Since several TBI models have been developed, it is important to choose a model based on the specific pathology that needs to be observed.
Brain injury caused by physical damage leads to primary and secondary brain injuries, which further exacerbate neuronal loss. Primary injury occurs immediately after the damage, resulting from the breakdown of the blood-brain barrier (BBB), hemorrhage, and hematoma. Therefore, minimizing hemorrhage and hematoma expansion is crucial, as these factors can exacerbate the severity of TBI symptoms. Secondary injury is triggered by intraparenchymal blood components, which subsequently lead to inflammation around the lesion5. The prognosis after brain injury depends on the inflammatory dynamics; therefore, it is crucial to rapidly mitigate both primary and secondary injuries for a favorable prognosis6,7,8.
The BBB is composed of pericytes, tight junctions between endothelial cells, and the endfeet of astrocytes, which work together to restrict the leakage of substances from the blood vessels in healthy brains9. In the presented stab-wound system, the BBB is physically disrupted. Common methods for evaluating the BBB integrity include staining for immunoglobulin G (IgG) and assessing the leakage of fluorescence tracers, such as Evans blue and dextran10,11. IgG staining labels blood components that leak from the lesion site and deposit in the brain. As the BBB recovers, leakage of blood components into the brain decreases, and these deposits are gradually degraded. Therefore, IgG staining is used to assess the extent of BBB recovery after brain injury. Additionally, the level of leakage of intravenously administered tracer into the brain parenchyma reflects the recovery of BBB. This method provides a clearer evaluation of the BBB dynamics, as tracer leakage directly indicates the transition of blood components from the bloodstream to the brain parenchyma. Furthermore, minimizing the hemorrhage leads to a milder primary injury, which is supported by prompt blood coagulation and timely fibrinolysis. Therefore, quantifying the expression of blood coagulation and fibrinolysis regulators is an effective way to analyze this process. Regarding the molecular mechanism underlying coagulation, hemorrhage after brain injury is stopped by fibrin formation. Subsequently, the fibrin-rich thrombus is degraded by tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA). In the stab-wound TBI mouse model, fibrin formation peaks on 1 day after the injury and reduces thereafter10. Thus, the recovery level of the BBB can be predicted by quantifying blood components and tracer extravasation into the brain parenchyma, as well as the expression of blood coagulation factors.
Quantification methods for inflammation in the secondary injury process include glial activation and inflammatory cytokine expression. Prolonged inflammation is mainly induced by excessive microglia and astrocyte accumulation around the lesion site. For example, in a stab-wound TBI model, stab-wounds stimulate the reactivation of glial cells around the lesion to remove the cell debris and blood components. This glial reactivation typically peaks 3 days after the stab-wound12,13. In addition to their phagocytosis function, reactivated glial cells secrete excessive inflammatory cytokines, resulting in neuronal loss around the lesion14. It has been reported that the attenuation of glial inflammation contributes to a favorable prognosis after brain injury12,14. Determining the level of inflammation is useful for evaluating the severity and prognosis. Therefore, it is essential to develop a TBI model suitable for assessing hemorrhage extension and inflammatory severity. This study introduces a stab-wound mouse model that mimics penetrating brain injury, with the aim of studying the mechanisms of hemorrhage, inflammation, and neuronal loss in TBI pathology.
Protocol
All animal care protocols were approved by the Institutional Animal Care and Use Committee of Ochanomizu University, Japan, and were performed in accordance with the guidelines established by the Ministry of Education, Science, and Culture in Japan. Six-week-old adult C57BL/6J female mice (20-25 g) were used. All mice were provided ad libitum access to food and water in a clean environment. Details of the reagents and equipment used are listed in the Table of Materials.
1. Stab-wound surgery to the cerebral cortex
- Preparation of reagents
NOTE: The anesthetic protocol is similar to that previously described15. If the animal facility recommends a specific anesthetic, please follow their instructions. It has been previously confirmed that pentobarbital sodium is effective for stab-wound surgery in the cerebral cortex4,13,16,17.- Combination anesthetic MMB: Dissolve 1.875 mL of 1 mg/mL medetomidine, 2.0 mL of 5 mg/mL midazolam, and 2.5 mL of 5 mg/mL butorphanol tartrate in 18.625 mL of phosphate-buffered saline (PBS) to create a total volume of 25 mL. This mixture will have a concentration of 75 mg/L medetomidine, 400 mg/L midazolam, and 500 mg/L butorphanol tartrate mixture. Store at 4 °C for up to 8 weeks prior to use.
- Anesthesia antagonist: Dissolve 150 μL of 5 mg/mL atipamezole hydrochloride in 9.85 mL of PBS to create a total volume of 10 mL, with a concentration of 75 mg/L atipamezole hydrochloride. Store at 4 °C, protected from light, for up to 8 weeks prior to use.
- Surgery procedure
- Mouse anesthesia: Temporarily anesthetize the mice using an isoflurane inhalation solution-soaked paper until they fall asleep. Then, inject 10 μL/g combination anesthetic MMB intraperitoneal using a 1-mL syringe with a 27 G x 3/4" needle. After 3-5 min, confirm the anesthesia effectiveness by performing a toe pinch and checking the righting reflex.
NOTE: Ensure to pay close attention not to give an overdose of anesthesia when using isoflurane inhalation solution. - Place the mouse on its ventral side into a paper towel. After the transfer, reconfirm the mouse is still anesthetized with a toe pinch.
- Shave the fur on the skin of the occipital region. Sterilize the fur on the back of the mouse's head with 70% ethanol using a cotton swab. Grasp the occipital skin with blunt forceps and make a 1.0-1.5 mm-wide incision to expose the occipital bone without damaging the skull or any organ. Gently and slowly open the incision to observe the boundary between the cerebral cortex and cerebellum through the skull (Figure 1B).
NOTE: Ensure no damage occurs to the skull or brain for the no wound mouse. - Mouse craniotomy: Create a small hole in the right hemisphere of the occipital bone using a 19 G x 1•1/2" needle. Gently rotate the needle to make an insertion point for stab-wound needling, taking care not to damage the brain parenchyma. The hole should be positioned on the midpoint of right hemisphere interaural line between lambda and edge (Figure 1B). This hole serves as a guide for the insertion of the stab-wound needle in the subsequent step.
- Stab-wound: Insert a 27 G x 3/4" needle from the insertion point and stab the cerebral cortex along the rostrocaudal axis (Figure 1B,C). The needle is inserted until it contacts the front of the skull, then gently withdrawn. The appropriate depth is one where the needle can be seen through the surface of the cerebral cortex. The left side of the cerebral cortex is used as the undamaged control.
- Suture the skin incision using a nylon 3-0 suture with a 1/2 round needle.
- Administer 10 μL/g of the anesthesia antagonist intraperitoneally as the combination anesthetic MMB. The mouse should be able to walk within 8-16 h after the anesthesia antagonist injection.
- Mouse anesthesia: Temporarily anesthetize the mice using an isoflurane inhalation solution-soaked paper until they fall asleep. Then, inject 10 μL/g combination anesthetic MMB intraperitoneal using a 1-mL syringe with a 27 G x 3/4" needle. After 3-5 min, confirm the anesthesia effectiveness by performing a toe pinch and checking the righting reflex.
2. Assessment of hemorrhage and recovery from BBB breakdown
- Immunoenzymatic staining of a stab-wounded brain section for mouse IgG leakage
NOTE: Here, slide-mounted frozen brain sections were used. Free-floating frozen brain sections are also suitable for this protocol.- Mouse anesthesia: Induce anesthesia by placing the mouse near an isoflurane inhalation solution-soaked paper until it falls asleep. Then, immediately administer 100 μL/kg combination anesthetic MMB intraperitoneally. Confirm the effect of anesthesia with a toe pinch and righting reflex.
- Place the mouse on its back and pin both arms and feet onto the dissection board.
- Mouse thoracotomy: Grasp the abdominal skin using blunt forceps and make a 1.0-1.5 cm incision through the skin and peritoneum to expose the ribcage. Carefully open the ribcage with scissors while avoiding damage to the heart to expose it for cardiac perfusion.
- Cardiac perfusion fixation: Prepare a peristaltic perfusion pump and attach a tube with a 19 G x 1•1/2" needle at one end. Fill the tube with PBS by turning on the pump before use. Then, while the pump is running, insert the needle tip into the left ventricle and secure it with forceps. Make a 0.5-1.0 mm incision in the right atrium. Perfuse the mouse18 at a rate of 4-6 mL/min for 5 min with at least 20 mL of PBS to remove the blood.
- Stop the pump and transfer the tube to a solution of 4% paraformaldehyde (PFA) in PBS. Restart the pump at a rate of 4-6 mL/min for 5 min with at least 20 mL of 4% PFA in PBS for fixation18.
NOTE: If the pump is not available, it is also possible to use a 50 mL syringe with a 19 G x 1•1/2" needle for perfusion. - Brain dissection: Place the mouse on its ventral side on the dissection board and expose the skull by making an incision through the midline of the skin from the occipital area to the nose. Carefully expose the brain completely by removing the skull with scissors, taking care not to damage the brain. Lift the brain with curved forceps and transfer it to a 15-mL tube filled with 10 mL of 4% PFA in PBS.
- Incubate the dissected brain overnight in the 4% PFA in PBS at 4 °C for complete fixation. Following this, incubate the brain overnight in 10 mL of 15% sucrose in PBS and transfer it to 10 mL of 30% sucrose in PBS for an additional day on a seesaw shaker. This gradual sucrose replacement helps to prevent tissue damage from ice when the brain is later stored in a freezer.
- Brain embedding: Embed the brain coronally in an embedding medium within a tissue embedding mold and then freeze using 2-methylbutane with dry ice19.
- Frozen brain sections: Using a cryostat, mount serial coronal sections (20 μm-thick) of the embedded brain on a positive-charged slide glass. The brain sections are air-dried completely at room temperature overnight and stored in a -80 °C freezer.
- Blocking buffer preparation: Mix 10% newborn calf serum, 30 mg/mL bovine albumin, 10 mg/mL glycine, and 0.4% Triton X-100 in TBS to create the blocking buffer. Store at 4 °C for up to 2 weeks prior to use.
- Mouse IgG staining: After the stab-wounded cerebral cortex sections are air-dried at room temperature for 1 h, post-fix them with 500 μL of 4% PFA per slide for 30 min. Then, wash the section with PBS (500 μL/slide) for 5 min, followed by two washes with Tris-buffered saline (TBS, 500 μL/slide) for 5 min each.
NOTE: Perform all procedures in a moisturizing box to prevent the buffers from evaporating. - Quench endogenous enzyme activity in sections with 10% methanol and 3% hydrogen peroxide in TBS for 5 min, followed by two washes with Tris-buffered saline (TBS, 500 μL/slide) for 5 min each.
- Perform blocking using a blocking buffer (500 μL/slide) for 1 h at room temperature to prevent non-specific binding of the antibody. Then, incubate the sections with a biotin-conjugated mouse IgG antibody (1:300 dilution with the blocking buffer, 300 μL/slide) for 1 h at room temperature.
- While incubating with the mouse IgG antibody, prepare the mixture of 1 μL of Reagent A and 1 μL of Reagent B from the avidin/biotin-based peroxidase system kit in 300 μL of the blocking buffer. Incubate this mixture for 30 min at room temperature before use. After the mouse IgG antibody incubation is complete, wash the sections with TBS for 5 min, three times. Then, incubate the sections with the prepared mixture of Reagent A and B (300 μL/slide) for 1 h at room temperature.
- Wash the sections with 0.1 M Tris-HCl pH 8.0 for 5 min, three times, over the course of 1 h. Then, develop color using 0.05% 3,3'-diaminobenzidine (DAB) in 0.05% hydrogen peroxide-added 0.1 M Tris-HCl pH 8.0 (500 μL/slide) for 5 min to 1 h (Figure 2A). Observe under a microscope for color change before stopping the reaction.
- Wash the sections with 0.1 M Tris-HCl pH 8.0 for 5 min three times, and air dry the sections for 15 min. Thereafter, dehydrate the sections by immersing them in 95% ethanol for 2 min twice, followed by 100% ethanol for 2 min, twice. Clear the sections in xylene for 5 min twice. Coverslip the sections using a water-insoluble mounting medium and allow them to dry.
- Analysis of mouse IgG staining intensity: Capture images of the sections using a microscope with a 20x objective.
- Using ImageJ software, quantify the staining intensity by measuring the mean gray values in five randomly selected fields (8 × 8 μm2 for each) from immunoenzymatically stained regions in each section. Normalize the staining intensity using the mean gray value from each contralateral; there is no wounded region as a background correction. Calculate the average of the normalized mean gray values from the five fields to determine the mean gray value of mouse IgG staining for each section.
- Assessment of Evans blue leakage into the brain parenchyma
NOTE: Evans blue protocol is similar to the method previously described11. Fluorescein isothiocyanate (FITC)-labeled dextran also works for recovery from BBB breakdown10.- Evans blue injection: Administer 2% Evans blue in PBS (3 mL/kg) to the stab-wounded mouse through the tail vein 1 h before dissection.
- Fresh brain extraction: 1 h after Evans blue administration, perfuse the mouse with PBS as described in steps 2.1.1-2.1.4. Then, dissect and slice the fresh brain coronally into 1 mm-thick sections using a brain slicer. Select four slices and cut out brain pieces (1 mm2 square, each) that include the stab-wound region or intact regions in the no-wound cerebral cortex, according to the graph paper (Figure 1D).
- Evans blue quantification: Collect the four brain pieces in a 1.5 mL tube and homogenize them with 200 μL of trichloroacetic acid using a tissue grinder. Centrifuge the homogenate at 10,000 x g at 4 °C for 20 min. Then, collect the supernatant with a pipette and dilute it with 600 μL of ethanol. Prepare a standard curve of Evans blue, ranging from 0-1.0 ng/mL, to quantify its concentration. Measure the fluorescence intensity at 680 nm with an excitation wavelength of 620 nm using a plate reader.
3. Assessment of inflammation level in the brain after stab-wound
- Immunoenzymatic staining of stab-wounded brain section for glial cells
- Preparation of the brain sections: Make the brain sections-mounted slide glasses as described in steps 2.1.1-2.1.9.
- Glial staining: After air-drying the stab-wounded cerebral cortex sections for 1 h, fix the sections again with 4% PFA (500 μL/slide) for 30 min. Then, wash the sections with PBS (500 μL/slide) for 5 min, followed by two washes with TBS (500 μL/slide) for 5 min each.
- Quench endogenous enzyme activity in sections with 10% methanol and 3% hydrogen peroxide in TBS for 5 min, followed by two washes with Tris-buffered saline (TBS, 500 μL/slide) for 5 min each.
- Perform blocking using a blocking buffer (500 μL/slide) for 1 h at room temperature to prevent non-specific binding of the antibody.
- Incubate the sections overnight with the primary antibody (300 μL/slide). Use the following dilutions for the primary antibody: 1:3,000 dilution for Iba1, and 1:1,000 dilution for GFAP, diluted in the blocking buffer.
- Wash the section three times with TBS for 5 min each. Then, incubate with the biotin-conjugated secondary antibody (1:300 dilution with the blocking buffer, 300 μL/slide) for 1 h at room temperature.
- While incubating with the secondary antibody, prepare the mixture of 1 μL of Reagent A and 1 μL of Reagent B from the avidin/biotin-based peroxidase system kit in 300 μL of the blocking buffer. Then incubate the mixture at room temperature for 30 min before use.
- After the secondary antibody incubation is complete, wash the sections three times with TBS for 5 min each, and incubate the sections with the prepared mixture of Reagent A and B (300 μL/slide) for 1 h at room temperature.
- Wash the sections with 0.1 M Tris-HCl pH 8.0 for 5 min, three times, over the course of 1 h. Then, develop color using 0.05% DAB in 0.05% hydrogen peroxide-added 0.1 M Tris-HCl pH 8.0 (500 μL/slide) for 30 min to 3 h. Observe under a microscope for color change before stopping the reaction.
- Wash the sections with 0.1 M Tris-HCl pH 8.0 for 5 min three times, and air dry the sections for 15 min. After that, dehydrate the sections by immersing them in 95% ethanol for 2 min twice, followed by 100% ethanol for 2 min, twice. Clear the sections in xylene for 5 min twice. Coverslip the sections using a water-insoluble mounting medium and allow them to dry. Capture the section images using the microscope with an objective of 20x.
- Immunofluorescent staining of stab-wounded brain section for glial cells
- Preparation of the brain sections: Make the brain sections-mounted slide glasses as described in steps 2.1.1-2.1.9.
- Glial staining: After air-drying the stab-wounded cerebral cortex sections for 1 h, fix the sections again with 4% PFA (500 μL/slide) for 30 min. Then, wash the sections with PBS (500 μL/slide) for 5 min, followed by two washes with TBS (500 μL/slide) for 5 min each. Perform blocking using a blocking buffer (500 μL/slide) for 1 h at room temperature to prevent non-specific binding of the antibody.
- Incubate the sections overnight with the primary antibody (300 μL/slide). Use the following dilutions for the primary antibody: 1:3,000 dilution for Iba1, and 1:1,000 dilution for GFAP, diluted in blocking buffer.
- Wash the section three times with TBS for 5 min each. Then, incubate with the fluorescent-conjugated secondary antibody (1:300 dilution with the blocking buffer, 300 μL/slide) for 1 h at room temperature.
- Wash the sections three times with TBS for 5 min each and incubate the sections with 0.4 μg/mL DAPI solution in TBS (500 μL/slide) for 5 min at room temperature.
- Wash the sections with TBS (500 μL/slide) for 5 min, and with distilled water (500 μL/slide) for 5 min. After air drying the sections for 15 min, coverslip them with a water-based mounting medium and allow them to dry. Capture the section images using a confocal microscope with a 20x objective.
- Real-time qPCR for glial cell markers and inflammatory cytokines
NOTE: The primer sequences16,20,21,22 used in the presented protocol are listed in Table 1.- Fresh brain tissue preparation: Perfuse the mouse with PBS as described in steps 2.1.1-2.1.4. Then, extract and slice the fresh brain coronally into 1 mm-thick sections using a brain slicer. Select four slices and cut out brain pieces (1 mm2 square, each) that include the stab-wound region or intact regions on the contralateral side of the cerebral cortex, according to the graph paper. Determine the region to cut out, which includes the lesion at the center (Figure 1D).
- RNA extraction: Collect the four brain pieces in a 1.5 mL tube and homogenize them with 1 mL of TRIzol reagent by pipetting. Incubate the mixture for 5 min at room temperature. Then, add 200 μL of chloroform, vortex well, and incubate for an additional 2 min at room temperature. Centrifuge the mixture at 12,000 x g for 15 min at 4 °C. Carefully transfer the upper, clear layer into a new 1.5 mL tube, avoiding any white precipitates, which contain DNA and proteins.
- Add 500 μL of isopropanol to the collected upper layer, mix well, and incubate for 10 min at room temperature. After centrifugation at 12,000 x g for 10 min at 4 °C, remove the supernatant and add 1 mL of 70% ethanol to wash the RNA pellet. Centrifuge at 7,500 x g for 5 min at 4 °C, remove the supernatant completely, and air dry the pellet for 10 min. Dissolve the RNA pellet in 15 μL of RNase-free water and denature the RNA for 10 min at 55°C.
- Measure the RNA concentration using a microvolume spectrophotometer. Store the RNA at -80 °C immediately.
- cDNA synthesis: For cDNA synthesis, use a commercially available DNA synthesis kit. Prepare a total of 10 μL of Reaction buffer in a 1.5 μL tube for each sample, which includes 2 μL of 5 x RT Buffer, 0.5 μL of RT Enzyme Mix, 0.5 μL of Primer Mix, 1 μg of RNA, and nuclease-free water. Incubate at 37 °C for 15 min for the reverse transcription reaction and at 98°C for 5 min for the enzyme inactivation reaction. Then, add 90 μL of nuclease-free water into the buffer and mix well. Store the cDNA solution at -20°C.
- Quantification of RNA expression: For real-time qPCR analysis, use a commercially available qPCR master mix kit. Prepare the Mix solution for each sample by combining 5 μL of KOD SYBR qPCR Mix, 0.4 μL of 5 μM forward primer, 0.4 μL of 5 μM reverse primer, and 0.2 μL of 50 x ROX in 2 μL of nuclease-free water.
- Make a mixture of 2 μL of cDNA solution and 8 μL of Mix solution in an 8-strip tube, mix well, and spin down.
- Initiate the PCR reaction with the following cycling conditions: 98 °C for 2 min, followed by 40 cycles of 98°C for 10 s, 60°C for 10 s, and 68°C for 30 s. Perform the Melting/Dissociation Curve Analysis step using a real-time qPCR machine.
- mRNA expression analysis: Analyze mRNA expression level using the 2-Delta-Delta-Ct method, which is performed by normalization with a housekeeping gene, Gapdh, then with that of the contralateral side23.
Results
To analyze recovery from BBB breakdown, hemorrhage level in the stab-wounded cerebral cortices was assessed by measuring the extravasation level of serum IgG at 1, 3, 5, and 7 days after brain injury. The mouse IgG staining images revealed blood leakage and deposition in the cerebral cortices following brain injury. This was reduced after more than 7 days, as the BBB recovered, and the IgG protein degraded (Figure 2B). IgG extravasation levels were quantified by measuring the intensity of IgG staining around the stab-wound area, which indicated that IgG extravasation peaked 1 day after the brain injury (Figure 2C). In addition to confirming recovery from BBB breakdown, Evans blue leakage into the brain parenchyma was quantified after stab-wounding. In the intact brain, the BBB prevents Evans blue from leaking from the blood vessels into the brain. However, after brain injury, BBB breakdown led to immediate Evans blue leakage around the wound site (Figure 2D). The quantification of Evans blue concentration, based on the fluorescence intensity, showed an exponential increase within 1 h after the stab-wound, followed by a decrease at 1 and 3 days post-injury (Figure 2E). Consistent with these data, it has been reported that the mRNA expression of BBB integrity and tight junction-related factors, such as alkaline phosphatase and claudin-5, is decreased 1 and 3 days after the injury17.
Immunoenzymatic and immunofluorescence staining for the microglial marker Iba1 and the astrocyte marker GFAP in the cerebral cortices at 1, 3, 5, and 7 days after the stab-wound revealed an accumulation of Iba1+ microglia and GFAP+ astrocytes around the lesion site. The peak accumulation of Iba1+ microglia occurred approximately 5 days post-injury, while GFAP+ astrocytes peaked approximately 3 days after the injury (Figure 3A,B). Consistent with the staining results, the mRNA expression levels of Iba1 and Gfap were also increased after the injury, with improvements observed after the 7th day (Figure 3C,D). Additionally, the stab-wound increased the mRNA expression levels of inflammatory cytokines, including tumor necrosis factor-α (Tnf-a), transforming growth factor-β1 (Tgf-b1), interleukin-6 (Il-6), and interleukin-1β (Il-1b) in the stab-wound regions of the cerebral cortices (Figure 3E-H). These increases in cytokine expression at 1 day for Tnf-a, Il-6, and Il-1b and at 3 days for Tgf-b1 after the stab-wound were concurrent with the rise in the astrocyte and microglial mRNA levels. Since the timing of glial activation and inflammatory cytokine expression overlapped, it is likely that activated astrocytes and microglia are the sources of these inflammatory cytokines. Additionally, immune cells that leaked into the brain due to the stab-wound may also contribute to the production of inflammatory cytokines alongside the glial cells24.
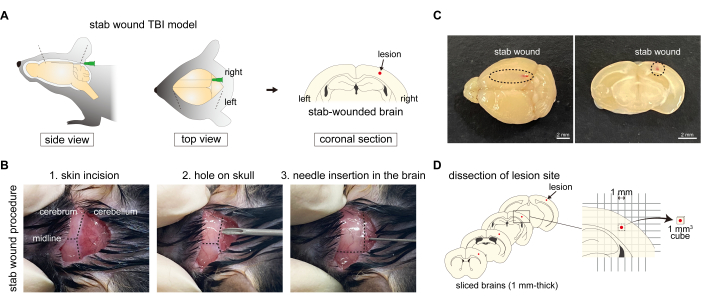
Figure 1: Procedure of generation of the stab-wound TBI mouse model. (A) Schematic diagram of the stab-wound TBI mouse model, showing the procedure of puncturing the right hemisphere of the cerebral cortex with needles, resulting in skull damage. (B) Overview of the stab-wound TBI protocol: (1) making a skin incision on the back of the head; (2) making a hole in the right hemisphere of the occipital bone using a needle; and (3) inserting a needle into the cerebral cortex of the right hemisphere through the hole in the occipital bone. (C) Macroscopic images of the stab-wound to the cerebral cortex from the caudal to the rostral axis. Scale bars: 2 mm. (D) Schematic diagram of lesion site dissection using the stab-wound TBI mouse model. Please click here to view a larger version of this figure.
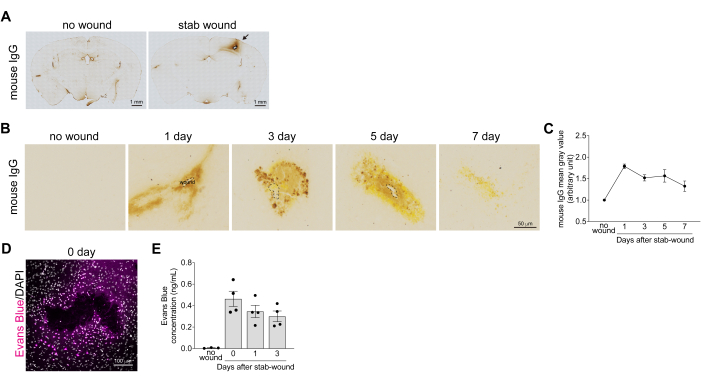
Figure 2: Quantification of the recovery from the breakdown of the blood-brain barrier in stab-wounded cortices. (A) Bright-field images of whole stab-wounded brain sections immediately after the injury were immunoenzymatically stained with an antibody against mouse IgG. No wound cortex is used as a negative control mouse. The leaked and deposited mouse IgG, which is abundant in the blood, is colored brown. Scale bars: 1 mm. (B) Bright-field images of the stab-wounded cerebral cortices at 1, 3, 5, and 7 days after the brain injury immunoenzymatically stained with an antibody against mouse IgG. No wound cortex is used as a negative control mouse. Scale bar: 50 µm. (C) The graph shows the reference example for the mean gray values of mouse IgG staining. All values were normalized with the mean gray value of the uninjured contralateral hemisphere. Data are presented as mean ± standard error of the mean, n > 3. (D) Confocal images of the stab-wounded cerebral cortex 1 h after the brain injury, after the mouse was injected with Evans blue solution. Images were acquired using a confocal microscope at a wavelength of 633 nm. Evans blue (magenta) and DAPI (gray). Scale bar: 100 µm. (E) The graph shows a reference example for the Evans blue concentration change in pieces of stab-wounded cerebral cortices. Data are presented as mean ± standard error of the mean, n > 3. Dots indicate the results from each tissue. Please click here to view a larger version of this figure.

Figure 3: Quantification of glial inflammation in stab-wounded cortices. (A,B) Images showing the stab-wounded cerebral cortices at 1, 3, 5, and 7 days after the brain injury after enzymic immunoenzymatically staining (upper, scale bar: 100 µm) and immunofluorescent staining (lower, scale bar: 40 µm) with antibodies against Iba1 as the microglial marker (A) and GFAP as the astrocyte marker (B). No wound cortex was used as the negative control. (C,D) The graphs show the reference examples for real-time qPCR analyses of Iba1 (C) and Gfap (D) mRNA expression changes after a stab-wound in the cerebral cortices. Iba1 and Gfap mRNA levels were normalized with that of Gapdh. The expression level in mice with no wound was considered as 1, and all other values were normalized with this value. Data are presented as mean ± standard error of the mean, n > 5. One-way ANOVA was used for statistical analysis. Dots indicate the results from each tissue. (E-H) The graphs show reference examples for real-time qPCR analyses of Tnf-a (E), Tgf-b1 (F), Il-6 (G), and Il-1b (H) mRNA expression changes after stab-wounds in the cerebral cortices. Each mRNA level was normalized with that of Gapdh. The expression level in mice with no wound was considered as 1, and all other values were normalized with this value. Data are presented as mean ± standard error of the mean, n > 5. One-way ANOVA was used for statistical analysis. Dots indicate the results from each tissue. Please click here to view a larger version of this figure.
| Gene name | Primer Sequence | ||
| Iba1 | Forward | 5′-GGATTTGCAGGGAGGAAAAG-3′ | |
| Reverse | 5′-TGGGATCATCGAGGAATTG-3′ | ||
| Gfap | Forward | 5′-GAAGGTCCGCTTCCTGGAA-3′ | |
| Reverse | 5′-GGCTCGAAGCTGGTTCAGTT-3′ | ||
| Tnf-a | Forward | 5′-ACAGAAAGCATGATCCGCG-3′ | |
| Reverse | 5′-GCCCCCCATCTTTTGGG-3′ | ||
| Tgf-b1 | Forward | 5′-CCCTATATTTGGAGCCTGGA-3′ | |
| Reverse | 5′-CTTGCGACCCACGTAGTAGA-3′ | ||
| Il-6 | Forward | 5′-CTGCAAGAGACTTCCATCCAGTT-3′ | |
| Reverse | 5′-GAAGTAGGGAAGGCCGTGG-3′ | ||
| Il-1b | Forward | 5′-GCACACCCACCCTGCAG-3′ | |
| Reverse | 5′-AACCGCTTTTCCATCTTCTTCTT-3′ | ||
| Gapdh | Forward | 5′-CGTGTTCTACCCCCAATGT-3′ | |
| Reverse | 5′-TGTCATCATACTTGGCAGGTTTCT-3′ | ||
Table 1: Primer sequences used for real-time qPCR analysis using the stab-wound TBI mouse model
Discussion
Here, a protocol for creating a TBI mouse model using needles was introduced. This protocol allows for a quantitative assessment of recovery from the breakdown of the BBB and inflammation after brain injury using histological and molecular biological approaches. Alternative protocols, such as the repetitive concussive TBI model and weight-drop TBI model, can also be used to analyze BBB breakdown and inflammation. These models replicate TBI pathology under controlled conditions, including specific parameters for impact strength, range, and brain region, using specialized machines. Additionally, they can cause a closed brain injury with an intact skull. Conversely, the proposed stab-wound TBI model mimics both skull and brain damage-induced penetrating brain injury. Furthermore, because the presented protocol can induce brain damage within a narrow range, it is important to clearly define the observation area for analyzing BBB breakdown and inflammation. This is the novelty of this protocol, which was made possible by using a thinner needle than usual25,26. Another unique feature is that the TBI mouse model can be easily created easily without the equipment of stereo taxic coordinate27.
TBI symptoms are classified as mild, moderate, or severe based on the level of consciousness impairment according to the Glasgow Coma Scale (GCS)28. The stab-wound TBI model is particularly effective in replicating symptoms corresponding to mild (GCS 13-15) to moderate (GCS 9-12) TBI and is especially suited for evaluating BBB breakdown and inflammation. However, the stab-wound TBI model technique may have some limitations, including a lower severity of brain damage, as it does not induce behavioral change after the stab-wound. We did not perform detailed behavioral tests using the stab-wound TBI model, but there were no visibly significant defects in motor ability or social behavior after wakening from anesthesia. Therefore, other TBI models are more suitable for tracking the behavioral changes after brain injury. However, the stab-wound TBI model might still reveal learning and memory dysfunction after a detailed analysis. For example, a repetitive concussive TBI mouse model exhibited behavioral impairments in the Morris water maze and social recognition test2,29,30. The weight-drop TBI mouse model also showed reduced learning and memory ability, despite having no effect on motor skills in the Morris water maze31,32. Therefore, TBI models that induce more severe damage are more suitable for behavioral analysis. Additionally, the stab-wound TBI model can exhibit significant variability in the damage conditions due to differences in the insertion position, angle, and depth of the needle inserted into the brain, which depend on the handing technique. However, it has already been demonstrated that the stab-wound TBI model protocol can provide reliable data for hemorrhage and inflammation analyses by increasing the number of replicates. Further refinement of the stab-wound protocol to reduce variability and improve reproducibility could enhance the model's reliability. This includes standardizing the technique and minimizing the risk of bleeding.
The protocol presented here facilitates the creation of a TBI mouse model, but there are important considerations for the stab-wound procedure. Occasionally, mice may bleed to death following the procedure. To minimize this risk, it is crucial to make the skin incision to expose the occipital bone as small as possible, avoiding damage to nearby blood vessels around the ears. Additionally, the size of the hole in the occipital bone should be just large enough to accommodate a 27 G x 3/4" needle.
During immunohistochemistry, non-specific binding is likely to occur around the stab-wound region because the antibody is easily captured by leaked blood components. Therefore, it is necessary to assess non-specific binding by staining with only the secondary antibodies in addition to the usual staining.
The stab-wound TBI model is useful for clarifying coagulation mechanisms in the brain, as it enables easy identification of the bleeding site. This makes it an appropriate model for analyzing the coagulation regulators. Minimizing hemorrhage extension, supported by immediate activation of the coagulation and fibrinolysis system, leads to the attenuation of inflammation after brain injury10,33. The presented stab-wound TBI protocol can be used to analyze platelet activation and fibrin formation in the lesion site10,33. Thereby, demonstrating the importance of rapid blood coagulant treatment after TBI. Additionally, it can be used to assess secondary injury mitigation by regulating hemorrhage after TBI.
Cerebral edema was not observed using the stab-wound TBI model. TBI induces vasogenic brain edema, which is caused by the physical damage-induced excess fluid accumulation in the extravascular and extracellular space, as well as cytotoxic brain edema, which results from intracellular fluid accumulation34. The stab-wound TBI model might be used to observe cerebral edema, including vasogenic and cytotoxic edema, since the stab-wound damages both the blood vessels and cells in the model.
It is possible to analyze neuronal apoptosis after brain injury using the stab-wound TBI model. A previous study indicated that neuronal apoptosis detected by cleaved caspase 3 is evident immediately and is limited to the brain area around the injury site, and peaking at 3 days after the brain injury. Furthermore, neuronal apoptosis detected by TUNEL assay showed a similar time point change as the cleaved caspase 3 analysis14.
It is advisable to use multiple TBI models for developing therapeutic approaches, as TBI can occur under various conditions and circumstances. This study demonstrated that the presented protocol is sufficient to quantify pattern changes in time-series data for glial activation, inflammatory cytokine expression, and neuronal loss after a stab-wound12,14. In addition, it can clearly demonstrate the time series change in the coagulation process in stab-wound TBI10,33. The application of the presented method will facilitate the examination of blood coagulants and anti-inflammatory compounds for the development of therapeutic agents for TBI. Additionally, the presented stab-wound TBI model allows for observing the activation of astrocytes and microglia immediately after the brain injury, with their subsequent quiescence occurring within 7 days in the cortex. Additionally, the inflammatory response and glial activation are both attenuated during glial quiescence. Furthermore, BBB breakdown is repaired within a period similar to the recovery of glial activation recovery17. These phenotypes indicated that the stab-wound TBI model reflects the acute phase of TBI rather than the chronic phase. Therefore, the presented stab-wound TBI model is a valuable approach for developing therapeutic methods for TBI, aiming to prevent the transition from the acute to the chronic phase.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Ayana Hamano, Minori Yamashita, Misaki Endo, Hirono Kobayashi, and Nito Nakahira for helping with the immunohistochemistry and real-time qPCR. This work was supported by the JSPS KAKENHI 19K16122, Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders, The Mitsubishi Foundation, Brain Science Foundation, and The Uehara Memorial Foundation to K.H.
Materials
| Name | Company | Catalog Number | Comments |
| 19 G x 1•1/2" needle | TERUMO | NN-1938R | |
| 27 G x 3/4" needle | TERUMO | NN-2719S | |
| anti-GFAP antibody | Sigma-Aldrich | G9269 | |
| anti-Iba1 antibody | Wako | 019-19741 | |
| Atipamezole Hydrochloride | Nippon Zenyaku Kogyo | Product name: Antisedan | |
| Biotin-conjugated mouse IgG antibody | Vector Laboratories | BA-9200 | |
| Biotin-conjugated rabbit IgG antibody | Vector Laboratories | BA-1000 | |
| Bovine albumin | Nacalai tesque | 01860-07 | |
| Brain Slicer | Visikol | BSLM-2 | |
| Butorphanol Tartrate | Meiji Animal Health | Product name: Vetorphale 5 mg | |
| Confocal microscope | Zeiss | LSM700 | |
| Cryostat | Leica | CM1520 | |
| DAB | Sigma-Aldrich | D5637-1G | |
| DAPI | Roche | 10236276001 | |
| Evans blue | Wako | 056-04061 | |
| Fluorescent-conjugated rabbit IgG antibody | Invitrogen | A-21206 | |
| Fluoromount-G | Invitrogen | 4958-02 | Water-based mounting medium |
| Isoflurane Inhalation Solution | Viatris | v002139 | |
| KOD SYBR qPCR Mix | TOYOBO | QKD-201 | qPCR master mix kit |
| Medetomidine | Nippon Zenyaku Kogyo | Product name: Domitor | |
| Microscope | Olympus | FSX100 | |
| Microvolume spectrophotometer | ThermoFisher Scientific | NanoDrop One | |
| Midazolam 10 mg/2 mL | Sandoz | 1124401A1060 | |
| MOUNT QUICK | Daido Sangyo | DM01 | Water insoluble mounting medium |
| Newborn calf serum | Gibco | 16010159 | |
| O.C.T. compound | Sakura Finetek Japan | 45833 | Embedding medium |
| Peel-A-Way, Truncated 22 mm Square Top | Ted Pella | 27118 | Tissue embedding mold |
| Peristaltic perfusion pump | ATTO | SJ-1211 | |
| Plate reader | Fisher Scientific | Cytation 3 | |
| Real-time qPCR machine | ThermoFisher Scientific | StepOne Plus | |
| ReverTra Ace qPCR RT Kit | TOYOBO | FSQ-101 | cDNA synthesis kit |
| Superfrost Plus Slide Glass | Fisher Scientific | 12-550-15 | Positive-charged slide glass |
| Suture with needle | Alfresa | HT2003NA75-KF2 | |
| TRIzol Reagent | Invitrogen | 15596026 | |
| VECTASTAIN ABC Standard Kit | Vector Laboratories | PK-4000 | Avidin/biotin-based peroxidase system kit |
References
- Narayan, R. K., et al. Clinical trials in head injury. J Neurotrauma. 19 (5), 503-557 (2002).
- Shitaka, Y., et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 70 (7), 551-567 (2011).
- Flierl, M. A., et al. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 4 (9), 1328-1337 (2009).
- Ikeshima-Kataoka, H., Shen, J. S., Eto, Y., Saito, S., Yuasa, S. Alteration of inflammatory cytokine production in the injured central nervous system of tenascin-deficient mice. In Vivo. 22 (4), 409-413 (2008).
- Zhou, Y., Wang, Y., Wang, J., Anne Stetler, R., Yang, Q. W. Inflammation in intracerebral hemorrhage: From mechanisms to clinical translation. Prog Neurobiol. 115, 25-44 (2014).
- Hijazi, N., et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 125 (16), 2558-2567 (2015).
- Kataoka, K., et al. Roles of urokinase-type plasminogen activator in a brain stab-wound. Brain Res. 887 (1), 187-190 (2000).
- Wang, Y., et al. Early posttraumatic csf1r inhibition via plx3397 leads to time- and sex-dependent effects on inflammation and neuronal maintenance after traumatic brain injury in mice. Brain Behav Immun. 106, 49-66 (2022).
- Kaplan, L., Chow, B. W., Gu, C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci. 21 (8), 416-432 (2020).
- Endo, M., et al. 2-carba cyclic phosphatidic acid regulates blood coagulation and fibrinolysis system for repair after brain injury. Brain Res. 1818, 148511 (2023).
- Goldim, M. P. S., Della Giustina, A., Petronilho, F. Using Evans blue dye to determine blood-brain barrier integrity in rodents. Curr Protoc Immunol. 126 (1), e83 (2019).
- Hashimoto, K., et al. 2-carba cyclic phosphatidic acid suppresses inflammation via regulation of microglial polarization in the stab-wounded mouse cerebral cortex. Sci Rep. 8 (1), 9715 (2018).
- Ikeshima-Kataoka, H., Abe, Y., Yasui, M. Aquaporin 4-dependent expression of glial fibrillary acidic protein and tenascin-c in activated astrocytes in stab-wound mouse brain and in primary culture. J Neurosci Res. 93 (1), 121-129 (2015).
- Nakashima, M., et al. The neuroprotective function of 2-carba-cyclic phosphatidic acid: Implications for tenascin-c via astrocytes in traumatic brain injury. J Neuroimmunol. 361, 577749 (2021).
- Kawai, S., Takagi, Y., Kaneko, S., Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 60 (5), 481-487 (2011).
- Ikeshima-Kataoka, H., Abe, Y., Abe, T., Yasui, M. Immunological function of aquaporin-4 in stab-wounded mouse brain in concert with a pro-inflammatory cytokine inducer, osteopontin. Mol Cell Neurosci. 56, 65-75 (2013).
- Ikeshima-Kataoka, H., Yasui, M. Correlation between astrocyte activity and recovery from blood-brain barrier breakdown caused by brain injury. Neuroreport. 27 (12), 894-900 (2016).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. J Vis Exp. (65), e3564 (2012).
- Botta, S., Chemiakine, A., Gennarino, V. A. Dual antibody strategy for high-resolution imaging of murine Purkinje cells and their dendrites across multiple layers. STAR Protoc. 3 (2), 101427 (2022).
- Amin, D. N., et al. Identification of stage biomarkers for human African trypanosomiasis. Am J Trop Med Hyg. 82 (6), 983-990 (2010).
- Jiang, J., et al. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis. 76, 126-136 (2015).
- Wei, J., et al. Microglia activation: One of the checkpoints in the CNS inflammation caused by angiostrongylus cantonensis infection in rodent model. Parasitol Res. 114 (9), 3247-3254 (2015).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 25 (4), 402-408 (2001).
- Hazy, A., et al. Divergent age-dependent peripheral immune transcriptomic profile following traumatic brain injury. Sci Rep. 9 (1), 8564 (2019).
- Xia, Y., et al. Osthole confers neuroprotection against cortical stab-wound injury and attenuates secondary brain injury. J Neuroinflammation. 12, 155 (2015).
- Cieri, M. B., Villarreal, A., Gomez-Cuautle, D. D., Mailing, I., Ramos, A. J. Progression of reactive gliosis and astroglial phenotypic changes following stab-wound-induced traumatic brain injury in mice. J Neurochem. 167 (2), 183-203 (2023).
- Barreda-Manso, M. A., Yanguas-Casas, N., Nieto-Sampedro, M., Romero-Ramirez, L. Neuroprotection and blood-brain barrier restoration by salubrinal after a cortical stab injury. J Cell Physiol. 232 (6), 1501-1510 (2017).
- Rapp, P. E., et al. Patient characterization protocols for psychophysiological studies of traumatic brain injury and post-TBI psychiatric disorders. Front Neurol. 4, 91 (2013).
- Brody, D. L., Benetatos, J., Bennett, R. E., Klemenhagen, K. C., Mac Donald, C. L. The pathophysiology of repetitive concussive traumatic brain injury in experimental models; new developments and open questions. Mol Cell Neurosci. 66 (Pt B), 91-98 (2015).
- Meehan, W. P., Zhang, J., Mannix, R., Whalen, M. J. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery. 71 (4), 885-891 (2012).
- Chen, C., et al. A novel simple traumatic brain injury mouse model. Chin Neurosurg J. 8 (1), 8 (2022).
- Machado, C. A., et al. Weight-drop model as a valuable tool to study potential neurobiological processes underlying behavioral and cognitive changes secondary to mild traumatic brain injury. J Neuroimmunol. 385, 578242 (2023).
- Hashimoto, K., Ikeda, N., Nakashima, M., Ikeshima-Kataoka, H., Miyamoto, Y. Vitronectin regulates the fibrinolytic system during the repair of cerebral cortex in stab-wounded mice. J Neurotrauma. 34 (22), 3183-3191 (2017).
- Unterberg, A. W., Stover, J., Kress, B., Kiening, K. L. Edema and brain trauma. Neuroscience. 129 (4), 1021-1029 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved