Ozonolysis of Alkenes
Przegląd
Source: Vy M. Dong and Zhiwei Chen, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate an example of an ozonolysis reaction to synthesize vanillin from isoeugenol (Figure 1). Ozonolysis of alkenes, an oxidation reaction between ozone and an alkene, is a common method to prepare aldehydes, ketones, and carboxylic acids. This experiment also demonstrates the use of an ozone generator and a low temperature (−78 °C) reaction.

Figure 1. Diagram showing the ozonolysis of isoeugenol to vanillin.
Zasady
The oxidative cleavage of alkenes to two carbonyl-group-containing compounds is called an ozonolysis reaction (Figure 2). The proposed mechanism (Figure 3) begins with a [3+2] cycloaddition between alkene 1 with ozone to generate the molozonide intermediate A. A is unstable and rearranges into the more stable ozonide C via the zwitterion B. C decomposes in the presence of a reductant such as dimethyl sulfide to furnish the two carbonyl products (2, 3) and dimethyl sulfoxide. When a nucleophilic solvent is used (e.g., methanol), the nucleophile attacks intermediate B to form a hydroperoxide E, which decomposes to the product 3 when dimethyl sulfide is added (Figure 4). The reaction is typically performed at −78 °C to prevent side reactions and in the presence of an indicator to determine when the reaction is complete. Sudan III is a commonly used indicator. Initially, the reaction mixture is red and turns to purple/blue when all of the alkene is consumed. When all of the alkene has reacted, the indicator, which has a N-N double bond, reacts with the ozone thereby giving the color change.

Figure 2. Diagram showing the general ozonolysis reaction of an alkene with a reductive workup.
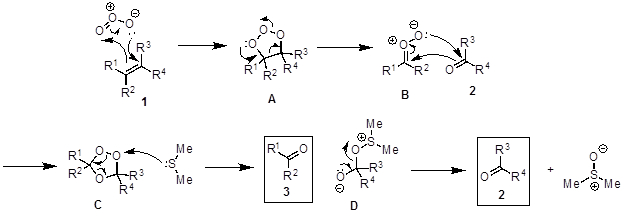
Figure 3. Diagram showing the general mechanism of alkene ozonolysis.

Figure 4. Diagram showing the formation of a hydroperoxide from intermediate B.
Procedura
- Add 200 mg of isoeugenol, 15 mL of MeOH, and ~ 2 mg of Sudan III to a 3-necked 50- mL round bottom flask equipped with a magnetic stir bar.
- Connect the reaction flask to an oxygen tank and a bubbler.
- Turn on the flow of oxygen.
- Cool the reaction mixture with a dry ice/acetone bath.
- Switch on the ozone generator, which converts the oxygen from the tank to ozone that goes into the reaction flask. The generator will be between the oxygen tank and the reaction flask. Allow the reaction mixture to stir until the red color changes to purple/blue.
- Turn off the ozone generator, and allow oxygen to purge the reaction mixture of ozone for 5 min.
- Remove the cooling bath and add 0.2 mL of dimethyl sulfide.
- Stir the reaction mixture while warming to room temperature for 1 h.
- Remove the solvent by rotary evaporation. Make a silica plug by placing silica gel into a Büchner funnel. Dissolve the residue in 10% ethyl acetate in hexanes and pass the solution through the silica plug under vacuum to remove impurities. Wash the silica plug 2 more times with 10% ethyl acetate in hexanes. Collect the filtrate and remove the solvent by rotary evaporation to obtain vanillin as a white solid.
- Calculate the percent yield of vanillin obtained and establish its purity and identity by melting point (m.p.) and 1H NMR.
Wyniki
Vanillin was obtained as a white solid (150 mg, 76% yield); m.p. 76-79 °C; 1H NMR (400 MHz, CDCl3) δ 9.82 (br s, 1H), 7.43-7.41 (m, 2H), 7.04 (d, J = 8.8 Hz, 1H), 6.30 (s, 1H), 3.96 (s, 3H).
Wniosek i Podsumowanie
In this experiment, we have demonstrated the synthesis of vanillin from isoeugenol using the ozonolysis reaction. Also, using an ozone generator while performing a low temperature reaction was shown.
Ozonolysis is a useful reaction to prepare aldehydes, ketones, and carboxylic acids from alkenes. It has been applied in natural product synthesis and industrial-scale preparation of pharmaceuticals. Artemisinin is a potent antimalarial agent and was one of the natural products recognized in the 2015 Nobel Prize in Medicine. In a 10-step synthesis from (R)-(+)-pulegone, ozonolysis was used in the last step to make the natural product (Figure 5). Ceftibuten and cefaclor are cephalosporin antibiotics produced on industrial scale. One commercial route uses ozonolysis to access a common key intermediate, which can be elaborated to both compounds (Figure 6).

Figure 5. Diagram showing ozonolysis as the last step in a synthesis of artemisinin.
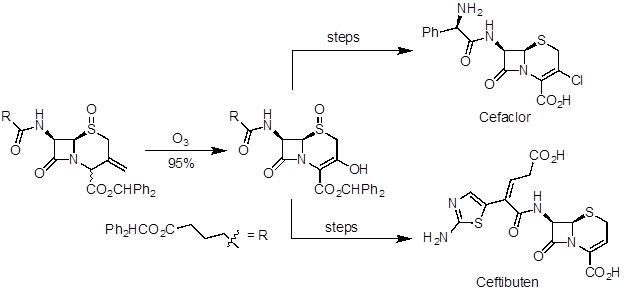
Figure 6. Diagram showing ozonolysis to prepare a key intermediate in the divergent synthesis of cefaclor and ceftibuten.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Ozonolysis of Alkenes
Organic Chemistry II
66.9K Wyświetleń

Cleaning Glassware
Organic Chemistry II
123.4K Wyświetleń

Nucleophilic Substitution
Organic Chemistry II
99.4K Wyświetleń

Reducing Agents
Organic Chemistry II
43.0K Wyświetleń

Grignard Reaction
Organic Chemistry II
148.9K Wyświetleń

n-Butyllithium Titration
Organic Chemistry II
47.7K Wyświetleń

Dean-Stark Trap
Organic Chemistry II
100.0K Wyświetleń

Organocatalysis
Organic Chemistry II
16.6K Wyświetleń

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.3K Wyświetleń

Solid Phase Synthesis
Organic Chemistry II
40.9K Wyświetleń

Hydrogenation
Organic Chemistry II
49.5K Wyświetleń

Polymerization
Organic Chemistry II
93.7K Wyświetleń

Melting Point
Organic Chemistry II
149.7K Wyświetleń

Infrared Spectroscopy
Organic Chemistry II
214.4K Wyświetleń

Polarimeter
Organic Chemistry II
99.8K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone