Method Article
5/6 Nephrectomy Using Sharp Bipolectomy Via Midline Laparotomy in Rats
W tym Artykule
Podsumowanie
This manuscript introduces a standardized protocol for a 5/6 nephrectomy by sharp bipolectomy via midline laparotomy in a rat model, aiming to induce a state of renal insufficiency via renal parenchyma reduction with a great degree of methodical precision and low risk of technical error.
Streszczenie
Chronic kidney disease (CKD) affects over 10% of the global population, amounting to more than 800 million individuals worldwide. The advances in the treatment of CKD have had a significant impact on patient outcomes. While in the past, CKD was often considered a death sentence, with many patients succumbing to the complications of end-stage renal disease, it is now increasingly being managed as a chronic condition with the availability of dialysis and kidney transplantation, as well as new pharmaceutical developments such as SGLT2 inhibitors or nonsteroidal mineralocorticoid receptor antagonists.
Yet, there continues to be a growing demand for further exploration into the pathophysiological processes and potential therapeutic interventions. Reliable biological models play a crucial role in facilitating this research. Given the multifaceted nature of renal disease, which encompasses not only cell biology but also vascular microanatomy and endocrine signaling, an appropriate model must possess a level of biological complexity that only an animal model can offer, rendering rodents an obvious choice.
This manuscript, therefore, provides an intricate, systematic protocol for surgically reducing renal parenchyma through midline laparotomy and combined total and partial nephrectomy in rats for survival as well as non-survival applications. It emphasizes the critical role of precise surgical techniques in ensuring consistent and reliable outcomes. Prime examples of potential applications for this model include biomolecular and pharmaceutical studies as well as the development of innovative intraoperative imaging modalities, such as hyperspectral imaging, to objectively visualize and differentiate renal malperfusion.
Wprowadzenie
Chronic kidney disease (CKD) is a progressive condition that affects a significant portion of the global population. It is characterized by the gradual loss of endocrine and filtrative kidney function over time, leading to the accumulation of waste products and fluid in the body and an imbalance in the endocrine system. Recent data suggest that 9.1% to 13.4% of the worldwide population (between 700 million and one billion people) has CKD1. The prevalence of CKD increases with age, affecting around 34% of people aged 65 years or older in the United States, compared to 12% in those aged 45-64 years and 6% in those aged 18-44 years2.
Therefore, CKD is a significant contributor to the global burden of disease and mortality rates. Early detection and management of CKD are crucial in slowing its progression and reducing the risk of complications, such as cardiovascular disease, anemia, and ultimately, end-stage renal disease, which requires dialysis or kidney transplantation for survival3.
Therapeutic interventions for end-stage CKD have undergone a remarkable evolution over the past few decades. Historically, the management of end-stage CKD was limited to supportive care, with dialysis emerging as a life-sustaining modality in the 1960s. Since then, significant advancements have been made in dialysis techniques, including the development of more biocompatible membranes, improved vascular access, and the advent of peritoneal dialysis4. Additionally, kidney transplantation has emerged as the optimal treatment for end-stage CKD, offering improved survival and quality of life compared to dialysis5. However, the shortage of donor organs remains a significant challenge, driving research into novel strategies such as xenotransplantation and regenerative medicine approaches. Furthermore, the management of end-stage CKD-associated complications, such as secondary hyperparathyroidism, has been enhanced by the introduction of calcimimetic agents like etelcalcetide, which effectively modulate parathyroid hormone levels6.
Despite these advancements, the quest for more effective and targeted therapies continues, fueled by ongoing research into the molecular mechanisms underlying end-stage CKD progression and associated comorbidities. Therefore, CKD persists as a significant concern in patient care, prompting a continued need for extensive research into biomedical processes and therapeutic approaches. Robust biological models are essential to facilitate such investigations. Given the multifaceted nature of CKD, which encompasses aspects ranging from cellular biology to interorgan endocrine signaling, vascular functional anatomy, and rheology, an ideal model must possess a level of biological complexity that only a comprehensive model organism can provide. Thus, rodents emerge as the preferred model due to their capacity to encompass these various biological dimensions effectively.
The 5/6 nephrectomy remnant kidney model serves as a common tool in CKD research for rat and murine experiments due to its stable induction of renal insufficiency7,8,9,10,11,12,13,14. This model entails the removal of one entire kidney and 2/3 of the other. The creation of the remnant kidney can be achieved through the surgical resection of renal poles, termed the polectomy model, or by ligating superior and inferior segmental renal arteries, resulting in pole infarction7,15,16,17,18,19,20.
While this 5/6 nephrectomy model with polectomy is an established technique, it has only been introduced as a transparent and comprehensible protocol with a dorsolateral retroperitoneal access21. This access can be advantageous for a unilateral procedure with renal parenchyma reduction on just one side or for a two-stage procedure with a temporal distance of a few days in order to increase postoperative survival of the animal22. However, the utilization of a midline laparotomy approach offers distinct advantages over the conventional laterodorsal retroperitoneal access route.
By employing a single midline abdominal incision, the surgeon gains unimpeded access to the entire abdominal cavity, thereby facilitating a comprehensive exploration and manipulation of the intra-abdominal organs. This expanded surgical field not only streamlines the nephrectomy procedure, but also enables the concurrent execution of additional interventions that may be required for specific experimental protocols, for example, procedures on the ureters, such as ligation, resection, or reconstruction, which may be essential for studying the pathophysiology of obstructive uropathy. Furthermore, this approach permits the simultaneous resection or manipulation of other abdominal organs, such as the liver, spleen, or gastrointestinal tract, thereby expanding the scope of experimental investigations into multi-organ interactions or systemic disease models.
Moreover, the midline laparotomy approach facilitates the construction of an ileum conduit or neobladder, a surgical procedure that involves the creation of a urinary diversion using a segment of the ileum, which is particularly relevant in studies investigating bladder dysfunction or reconstructive urology techniques. This versatility in combining nephrectomy with other surgical interventions within the same operative field not only streamlines experimental protocols but also minimizes cumulative surgical trauma and associated risks to the animal subjects. Therefore, in the case of single-stage bilateral renal surgery or simultaneous additional intraabdominal procedures, the ventral access via midline laparotomy should be the preferred option.
Currently, there is no publication or protocol available describing this surgical strategy. Therefore, with this work, our objective is to present a detailed procedural guide for conducting renal resection and surgical induction of CKD via midline laparotomy in rats, applicable to both survival and non-survival studies. This experimental model creates a regulated environment conducive to investigating the complex dynamics of CKD, mimicking clinically significant scenarios. This protocol was specifically designed to illustrate the surgical technique. The intervention was therefore performed in a non-survival setting on a homogeneous group of 10 male rats. As there was no meaningful reason for the comparison to a baseline or alternative intervention, the inclusion of a control group was not necessary. 5/6 nephrectomy explicitly refers to the extent of surgical parenchyma resection. This certainly translates to a functional reduction in the sense of a reduction of glomerular filtration rate. However, the exact functional degree cannot be predicted but will have to be measured individually for each animal, for example, by using inulin or p-aminohippuric acid clearance23,24 if required.
Protokół
All animal procedures outlined in this document were carried out within accredited facilities and have been granted approval by the institutional animal care and use committee (IACUC) of the Baden-Württemberg Regional Council in Karlsruhe, Germany (35-9185.81/G-62/23). Experimental animals were handled in accordance with institutional protocols and in compliance with German legislation governing animal welfare, as well as adhering to the guidelines set forth by the European Community Council (2010/63/EU) and the ARRIVE guidelines. Male Sprague Dawley rats with an initial weight of 400 g were utilized following a 1-week acclimatization period.
1. Anesthesia and analgesia
- Narcotize the rat model with the pharmaceuticals of choice. To follow this protocol, perform volatile induction of sedation with isoflurane followed by an intraperitoneal injection of 100 mg/kg body weight ketamine for dissociative anesthesia and 4 mg/kg body weight xylazine. Achieve analgesia with subcutaneous injections of 5 mg/kg body weight carprofen.
NOTE: A detailed protocol can be found in the cited literature25. - Ensure adequate analgesic depth by looking for pain reflexes during the toe pinch test with surgical forceps and regularly reassess the depth of anesthesia during the procedure.
- Apply ophthalmic lubricant to the eyes to prevent corneal desiccation.
2. Procedure preparation
- Prepare the operating site with all the materials and instruments required, including polyfilament ligatures, silicone vessel loops, blunt overholt clamps, fine preparation scissors and forceps, as well as hemostatic patches cut into 0.8 x 0.6 cm pieces (Figure 1A-G). Prepare the rodent surgical exposure apparatus, a heating pad, and the surgical preparation hooks as specified in cited literature25.
- Shave the length of the desired access, disinfect the surgical site by three alternating scrubs with 70% ethanol and iodine-based or chlorhexidine-based scrubbing swabs in a circular motion, and achieve proper oxygenation via the inhalation of 100% oxygen using a neonatal face mask (Figure 1H-J). Cover the rest of the body outside the surgical situs with drapes to avoid contamination.
NOTE: The representative animal that was used to obtain figure images was not draped in order to permit better anatomical landmark visualization. - Perform a median mini-laparotomy via an initial median cutaneous incision over the desired abdominal length of ~3 cm and a subsequent slightly smaller incision of the fascia along the linea alba (Figure 1K-M).
- Achieve surgical exposure of the kidney using surgical compresses and surgical preparation hooks (Figure 1N-O). Only touch the renal parenchyma with atraumatic preparation instruments such as a moistened cotton swab or a moistened compress using forceps or blunt overholt clamps.

Figure 1: Experimental instruments, materials, and setup. (A) Surgical instruments required; (B) polyfilament ligature; (C) silicone vessel loop; (D) fine preparation scissors. (E-G) Hemostatic patch cut into 0.8 x 0.6 cm pieces. (H-J) Rat model shaven and oxygenated with face mask. The representative animal that was used to obtain figure images was not draped in order to permit better anatomical landmark visualization. (K,L) Median cutaneous incision over the desired abdominal length of ~3 cm. (M) Median mini-laparotomy; (N) exposure of the left kidney using a surgical compress, surgical preparation hooks, and a metal stand; (O) analogous exposure of the right kidney and resection of the Gerota's fascia. Please click here to view a larger version of this figure.
3. Partial nephrectomy
- Expose the respective kidney, grab the perirenal fat that is attached to the thin Gerota's fascia, and apply some tension to locally lift the fascia off the renal parenchyma (Figure 2A,B.2).
- Incise and undermine the fascia with the sharp end of one scissor's edge and continue with a longitudinal dissection of the Gerota's fascia (Figure 2B).
- Perform a blunt degloving of the Gerota's fascia using closed scissors by gradually undermining the fascia all around the parenchyma and folding the fascial capsule medially-optionally partially resecting it (Figure 2C).
- Sling the renal hilum using a silicone vessel loop for improved vascular control (Figure 2D).
- Place the tip of the forceps into the retroperitoneal space to stabilize the kidney to avoid a dorsal escape of the kidney during the cutting process and perform the cranial sharp 1/3 renal polectomy in one precise aimed stroke using scissors (Figure 2E).
NOTE: The dissection line should be chosen by clinical evaluation so that it separates the organ in 1/3 of organ height cranially and caudally. - Achieve hemostasis by applying a hemostatic patch, by manual compression, by compression using blunt instruments, or by hilar tension via the silicone vessel loop, effectively reducing hilar blood flow (Figure 2F-J).
NOTE: Hemostasis was regularly achieved by simultaneous pulling of the vascular loop and the application of the hemostatic patch for 2.5-3 min. - Follow with a caudal sharp 1/3 renal polectomy in an analogous fashion (Figure 2K-N). For both scissor strokes, aim for a slightly angulated dissection plane that will leave more renal parenchyma at the hilar side and less tissue on the lateral side to avoid unintended hilar injury and reduce urinary leakage from the pelvicocaliceal system (Figure 2O). If a higher standardization of precise 5/6 parenchyma resection is desired, weigh the resectate of the total nephrectomy on the contralateral side described further below, weigh the resectates of the partial 2/3 nephrectomy, and repeat the dissection in a "salami-slicing" technique until exactly 2/3 of weight are obtained.

Figure 2: Partial nephrectomy. (A) Surgical exposure of one kidney. (B) Longitudinal incision of the Gerota's fascia using sharp scissors. (C) Blunt degloving of the Gerota's fascia using closed scissors. (D) Slinging the renal hilum using a silicone vessel loop. (E) Cranial sharp 1/3 polectomy using scissors and forceps as guidance. (F) Achieving hemostasis by applying an hemostatic patch; (G) achieving hemostasis by manual compression; (H-J) achieving hemostasis by compression using blunt instruments and hilar tension via the silicone vessel loop. (K-N) Caudal sharp 1/3 polectomy in analogy. (O) Schematic depiction of recommended dissection planes to avoid unintended hilar injury (black lines). Please click here to view a larger version of this figure.
4. Total nephrectomy
- Mobilize the kidney in analogy to the steps described above and tunnel the renal hilum using blunt overholt clamps (Figure 3A,B).
- Sling the renal hilum using a polyfilament ligature and place a secure sliding knot on the renal hilum rather closer to the abdominal vessels to occlude the renal blood flow and ureter (Figure 3C,D).
- Sharply dissect the hilum using scissors and remove the kidney (Figure 3E,F).
- Control for hemostasis and cut off the ligature ends (Figure 3G-J).

Figure 3: Total nephrectomy. (A) Surgical exposure of the contralateral kidney; (B) analogous removal of the Gerota's fascia and tunneling of the renal hilum using blunt overholt clamps; (C) slinging the hilum using a polyfilament ligature. (D) Placement of a sliding knot ligature on the renal hilum; (E,F) sharp dissection of the hilum using scissors and removal of the kidney; (G-I) control for hemostasis and cutting of the ligature ends. (J) Schematic depiction of recommended ligature height (dashed line) and dissection plane (black line). Please click here to view a larger version of this figure.
5. Abdominal wall closure
- Place a corner suture on the abdominal fascia using a polyfilament suture (Figure 4A-D).
- Continue suturing the abdominal fascia with a running suture with ~2 mm of tissue grabbed with each bite and about 4 mm between the bites (Figure 4E-I).
- Suture the cutaneous layer using single stitches with ~3 mm of tissue grabbed with each bite and ~6 mm between the bites (Figure 4J-Q).
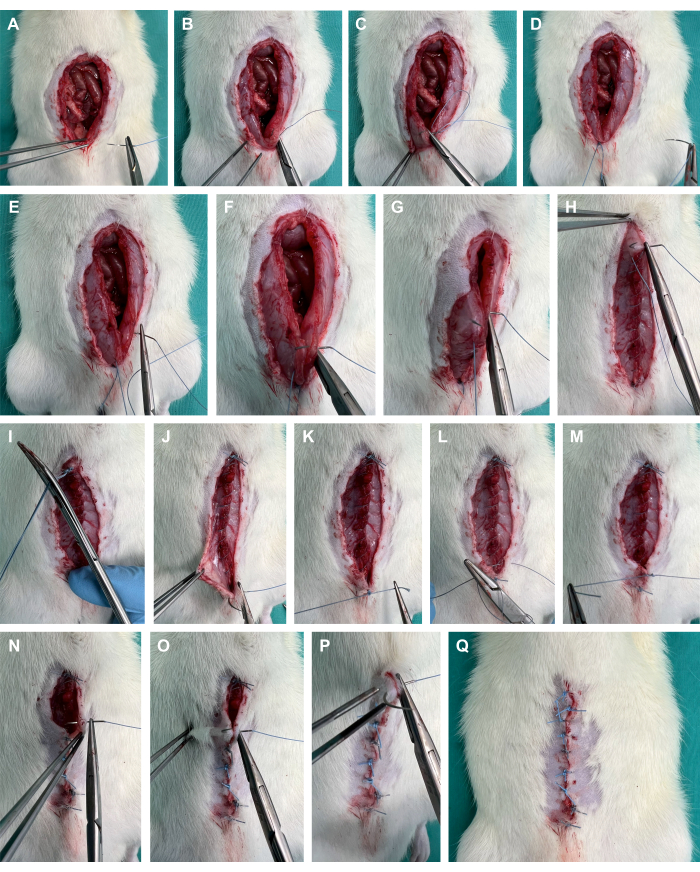
Figure 4: Abdominal wall closure. (A-D) Placement of a corner suture on the abdominal fascia using a polyfilament suture. (E-I) Running suture of the abdominal fascia; (J-Q) suturing of the cutaneous layer using single stitches. Please click here to view a larger version of this figure.
6. Further steps
- Depending on the desired scenario, research purpose, and the degree of renal insufficiency desired, consider deviations from this protocol, including a variation in the amount of resected renal parenchyma, for example, unilateral total nephrectomy (3/6 nephrectomy), bilateral total nephrectomy (6/6 nephrectomy), unilateral monopolectomy (1/6 nephrectomy), bilateral monopolectomy (2/6 nephrectomy), unilateral bipolectomy (2/6 nephrectomy), and bilateral bipolectomy (4/6 nephrectomy).
- In general, either euthanize the animals by sharp cardiectomy without prior abdominal wall closure for non-survival applications or perform stepwise abdominal closure as described above in case of planned survival experiments with follow-ups.
- In the case of survival applications, maintain sterile conditions at all times during surgery. Before surgery, aseptically prepare skin at the surgical site using both a scrub (iodine-based or chlorhexidine-based) and alcohol. Use a fresh autoclaved pack of instruments for each animal.
- Post surgery, monitor the animal until it is conscious enough to maintain sternal recumbency and isolate it until fully recovered.
- As postsurgical treatment of the animal, include daily visits through medical staff as well as postsurgical pain treatment using subcutaneous injections of 5 mg/kg body weight carprofen 2x a day for 2 days.
- Use score sheets with clearly defined termination criteria for the duration until full postoperative recovery. Use the Rat Grimace Scale26 or the Body Condition Score27 from cited literature. Terminate the experiment if Grimace Score ≥ 6 or a Body Condition score = 1 after application of carprofen. Additionally, terminate in case of a surgical complication such as a postoperative wound infection or abdominal wall closure insufficiency.
Wyniki
This protocol was conducted in 10 male rats (mean weight 398 ± 35 g) in a non-survival setting and the procedure was performed by a third-year surgical resident. The success rate defined by survival over 20 min after abdominal wall closure was 100%. The mean duration of the preparation from skin incision until skin closure was 18 min 34 s ± 7 min 31 s.
Unfortunately, due to the non-survival nature of this manuscript, there are no data on the postoperative renal function. Future animal studies with survival settings should associate the degree of parenchyma loss with renal function parameters to provide a better understanding of the degree and variation of renal insufficiency corresponding to the amount of renal resection performed.
For validation of the viability of the renal parenchyma remnant, index parameters of the physiological kidney (before resection), of the perfused remnant, as well as of the resected organ parenchyma for oxygenation (StO2) and perfusion (NIR) were measured using hyperspectral imaging (HSI) and compared (Figure 5). Values were provided in arbitrary units and showed physiological values of the remnant, indicating viability of the remaining renal parenchyma (Table 1).
The hyperspectral results were consistent with recent publications, indicating that tissue viability and perfusion can be assessed using organ-specific HSI StO2 cut-off values. These values matched those observed in this study with values of 60.1% (±7.2%) for physiological perfusion and 20.9% (±2.7%) for malperfused renal tissue. Since these were non-survival experiments, there are no experimental data on the long-term outcomes of the animals.
| parameter | physiological | physiological remnant | resected organ |
| StO2 | 60.1% (±7.2%) | 60.7% (±5.2%) | 20.9% (±2.7%) |
| NIR | 37.6% (±11.2%) | 35.5% (±11.5%) | 1.5% (±1.4%) |
Table 1: Tissue parameters. HSI StO2 oxygenation and NIR perfusion values in arbitrary units across three different renal tissue states. Abbreviations: StO2 = tissue oxygen saturation; NIR = near-infrared spectroscopy; HSI = hyperspectral imaging
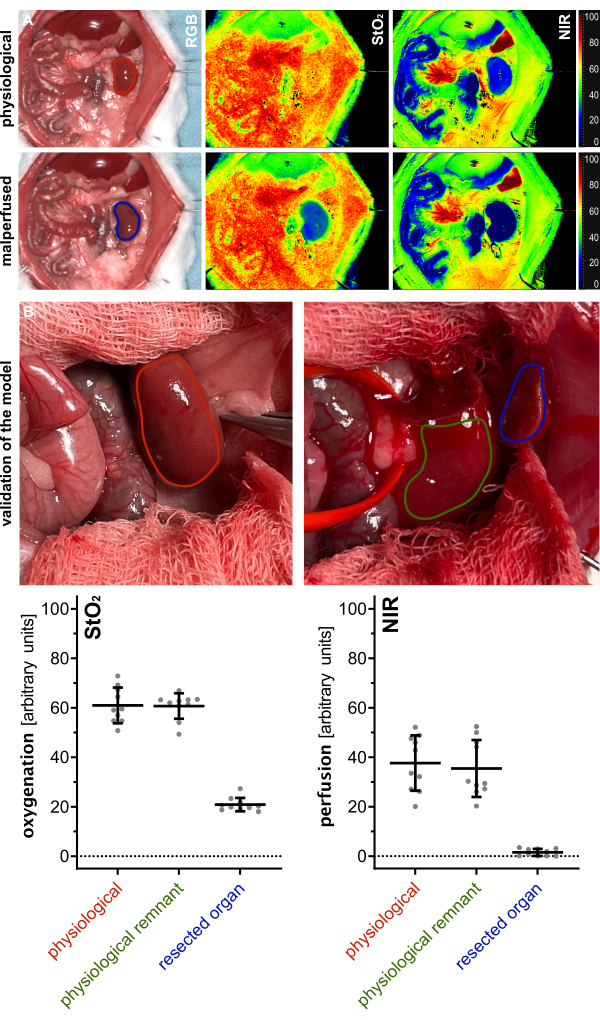
Figure 5: Validation of the model. (A) Visualization of physiological (red) and malperfused (blue) kidney using color-coded index pictures for oxygenation (StO2) and perfusion (NIR) from hyperspectral imaging. (B) RGB images of physiological kidney (red), renal physiological remnant after polectomy (green), and resected renal tissue (blue) with quantification of respective HSI oxygenation (StO2) and perfusion (NIR) values with n = 10 animals. Abbreviations: StO2 = tissue oxygen saturation; NIR = near-infrared spectroscopy; HSI = hyperspectral imaging. Please click here to view a larger version of this figure.
Dyskusje
CKD is defined by kidney damage or reduced kidney function for at least 3 months, regardless of the cause28,29. Kidney damage encompasses pathologic anomalies in the native or transplanted kidney, identified via imaging, biopsy, or deduced from clinical markers like increased albuminuria (albumin-to-creatinine ratio > 30 mg/g or 3.4 mg/mmol) or urinary sediment alterations. Reduced kidney function implies a reduced glomerular filtration rate, which is usually estimated from the serum concentration of creatinine.
According to the US Centers for Disease Control and Prevention, ~37 million people in the United States alone, correlating to ~15% of the adult US population, are estimated to have CKD29 associated with substantial healthcare expenditures, with end-stage renal disease alone causing over $30 billion in excess costs for Medicare beneficiaries in the United States30.
Therefore, establishing suitable and replicable animal models is imperative for tackling emerging research inquiries within this domain. Furthermore, beyond unraveling foundational pathophysiological pathways, the capability to diminish kidney parenchyma and instigate renal insufficiency acts as an essential foundation for appraising the effectiveness of pharmacological treatments, surgical methodologies, and pioneering therapeutic approaches. By exploiting this experimental framework, scientists can accelerate the conversion of preclinical discoveries into practical clinical approaches, thereby mitigating the morbidity and mortality linked with various renal conditions.
For instance, researchers can utilize this model to assess the effectiveness of pharmaceutical compounds aimed at enhancing renal protection and regeneration pathways, as was the case with Sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin or dapagliflozin31, nonsteroidal mineralocorticoid receptor antagonists (MRAs), such as finerenone32,33, endothelin receptor antagonists (ERAs), like sparsentan31 or Hypoxia-inducible factor (HIF) stabilizers31. Additionally, this model facilitates the assessment of novel surgical approaches, such as allotransplantation with advanced immunosuppression regimens or recently even xenotransplantation - with the first successful transplantation of genetically modified pig kidneys to brain-dead human patients in 202134,35,36.
While utilizing this 5/6 nephrectomy model, it is essential to consciously determine how exactly the partial nephrectomy should be conducted-either by surgical resection of poles, termed the polectomy model as introduced here, or by ligating superior and inferior segmental renal arteries or the parenchyma itself, resulting in pole infarction7,15,16,17,18,19,20.
While sharing many characteristics, these two models (resection vs. ligation) exhibit significant phenotypic variations, each capturing distinct aspects of human CKD. Regarding phenotypic similarities, both models exhibit general characteristic features akin to human CKD, such as uremia, fibrosis, capillary rarefaction, and a progressive decline in renal function. Additionally, they both experience hypertrophy of the entire organ, hyperfiltration in functional nephrons, decreased renal expression of vascular endothelial growth factor, heightened renal expression of the antiangiogenic thrombospondin-1, impaired autoregulation of the kidneys, and irregularities in vascular nitric oxide physiology. Therefore, both models, in general, enable the study of a variety of aspects including arterial blood pressure, the renin-angiotensin-aldosterone system, autoregulation, nitric oxide dynamics, single-nephron physiology, angiogenic and antiangiogenic factors, capillary rarefaction, uremia, fibrosis, capillary rarefaction, renal expression of vascular endothelial growth factor, increased renal expression of antiangiogenic thrombospondin-1, impaired renal autoregulation, and abnormal vascular nitric oxide physiology.
Histological features found regularly include tubulo-interstitial damage reflected by inflammation, tubular atrophy and fibrosis, and focal glomerulosclerosis leading to a massive reduction of healthy glomeruli within the remnant population (<10%)21.
Contrarily, notable phenotypic distinctions emerge between the two models: the infarction model presents a swift onset of moderate to severe systemic hypertension, while the polectomy model initially maintains normotension before transitioning to mild to moderate hypertension. Furthermore, it is known that rats subjected to the infarction model demonstrate significantly heightened activity in the renin-angiotensin-aldosterone system7. Moreover, genotypic differences in the sense of upregulated and downregulated genes have been proven to exist, so that the decision whether to use a resection or ligation model for the induction of CKD has to be done consciously and in full comprehension of the biological implications and desired research question37.
While there are several publications on 5/6 nephrectomy with dorsolateral access, there is a lack of scientific literature addressing 5/6 nephrectomy via midline laparotomy and explicitly no methodical protocol. This is, therefore, the claim of this manuscript. Limitations of the presented technique mainly include the invasiveness of the procedure and the difficulty of estimating the correct amount of parenchyma resected to achieve the desired level of renal insufficiency.
When addressing common challenges encountered during the procedure, we wish to highlight the following points and recommendations: Thoroughly gather equipment and medications in advance, and meticulously execute hemostatic control through precise preparation and dissection along avascular planes. Limit contact with the renal parenchyma to non-traumatic instruments, such as moistened cotton swabs or surgical compresses handled with forceps. Start with the partial nephrectomy so that in case of excessive bleeding of the resection surfaces at the poles, the resection can be converted to a total nephrectomy for hemostatic control and the partial nephrectomy can be performed on the contralateral side.
For even improved hemostatic control, a Yasargil aneurysm clamp could be placed on the hilar vessels just before parenchyma dissection. The cautious gradual release of this Yasargil clamp could facilitate the identification of single arterial bleeders on the resection surface and enable the precise bipolar electrocauterization of these.
Typically, because of the elevated risk of bleeding during vascular preparation, it is advisable to utilize blunt overholt clamps for most of the surgical preparation, rather than relying on sharp dissection. Additionally, it is beneficial to moisten the instruments and silicone vessel loops before use to minimize surface friction.
The most hazardous preparation step is the ligature of the renal hilum and its dissection. An insufficient sliding knot technique can cause renal hemorrhage. Consequently, we recommend preparing the hilum over a slightly longer distance so that there is sufficient tissue for reclamping and resuturing.
The risk for postoperative bleeding is relevant so we recommend prolonging abdominal wall closure for an extensive period (e.g., 20 to 30 min) to observe the remnant over a longer period. Applying hypertensive agents after initial hemostasis, for example, subcutaneous norepinephrine to increase systemic blood pressure and identify possible bleeders can be considered.
Beyond illuminating fundamental biomolecular mechanisms, the ability to induce renal malperfusion, organ resectates, and organ remnants in rats provides a valuable tool for evaluating pharmacological interventions and innovative imaging modalities such as HSI38,39,40,41,42. This model is, therefore, crucial for providing the biological tissue ground truth behind renal insufficiency necessary to fully utilize HSI for tissue evaluation in the urological context of CKD.
While this protocol is thought to be a step-by-step guide for a general nephrectomy model in rats, the amount of renal parenchyma resected can be adjusted according to the specific research question as described above. In case of a specific degree of renal failure, that is required for the experiments, the level of CKD can be followed over time by measuring plasma urea, systolic blood pressure, proteinuria, and clearance, for example, using the gold standard inulin and para-amino hippuric acid (PAH). It is generally recognized that 6 weeks after the procedure, renal failure, defined by a reduction in glomerular filtration rate (GFR), has stabilized. To achieve even higher levels of CKD, a combination therapy comprising of a high salt diet and the application of nitric oxide synthase inhibition - using NG-nitro-L-Arginine) (L-NNA) - can be applied as previously described in the cited literature21.
By providing a comprehensive and replicable methodology, this protocol streamlines the process of nephrectomy and chronic kidney disease (CKD) modeling in rat subjects. This standardization enhances the reliability and robustness of data, promotes researcher autonomy, and facilitates comparability across future animal studies. As a result, it emerges as an essential instrument in the biomedical research toolkit, shedding light on the intricate relationship between the remaining functional renal tissue and the systemic impacts of CKD. Leveraging the adaptability of this experimental framework, investigators can chart new territories in translational medicine and ultimately advancing patient outcomes in the realm of renal health.
Ujawnienia
The authors have no conflicts of interest to declare.
Podziękowania
There was no special funding for this project. The authors gratefully acknowledge the data storage service SDS@hd supported by the Ministry of Science, Research and the Arts Baden-Württemberg (MWK) and the German Research Foundation (DFG) through grant INST 35/1314-1 FUGG and INST 35/1503-1 FUGG. Furthermore, the authors gratefully acknowledge the support from the NCT (National Center for Tumor Diseases in Heidelberg, Germany) through its structured postdoc program and the Surgical Oncology program. We also acknowledge the support through state funds approved by the State Parliament of Baden-Württemberg for the Innovation Campus Health + Life Science Alliance Heidelberg Mannheim from the structured postdoc program for Alexander Studier-Fischer: Artificial Intelligence in Health (AIH) - A collaboration of DKFZ, EMBL, Heidelberg University, Heidelberg University Hospital, University Hospital Mannheim, Central Institute of Mental Health, and the Max Planck Institute for Medical Research. Furthermore, we acknowledge the support through the DKFZ Hector Cancer Institute at the University Medical Center Mannheim. For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme "Open Access Publikationskosten" as well as by Heidelberg University.
Materiały
| Name | Company | Catalog Number | Comments |
| atraumatic preparation forceps | Aesculap | FB395R | DE BAKEY ATRAUMATA atraumatic forceps, straight |
| blunt overholt clamp | Aesculap | BJ012R | BABY-MIXTER preparation and ligature clamp, bent, 180 mm |
| cannula | BD (Beckton, Dickinson) | 301300 | BD Microlance 3 cannula 20 G |
| fixation rods | legefirm | 500343896 | tuning forks used as y-shaped metal fixation rods |
| heating pad | Royal Gardineer | IP67 | Royal Gardineer Heating Pad Size S, 20 Watt |
| plastic perfusor tube | M. Schilling GmbH | S702NC150 | connecting tube COEX 150 cm |
| polyfilament suture | Covidien | CL-769 | Covidien Polysorb Braided Absorbable Suture 2-0 75 cm |
| preparation scissors | Aesculap | BC177R | JAMESON preparation scissors, bent, fine model, blunt/blunt, 150 mm (6") |
| sealing hemostat patch | Baxter | 1506257 | Hemopatch Sealing Hemopatch Baxter 45 x 90 mm |
| silicone vessel loop tie | SERAG WIESSNER | SL26 | silicone vessel loop tie 2.5 mm red |
| Spraque Dawley rat | Janvier Labs | RN-SD-M | Spraque Dawley rat |
| steel plate | Maschinenbau Feld GmbH | C010206 | Galvanized sheet plate, 40 x 50 cm, thickness 4.0 mm |
| Yasargil clip | Aesculap | FE795K | YASARGIL Aneurysm Clip System Phynox Temporary (Standard) Clip |
| Yasargil clip applicator | Aesculap | FE558K | YASARGIL Aneurysm Clip Applicator Phynox (Standard) |
Odniesienia
- Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int Suppl (2011). 12 (1), 7-11 (2022).
- Sundström, J., et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The careme ckd study. Lancet Reg Health Eur. 20, 100438(2022).
- Webster, A. C., Nagler, E. V., Morton, R. L., Masson, P. Chronic kidney disease. Lancet. 389 (10075), 1238-1252 (2017).
- Fassett, R. G. Current and emerging treatment options for the elderly patient with chronic kidney disease. Clin Interv Aging. 9, 191-199 (2014).
- Tarun, T., et al. Updates on new therapies for patients with ckd. Kidney Int Rep. 9 (1), 16-28 (2024).
- Dudar, I., Shifris, I., Dudar, S., Kulish, V. Current therapeutic options for the treatment of secondary hyperparathyroidism in end-stage renal disease patients treated with hemodialysis: A 12-month comparative study. Pol Merkur Lekarski. 50 (299), 294-298 (2022).
- Adam, R. J., Williams, A. C., Kriegel, A. J. Comparison of the surgical resection and infarct 5/6 nephrectomy rat models of chronic kidney disease. Am J Physiol Renal Physiol. 322 (6), F639-F654 (2022).
- Huang, Y., et al. The impact of senescence on muscle wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 14 (1), 126-141 (2023).
- Makhloufi, C., et al. Assessment of thrombotic and bleeding tendency in two mouse models of chronic kidney disease: Adenine-diet and 5/6th nephrectomy. TH Open. 4 (2), e66-e76 (2020).
- Liu, J., Lilly, M. N., Shapiro, J. I. Targeting na/k-atpase signaling: A new approach to control oxidative stress. Curr Pharm Des. 24 (3), 359-364 (2018).
- Laget, J., et al. Cafeteria diet-induced obesity worsens experimental CKD. Nutrients. 15 (15), 3331(2023).
- Gritter, M., et al. Chronic kidney disease increases the susceptibility to negative effects of low and high potassium intake. Nephrol Dial Transplant. 39 (5), 795-807 (2024).
- Bovée, D. M., et al. Dietary salt modifies the blood pressure response to renin-angiotensin inhibition in experimental chronic kidney disease. Am J Physiol Renal Physiol. 320 (4), F654-F668 (2021).
- Vettoretti, S., et al. Renal endothelial function is associated with the anti-proteinuric effect of ace inhibition in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 310 (10), F1047-F1053 (2016).
- Zhang, Y., Kompa, A. R. A practical guide to subtotal nephrectomy in the rat with subsequent methodology for assessing renal and cardiac function. Nephrology (Carlton). 19 (9), 552-561 (2014).
- Ibrahim, H. N., Hostetter, T. H. The renin-aldosterone axis in two models of reduced renal mass in the rat. J Am Soc Nephrol. 9 (1), 72-76 (1998).
- Griffin, K. A., Picken, M., Bidani, A. K. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 4 (12), 2023-2031 (1994).
- Garber, S. L., et al. Effect of relaxin in two models of renal mass reduction. Am J Nephrol. 23 (1), 8-12 (2003).
- Griffin, K. A., Picken, M. M., Churchill, M., Churchill, P., Bidani, A. K. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol. 11 (3), 497-506 (2000).
- Vavrinec, P., et al. Vascular smooth muscle function of renal glomerular and interlobar arteries predicts renal damage in rats. Am J Physiol Renal Physiol. 303 (8), F1187-F1195 (2012).
- Van Koppen, A., Verhaar, M. C., Bongartz, L. G., Joles, J. A. 5/6th nephrectomy in combination with high salt diet and nitric oxide synthase inhibition to induce chronic kidney disease in the lewis rat. J Vis Exp. (77), e50398(2013).
- Wang, X., et al. A mouse 5/6th nephrectomy model that induces experimental uremic cardiomyopathy. J Vis Exp. (129), e55825(2017).
- Harvey, A. M., Malvin, R. L. Comparison of creatinine and inulin clearances in male and female rats. Am J Physiol. 209 (4), 849-852 (1965).
- Gloff, C. A., Benet, L. Z. Differential effects of the degree of renal damage on p-aminohippuric acid and inulin clearances in rats. J Pharmacokinet Biopharm. 17 (2), 169-177 (1989).
- Studier-Fischer, A., et al. Endotracheal intubation via tracheotomy and subsequent thoracotomy in rats for non-survival applications. J Vis Exp. (205), e66684(2024).
- Sotocinal, S. G., et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 7, 55(2011).
- Hickman, D. L., Swan, M. Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. J Am Assoc Lab Anim Sci. 49 (2), 155-159 (2010).
- Levey, A. S., et al. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (kdigo). Kidney Int. 67 (6), 2089-2100 (2005).
- Wilson, S., Mone, P., Jankauskas, S. S., Gambardella, J., Santulli, G. Chronic kidney disease: Definition, updated epidemiology, staging, and mechanisms of increased cardiovascular risk. J Clin Hypertens (Greenwich). 23 (4), 831-834 (2021).
- Dobaria, V., et al. Clinical and financial impact of chronic kidney disease in emergency general surgery operations. Surg Open Sci. 10, 19-24 (2022).
- Copur, S., et al. Novel strategies in nephrology: What to expect from the future. Clin Kidney J. 16 (2), 230-244 (2022).
- Pradhan, N., Dobre, M. Emerging preventive strategies in chronic kidney disease: Recent evidence and gaps in knowledge. Curr Atheroscler Rep. 25 (12), 1047-1058 (2023).
- Dietrich, M., et al. Hyperspectral imaging for perioperative monitoring of microcirculatory tissue oxygenation and tissue water content in pancreatic surgery - an observational clinical pilot study. Perioper Med (Lond). 10 (1), 42(2021).
- Stone, L. Kidney xenotransplantation. Nat Rev Urol. 20 (11), 641-641 (2023).
- Xu, H., He, X. Developments in kidney xenotransplantation. Front Immunol. 14, 1242478(2023).
- Dos Santos, R. M. N. Kidney xenotransplantation: Are we ready for prime time. Curr Urol Rep. 24 (6), 287-297 (2023).
- Nasci, V. L., et al. Mir-21-5p regulates mitochondrial respiration and lipid content in h9c2 cells. Am J Physiol Heart Circ Physiol. 316 (3), H710-H721 (2019).
- Nickel, F., et al. Optimization of anastomotic technique and gastric conduit perfusion with hyperspectral imaging and machine learning in an experimental model for minimally invasive esophagectomy. Eur J Surg Oncol. S0748-7983 (23), 00444-00454 (2023).
- Seidlitz, S., et al. Robust deep learning-based semantic organ segmentation in hyperspectral images. Med Image Anal. 80, 102488(2022).
- Studier-Fischer, A., et al. Icg-augmented hyperspectral imaging for visualization of intestinal perfusion compared to conventional icg fluorescence imaging: An experimental study. Int J Surg. 109 (12), 3883-3895 (2023).
- Studier-Fischer, A., et al. Heiporspectral - the heidelberg porcine hyperspectral imaging dataset of 20 physiological organs. Sci Data. 10 (1), 414(2023).
- Studier-Fischer, A., et al. Spectral organ fingerprints for machine learning-based intraoperative tissue classification with hyperspectral imaging in a porcine model. Sci Rep. 12 (1), 11028(2022).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone