Method Article
Addressing Practical Issues in Atomic Force Microscopy-Based Micro-Indentation on Human Articular Cartilage Explants
W tym Artykule
Podsumowanie
We present a step-by-step approach to identify and address the most common problems associated with atomic force microscopy micro-indentations. We exemplify the emerging problems on native human articular cartilage explants characterized by various degrees of osteoarthritis-driven degeneration.
Streszczenie
Without a doubt, atomic force microscopy (AFM) is currently one of the most powerful and useful techniques to assess micro and even nano-cues in the biological field. However, as with any other microscopic approach, methodological challenges can arise. In particular, the characteristics of the sample, sample preparation, type of instrument, and indentation probe can lead to unwanted artifacts. In this protocol, we exemplify these emerging issues on healthy as well as osteoarthritic articular cartilage explants. To this end, we first show via a step-by-step approach how to generate, grade, and visually classify ex vivo articular cartilage discs according to different stages of degeneration by means of large 2D mosaic fluorescence imaging of the whole tissue explants. The major strength of the ex vivo model is that it comprises aged, native, human cartilage that allows the investigation of osteoarthritis-related changes from early onset to progression. In addition, common pitfalls in tissue preparation, as well as the actual AFM procedure together with the subsequent data analysis, are also presented. We show how basic but crucial steps such as sample preparation and processing, topographic sample characteristics caused by advanced degeneration, and sample-tip interaction can impact data acquisition. We also subject to scrutiny the most common problems in AFM and describe, where possible, how to overcome them. Knowledge of these limitations is of the utmost importance for correct data acquisition, interpretation, and, ultimately, the embedding of findings into a broad scientific context.
Wprowadzenie
Due to the ever-shrinking size of electronic devices and systems, the rapid development of micro- and nano-based technology and equipment has gained momentum. One such device is atomic force microscopy (AFM), which can scan biological surfaces and retrieve topographic or biomechanical information at both nano- and micrometer scales1,2. Among its vast features, this tool can be operated as a micro- as well as a nano-indenter to obtain information about the mechanical properties of various biological systems3,4,5,6. The data are collected by physical contact with the surface through a mechanical probe, which can be as small as about 1 nm at its tip7. The resulting deformation of the sample is then displayed based on the indentation depth of the cantilever tip and the force applied on the sample8.
Osteoarthritis (OA) is a long-term degenerative chronic disease characterized by deterioration of the articular cartilage in the joints and surrounding tissues, which can lead to complete exposure of the bone surfaces. The burden of OA is substantial; currently, half of all women and one-third of all men aged 65 and over suffer from OA9. Traumas, obesity, and the resulting altered biomechanics of the joint10 determine the articular cartilage degeneration, which is viewed as a common end result. The pioneering study of Ganz et al. posited that the early steps of the OA process may involve the biomechanical properties of cartilage11, and since then researchers have confirmed this hypothesis12. Likewise, it is generally accepted that the biomechanical properties of the tissue are functionally orchestrated by the ultrastructural organization as well as cell-cell and cell-matrix crosstalk. Any alterations can dramatically impact the overall tissue biomechanical functioning13. To date, OA diagnosis is clinical and is based on plain film radiography14. This approach is two-sided: firstly, the lack of a defined degenerative cut-off threshold to formulate the diagnosis of OA makes the condition difficult to quantify, and, secondly, imaging methods lack sensitivity and standardization and cannot detect localized cartilage damage15,16,17. To this end, the assessment of the mechanical properties of the cartilage has the decisive advantage that it describes a parameter that changes during the course of OA regardless of the etiology of the disease and has a direct influence on tissue functionality at a very early stage. Indentation instruments measure the force by which the tissue resists the indentation. This is, in fact, not a new concept; the earliest studies date back to the 1980s and 1990s. In this period, numerous studies suggested that indentation instruments designed for the arthroscopic measurements of articular cartilage could be well suited to detect degenerative changes in the cartilage. Even 30 years ago, some studies were able to demonstrate that indentation instruments were able to detect in vivo changes in the cartilage surface during tissue degeneration by conducting compressive stiffness measurements during arthroscopy18,19,20.
AFM indentation (AFM-IT) of the articular cartilage provides information about a pivotal mechanical property of the tissue, namely, stiffness. This is a mechanical parameter that describes the relation between an applied, nondestructive load and the resultant deformation of the indented tissue area21. AFM-IT has been shown to be capable of quantifying age-dependent modifications in stiffness in macroscopically unaffected collagen networks, thus, differentiating between the pathological changes associated with OA onset (grade 0 on the Outerbridge scale in articular cartilage)22. We have previously shown that AFM-ITs, on the basis of spatial chondrocyte organization as an image-based biomarker for early cartilage degeneration, allow for not only quantifying but also actually pinpointing the earliest degenerative mechanical changes. These findings have already been confirmed by others23,24. Hence, AFM-IT acts as an interesting tool to diagnose and identify early degenerative changes. These changes can be already measured at a cellular level, reshaping the understanding of the OA pathophysiological process.
In this protocol, we demonstrate a complete histological and biomechanical grading procedure of articular cartilage explants, from native cartilage explant preparation to AFM data acquisition and processing. Through a step-by-step approach, we show how to generate, grade, and visually classify articular cartilage tissue according to different stages of degeneration by means of 2D large mosaic imaging, followed by micro-AFM indentations.
Even though, currently, AFM-IT is one of the most sensitive tools to measure biomechanical changes in cartilage7, like any other instrumental technique, it has limitations and practical peculiarities25 that can lead to erroneous data acquisition. To this end, we subject to scrutiny the most common problems that arise during AFM measurements of the cartilage explants and describe, where possible, how to minimize or overcome them. These include topographical aspects of the samples and the difficulties to stabilize them in an AFM-compatible environment, physical peculiarities of the tissue's surface, and the resulting difficulties in performing AFM measurements on such surfaces. Examples of erroneous force-distance curves are also presented, emphasizing the conditions that may cause them. Additional limitations inherent to the geometry of the cantilever tip and the use of the Hertz model for the data analysis are also discussed.
Protokół
Femoral condyles collected from patients undergoing total knee arthroplasty at the University Hospital of Tübingen, Germany, were used. Only articular cartilage samples from patients with degenerative and posttraumatic joint pathologies were included in this study. Departmental, institutional, as well as local ethical committee approval were obtained before the commencement of the study (Project no.674/2016BO2). Written informed consent was received from all patients before participation.
NOTE: A flowchart of the experiment steps in their chronological order is given in Figure 1.
1. Tissue processing and generation of cartilage discs
- Tissue preparation
- Following post-operative resection, place the cartilage samples in a container filled with Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% (v/v) penicillin-streptomycin. Make sure the samples are completely submerged in the medium. The duration between surgical resection and further processing of the cartilage should not exceed 24 h. Ensure that, throughout the entire processing, the samples are fully submerged in media to avoid sample drying.
- Cut the cartilage away from the bone using a scalpel.
- Cartilage disc generation
- Generate cartilage discs of 4 mm in diameter using a biopsy punch.
NOTE: It is important to select and resect the areas of the condyle where the cartilage layer thickness exceeds 1 mm. This might be problematic, especially around loadbearing zones, where the cartilage layer typically loses its thickness due to wear and tear processes or degeneration. - Place the previously generated 4 mm cartilage discs on a custom-made cutting device and fix and hold the cartilage discs stable by means of a spatula. When placing the cartilage discs on the cutting device, care must be taken. Position the samples so that the cartilage's topmost layer (the superficial zone of the articular cartilage) does not face the blade
- Cut the cartilage discs with a razor blade. Disc-shaped cartilage samples of 4 mm x 1 mm are, thus, generated. To prevent sample drying, perform tissue cutting as quickly as possible.
- Collect each disc with the help of a spatula and place the generated cartilage discs into 1.5 mL tubes containing 1 mL of DMEM supplemented with 5% (v/v) penicillin-streptomycin. Place approximately 15 discs in one tube.
- Generate cartilage discs of 4 mm in diameter using a biopsy punch.
- Cryotome sectioning of the cartilage discs (for perpendicular slices)
NOTE: This step is optional, and it can be employed if a side-view visualization of the cellular pattern distribution within the cartilage discs is desired. It can be used as a verification method as the distribution of cellular pattern is a 3D feature of articular cartilage26. Optical sectioning and 3D reconstructions of the entire cartilage discs using a confocal microscope can also be used, removing, thus, the need to section the samples as described in the protocol.- Cover the cartilage disc with water-soluble embedding medium and place it on its edge on the cryotome knob (with the surface of the disc perpendicular to the surface of the knob). In the cryotome device, the embedding medium freezes at low temperatures.
- Using a standard cryotome, section the tissue sideways at a 60 µm thickness until the middle of the disc is reached (i.e., when cryosections reach a length of 4 mm) and collect the slices. By sectioning the disc explant perpendicularly, all zones of the cartilage (superficial, middle, and deep) can be visualized.
- Collect the sections on a glass slide and remove the water-soluble embedding medium by washing three times with phosphate-buffered saline (PBS).
2. Cartilage disc sorting as a function of the cellular spatial pattern
- Staining of the disc-shaped cartilage samples
- Place one cartilage disc (section 1.2) in each well of a 96-well plate and add 130 µL of cell permeable fluorescence dye at a dilution of 1:1,000 to each well.
- Visually inspect the entire plate and make sure that only one disc is placed in each well. Incubate the plate for 30 min in the standard cell culture incubator at 37 °C.
- Staining of the 60 µm cartilage slices
- Gently place the cartilage disc sections (section 1.3) on glass microscope slides with the help of forceps.
- Cover the cartilage sections with mounting medium containing nuclear DAPI counterstaining and gently place coverslips that are suitable for fluorescence microscopy.
- Seal the edges of each coverslip with regular transparent nail polish and allow to dry for 3 min.
- Top-down and side-view cartilage sorting and imaging
NOTE: Each disc must be examined under a fluorescent microscope. The aim of this step is to sort the discs based on their predominant cellular pattern (single strings, double strings, small clusters, big clusters, or diffuse).- Place the 96-well plate on the plate holder of the fluorescence microscope.
- Select the appropriate fluorescence filter of either Em 495 nm/Ex 515 nm (for top-down imaging of the cartilage discs prepared in section 2.1.) or Em 358 nm/Ex 461 nm (for side-view imaging of the cartilage sections prepared in section 2.2) and the 10x objective.
NOTE: Using the 10x objective allows the whole circumference of the disc to be inspected, and samples with inhomogeneous or improper staining can be excluded. However, using only the top-down view may result in the perception of changes in cellular organization as a result of analysis of the deeper tissue layers made visible to top-down observation by superficial erosion. As an example, an ascending string following the collagen arcades could be perceived as a single cell or scattered cells (diffuse pattern)26. As a result, both sides of the discs must be inspected to ensure proper cellular pattern selection. - Determine visually the cellular pattern displayed in each cartilage disc. It is unlikely that a disc will have only one type of cellular pattern. For the portion of the disc where the chondrocyte arrangement does not match the pattern of interest, only accept the samples if the undesired pattern is at the very periphery, where AFM measurements are not taking place (i.e., up to 0.5 mm from the disc border), and ensure that this does not exceed 10% of the total surface of the disc27,28.
- Image acquisition of the entire cartilage discs
- Select the 10x objective of the microscope and position it underneath the preselected well containing an individual cartilage disc. Focus on the disc to see the cellular pattern.
- Select the Navigator Function to get an overview of the entire well. Use the left mouse button and drag to navigate to a different stage position. With the mouse wheel, zoom in and out.
NOTE: At this point, a preview of the well with the entire sample can be seen by double-clicking on each area of interest sequentially. - Select a square that encompasses the area of interest to be scanned; at this point, all single tiles that compose the mosaic will become visible.
- Adjust the exposure/light intensity so that the cells can be clearly visualized from the background. At this point, the picture's brightness/contrast has been adjusted for all the tiles and can no longer be customized individually for each tile.
NOTE: As the cells near the disc's edge often emit a higher fluorescence signal than the cells in the center, the exposure/light intensity settings must be adapted.- To evaluate if the exposure time is appropriate for a particular channel, examine the distribution of the signal in the histogram. By using the automatic exposure mechanism included in the microscope's image software, visualize all the cells residing within the disc.
- Select the software's Focus Map Point option, and then select each individual tile by left-clicking in the center of it.
- Select the option Focus Map. A window is displayed with all the previously selected tiles. Double-click on a tile in the list to display and bring it into proper focus.
- Click Set Z to save the focal plan and proceed to the next tile. After adjusting the focal plan for each individual tile, begin image acquisition by pressing Start Scan.
- If the scan is displaying darker horizontal and/or vertical bars, this may be due to improper and uneven illumination of the single frames. Resolve this by using the Linked Shading option incorporated in the software prior to the actual scan.
- Save, export, and correctly annotate the images.
3. Biomechanical approach of cartilage explants
- Sample preparation for AFM measurements
- Fix each preselected cartilage disc containing a cellular pattern (section 2) in Petri dishes by means of biocompatible glue.Add sufficient sample glue on the top, bottom, left, and right sides of the disc.
- Cover the discs with 2.5 mL of Leibovitz's L-15 medium without L-glutamine. Add the Leibowitz medium gently onto the samples to avoid sample detachment from the surface due to waves created by the medium.
- Loading of the samples into the AFM
- Position the Petri dish in the AFM device's sample holder and turn on the Petri dish heater set to 37 °C. Allow the tissue culture dish to reach the desired temperature. This is done to exclude possible artifacts caused by temperature variation.
- AFM-cantilever calibration
- Initialize the software setup as previously described by Danalache et al.29.
- Select a suitable glass block cantilever holder for liquid measurements and carefully place it on the AFM head. A locking mechanism secures the glass block in the AFM head. Ensure that the glass block's reflective surface is straight and parallel to the AFM holder.
- Place the cantilever on the surface of the glass block cantilever holder with care. The cantilever itself should rest on the polished optical plane, in the center of the glass block.
- Carefully place a silicone skirt (silicone membrane) on the base of the cantilever holder in order to prevent medium condensation in the AFM head.
- Lower the cantilever in 100 µm steps using the stepper motor function until it is completely submerged in the medium.
- Run a scanner approach with the approach parameters described by Danalache et al.29. Retract the cantilever by 100 µm once the bottom of the Petri dish is reached.
- Calibrate the cantilever using the exact steps and run the parameters described by Danalache et al.29. At the end of the calibration, the vertical deflection is saved and displayed in newton (N) units of force rather than volts (V)-the unit of the original registration by the photodiode detector. In the experiments here, a set point of 4.47 nN resulted after calibration.
- Using the stepper motor function, retract the cantilever to 1,000 µm.
- Identifying the desired cartilage measurement site under the AFM
NOTE: Due to the 1 mm thickness of the cartilage discs, the cantilever is not visible in the field of view while navigating over the sample.- Use the CCD camera of the microscope to identify the cantilever. The AFM cantilever should be positioned in a sample-free area of the Petri dish.
- Start a scanner approach with the cantilever on a clean, sample-free area of the Petri dish, using the same parameters described by Danalache et al.29.
- Further retract the cantilever 1.5 mm away from the bottom of the plate with the stepper motor control. This step is crucial in order to avoid a direct collision between the cantilever and the sample.
- Switch from brightfield to fluorescence view and visually identify the top of the disc.
- Move the AFM sample holder exactly 2 mm toward the middle of the disc. This point is considered to be the center of the cartilage disc.
- Run a scanner approach and, once the surface of the cartilage disc is reached, retract the cantilever by 100 µm.
- Force-distance curve measurements
- Focus on the cells positioned in the desired measurement site. Click the Run button to start the measurements and the generation of force-distance curves in the targeted position.
- Acquire five force-distance curves on each measurement site. Retract the cantilever by 500 µm and move the cantilever to the next measuring site.
NOTE: The retraction of the cantilever is a crucial step, as the cartilage disc surface is not homogenous and has irregularities. A high hillock on the surface of the sample can result in a dramatic collision, leading to unwanted cantilever tip/sample damage. We recommend selecting a minimum of five different measurement sites dispersed across the surface of the disc and acquiring a minimum of five force-distance curves at each site. - Inspect the force-distance curves and save them.
- Estimation of Young's moduli using the Hertz fit model
- Open the generated force-distance curves to be analyzed (.jpk file) in the data analysis software using the Open a Batch of Spectroscopy Curves option.
- Select the Hertz fit model and then select the Elasticity fit option.
- The elasticity fit option automatically performs the following computations on the selected force-distance curve: calculates the baseline and subtracts from the whole curve to remove the baseline offset (the baseline is brought back to zero on the y-axis); determines the contact point by detecting the point where the force-distance curve crosses the zero force-line (the contact point is set to zero on the x-axis); calculates the tip-sample separation (the height signal of the piezo accounting for the bending of the cantilever is subtracted); and fits the force-distance curve automatically with the selected model. If desired, each of these steps can also be carried out independently.
- Adapt using the following fit parameters: Poisson ratio of 0.5 and the appropriate cantilever tip radius.
NOTE: When using a cantilever with a spherical cantilever tip, the Hertz fit model should be used. The cantilever used in this study had a spherical tip with a radius of 5 µm. We recommend fitting the force-distance curve until the maximum applied force is reached (setpoint). - Check visually the force-distance curve fit to ensure correctness. This step has to be done for each of the force-distance curves analyzed.
- Indentation depth determination
NOTE: Depending on the data analysis tool being used, this process may differ. The experimenter can easily read the indentation depth by following a series of steps that are included in the data analysis program.- Open each of the generated force-distance curves in the data analysis software and select the Hertz fit Model as the analysis process.
- Apply the Subtract Baseline Offset option to zero the vertical deflection axis (y-axis) and select the Offset + Tilt function.
- Use the Find Contact Point function to automatically identify the contact point, which is automatically brought to an x-coordinate of zero.
- Subtract the distance accounting solely for cantilever deflection from the raw piezo height during the indentation using the Vertical Tip Position function.
- Select the Elasticity Fit option to display the processed force-distance curve and select the area of the graph so that it lines up with the most negative value on the Vertical Tip Position Axis (x-axis).
- Read and document the indentation from the X Min box in the parameter tab. Save and document the results.
4. Statistical analysis
- Open the statistical software. Select New Dataset from the drop-down menu.
- Open the Variable View tab after selecting the DataSet file. Define the numerical variables for each cellular pattern category: single strings = SS, double strings = DS, small clusters = SC, big clusters = BC, diffuse and the Young's moduli.
- In the data view tab, enter the measured Young's moduli data for each of the corresponding cellular pattern categories. Analyze the data distribution by selecting Analyze from the menu bar, and then Exploratory Data Analysis.
- Select Young's Moduli as the dependent variable and Cellular Pattern as the factor list. A box plot used for the results section is displayed among the results in the output file.
- To conduct a statistical analysis, choose Dependent Samples in the nonparametric test section of the analyze menu bar tab. Select Young's Moduli as Test Fields and Cellular Pattern as Groups under the fields tab. Press Run.
NOTE: The results are displayed in the output file. For the statistical analysis, a Friedman test is performed. - Incorporate the nonparametric test's p-values into the box plot that was created in step 4.4. Save the results by clicking File in the menu bar and selecting Save.
Wyniki
Using a self-made cutting device, we were able to explant and generate small (4 mm x 1 mm) cartilage discs from fresh human condyles containing a single cellular spatial pattern30 of single strings (SS, Figure 2A), double strings (DS), small clusters (SC), big clusters (BC; Figure 2A), and diffuse (Figure 2B). A representative cartilage explant is depicted in Figure 3A. The selection of discs displaying only one type of pattern was done using top-down fluorescence imaging (Figure 2). The topographic variation of the cartilage surface disc was further illustrated by side-view imaging of 60 µm thick sections generated from the cartilage discs (Figure 2C). On the surface of the explanted osteoarthritic cartilage discs, superficial fibrillation and matrix clefting were present (Figure 3B,C). This was particularly notable in the discs representative of advanced OA progression-represented by the presence of BC (Figure 2A). Following post-fluorescence sorting, the stiffness of the discs was assessed by AFM micro-indentations. To this end, the generated cartilage discs were successfully fixed in the AFM Petri dish by means of biocompatible glue (Figure 3D) to avoid sample drifting during the measurements (Figure 3E). The amount of glue used has to be adjusted. An insufficient amount of glue will result in disc instability, while adding too much glue may lead to an unwanted spreading of the glue either under and/or over the cartilage disc. The latter leads to measurement artifacts and poor identification of the disc under the fluorescence microscope. Inadequate glue application or sudden movements of the sample during fixation are frequent issues that cause the tissue to detach from the Petri dish and should be avoided.
A representative gradual stiffness reduction alongside the cellular pattern arrangement is displayed in Figure 4A. The stiffness values were higher in the discs containing SS (median of 2.6 kPa)-representative of uncompromised heathly cartilage areas. With OA onset and progression, the AFM measurements showed a strong stepwise decrease in stiffness of 42% in DS (1.5 kPa), 77% in SC (0.6 kPa), and, ultimately, 88% in advanced stages represented by BC (0.3 kPa; Figure 4A). The discs containing a diffuse pattern displayed an elevated elasticity with an important variation of the Young's modulus single values. For all of the cartilage discs with assigned predominant cellular pattern organization, the indentation depth associated with the employed setpoint (4.477 nN) was found to be inversely proportional to stiffness (Figure 4B). A representative generated force-distance curve is shown in Figure 4C, and a Hertz fit as well as the identification of the contact point is shown in Figure 4D.
The correct fit depends on, among other factors, the correct determination of the baseline. If the automatic baseline detection is erroneous (for example, because of a turbulent baseline), the fit can also be determined manually, and it allows the user to select a more representative baseline for the measurements. However, if the generated force-distance curve does not allow a proper fit, it has to be discarded. Figure 5 shows examples of incorrect force-distance curves. Generating suitable force-distance curves on osteoarthritic articular cartilage explants can be difficult due to the tissue's irregular surface, on the one hand (examples are shown in Figure 5A,B), and the sample's instability caused by improper sample fixation (examples displayed in Figure 5C,D). Artifacts may be caused by multiple probe-sample contact points (due to the uneven surface of degenerated cartilage) or unwanted tissue movement (visible through changes in the focal plane). These artifacts can be seen in the generated force-distance curves and are indicative of either a suboptimal contact between the AFM cantilever and the cartilage surface or improper sample fixation to the Petri dish (see Figure 5A-D).

Figure 1: Flow chart of the experimental procedure. Summary of the experimental steps in chronological order, starting from intraoperatively resected cartilage samples to the generation of 4 mm x 1 mm cartilage discs, fluorescence staining and sorting of the discs on the basis of the cellular pattern organization by means of top-down and side-view imaging, and, ultimately, elasticity assessment by means of atomic force measurements. Please click here to view a larger version of this figure.

Figure 2: Fluorescence imaging of representative cartilage discs. (A) Mosaic 2D images and a zoomed exemplary field of cartilage discs stained with cell membrane permeable dye at 100x magnification. The top disc displays a representative single strings disc, while the lower one is representative of a big cluster disc (bottom). (B) Mosaic picture of a diffuse pattern disc seen from the surface (top), and the same disc imaged from the underside (bottom). (C) Side view of nuclear staining of 60 µm slices of cartilage discs. The white scale bar represents 500 µm for the mosaic pictures (larger field of view, A [left panel], B, C) and 100 µm for the zoomed in, focused pictures (A[right panel]). Please click here to view a larger version of this figure.
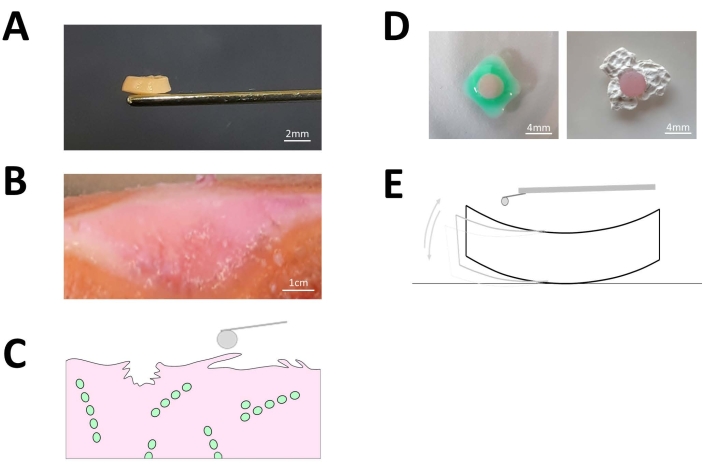
Figure 3: Explanted articular cartilage discs. (A) Representative image of a generated 4mm x 1mm cartilage disc explant from fresh human articular cartilage. The scale bar depicted in white represents 2mm. (B) Representative image of native osteoarthritic cartilage where the tissue surface presents macroscopically visible superficial fibrillation and clefting. The scale bar depicted in white represents 1,000 mm. (C) Schematical depiction of the fibrillated cartilage surface. (D) Prior to the AFM measurements, each of the cartilage disc explants was properly fixed by means of bio-compatible sample glue to the surface of the AFM Petri dish to avoid artifacts due to sample drifting during the actual indentation measurements as shown in (E). The white scale bar represents 4 mm. Please click here to view a larger version of this figure.
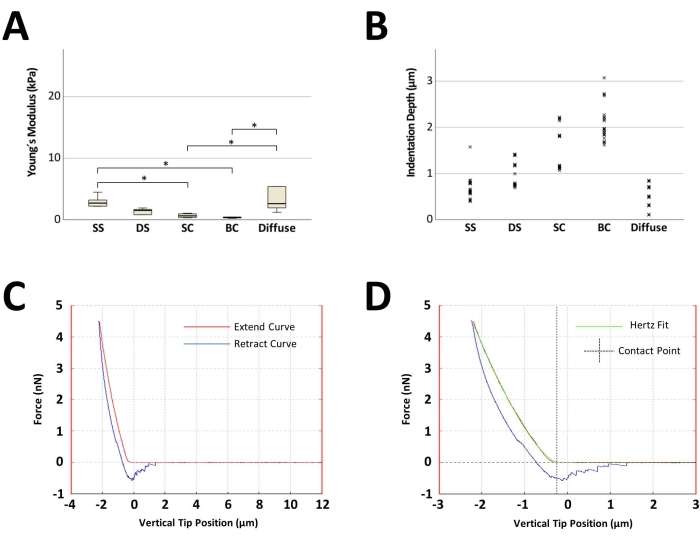
Figure 4: Representative results of atomic force microscopy measurements of articular cartilage discs sorted on the basis of their predominant cellular pattern organization. (A) Boxplot displaying the median of the computed Young's moduli of five discs, one for each cellular pattern, originating from one patient. A total of 25 measurements were performed on each disc (five measurements for five distinct measurement sites). The black line inside the rectangle represents the median value, the lower and upper extremities of the rectangle represent the first and third quartiles, respectively, and the error bars represent the lowest and highest values for each group. (B) Point-plot depicting the 125 indentation depth points for each cellular pattern. (C) Exemplary force-distance curves acquired with the AFM with a computed Young's modulus of 0.4 kPa. (D) A representative Hertz fit and contact point determination for the force-distance curve shown in (C). The x-axis displays the vertical tip position (which is the distance crossed by the piezo, with the length accounting for the cantilever bending being automatically subtracted from the contact part of the force-distance curve). *p < 0.05. For statistical analysis, the Friedman test was used. Abbreviations: SS = single strings; DS = double strings; SC = small clusters; BC = big clusters. Please click here to view a larger version of this figure.

Figure 5: Examples of erroneous force-distance curves. (A) The force-distance curve shows a massive deviation followed by recovery near to the baseline level before a continuous indentation of the surface is observed. This phenomenon can be attributed to a relatively large obstacle (e.g., large surface clefts protruding from the topmost layer of the cartilage). (B) The extended force-distance curve shows multiple small peaks. These curves are thought to be caused by micro-scale irregularities on the cartilage surface (e.g., fibrillation). Both (C) and (D) display force-distance curves with biphasic courses. Both force-distance curves are representative of poor sample fixation and sample drift. It is also quite common in these cases to see a sudden change in the focal plane. Please click here to view a larger version of this figure.
Dyskusje
As a progressive and multifactorial disease, OA triggers structural and functional changes in the articular cartilage.Throughout the course of OA, impairments in mechanical features are accompanied by structural and biochemical changes at the surface of the articular cartilage27,31. The earliest pathological events occurring in OA are proteoglycan depletion coupled with collagen network disruption32,33,34. Such early subtle surface changes are difficult to pinpoint and identify with bulk testing, because the mechanical behavior is averaged over the whole tissue depth. Additionally, a still unaddressed question is whether functional changes at the organ and tissue levels relate to micro- or nano-scale structural and functional changes. To this end, AFM is considered to be one of the most sensitive methods, capable of detecting the earliest biomechanical changes occurring with OA onset7. It allows stiffness measurements on both micro- and nanometer scales in native samples, providing information on the mechanical properties of articular cartilage35,36. In this protocol, using micro-AFM indentations, we measured the elastic properties of healthy and osteoarthritic articular cartilage human explants. The results showed that the cartilage explants are highly representative of early local OA events with a notable gradual decrease in stiffness occurring in pattern-specific cartilage explants. Furthermore, the results are in line with previous published research that showed a notable stiffness decrease alongside the cellular pattern organization23,24,27,37.
Corroborated native human models that mimic various aspects of OA pathogenesis and progression are currently needed to address the shortfalls of translational research and the challenges of translating in vitro data to a clinical setting.To date, no model can accurately represent the complex native human cartilage compartment, let alone the age-related joint tissues which are prone to OA in response to disease-initiating stimuli38. The most commonly used explant-based models thus far were of bovine or cattle origin and applied either a strong inflammatory cytokine treatment or mechanical loading39,40,41.This protocol, on the other side, demonstrates how to generate small (4 mm x 1 mm) explanted disc-shaped human cartilage samples, which are indicative of the individual stages of specific OA events. The cartilage explants are sorted and stage assigned using the cellular spatial organization as an image-based biomarker30,42. Since early changes in biomechanical properties can be already identified and quantified as soon as double strings start arising23,27, at a stage where the cartilage surface still appears macroscopically intact26, this explant-based model allows the investigation of a local native cartilage compartment and may provide insightful information on early OA. Furthermore, this cartilage model could be useful in investigating the cells and matrix response to mechanical and inflammatory alterations in a 3D native local habitat38,39. Being relatively simple and easy to generate, these cartilage explants can be also used to study OA heterogeneity, which is a limiting factor in developing and testing disease-modifying OA drugs43. It also has to be noted that scalability and dependency on patients undergoing joint replacement surgery are two of the model's shortcomings.
It is well known that articular cartilage presents a peculiar behavior depending on the scale level that is tested. As indicated by Loparic et al., at the micro-scale, the cartilage behaves as a nonstructured and uniform material35, and such an approach gives an approximation of localized overall cartilage stiffness. With respect to whether micro- or nano-indentations are better suited, a 2004 study by Stolz et al.44 compared both micro- and nano-scale indentations in assessing the structure-mechanical properties of articular cartilage. The authors emphasized that for micro-scale spherical indentation of the articular cartilage, the nano-scale fine structural components (i.e., individual collagen fibers and proteoglycans) commonly share the task of load bearing. In such, the aggregate mechanical properties differ markedly from those of the individual nanocomponents. The same authors proposed that a combination of micro- and nano-indentations could be used to assess the overall local stiffness profiles of articular cartilage, as well as the stiffness related to the fine structural components44.
Numerous AFM-based indentation experiments have used sharp pyramidal cantilever tips (radius = 15-20 nm)22,36,44 for assessing cartilage mechanics. Although the nanoindentations with sharp cantilevers are currently regarded to be more suitable for assessing the finest mechanical properties, spherical cantilever tips produce results that are more consistent and easier to model and interpret when testing soft biological samples44,45. Furthermore, Stolz et al. demonstrated that AFM nano-indentations of enzymatically (i.e., elastase) degraded articular cartilage are not possible because the tissue becomes so sticky that tip-sample adhesion dominates the force-distance curves, rendering the data unfeasible44.
In the present AFM measurements, a cantilever with a spherical cantilever tip of 5 µm radius was used. The cantilever choice was motivated by the intention to average the mechanical properties of the tissue over a fairly large surface while minimizing the inflicted damage on the cartilage surface. The relationship between the cantilever-applied force and the resulting sample indentation was fitted with the Hertz fit model. When using spherical indenters, the Hertz fit model is recommended, with the attractive forces acting between the cantilever tip and the sample surface being neglected46. The equations for the Hertzian model are shown in Eq.1 and Eq.2.
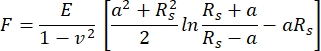 Eq.1
Eq.1
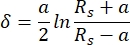 Eq.2
Eq.2
Where F = force; E = Young's modulus; v = Poisson's ratio; δ = indentation; a = radius of contact circle; and Rs = radius of sphere.
The model ultimately computes the cellular/tissue elasticity47, formally expressed as the Young's modulus (E). The Hertz fit model takes into consideration several characteristics such as the tip shape and size, indentation, and sample deformability. If these requirements are not ideally met, the model may provide an inaccurate estimate of the Young's modulus46.
The Hertz fit model assumes that the strain and elastic stress depend linearly on the elastic modulus, which implies that the indentation in the sample remains much smaller than the sample thickness itself46. This assumption was easily met in this setup, where the cartilage explants had a 1 mm thickness compared to indentations of few micrometers.
Articular cartilage can be modeled as a porous viscoelastic material48,49. The viscoelastic behavior results from friction between the intracellular/cytoplasmic or matrix constituents such as molecules, organelles, and the collagen-proteoglycan network50,51. As the name implies, viscoelastic materials combine two distinct properties: viscous-the material deforms slowly when subjected to an external load-and elastic-the material returns to its initial configuration once the applied load is removed52,53. The viscoelastic behavior is manifested as a hysteresis between the approach (extended) and retraction curves in the force-distance curves46,52, similar to the ones obtained in this study (Figure 4C). Furthermore, a characteristic of viscoelastic materials is that their mechanical properties depend on the deformation rate, with the material's stiffness increasing with the rate at which loading is applied (indentation speed)54. Thus, by selecting different loading rates, a family of force-distance curves is generated, each of which represents the mechanical properties of the tested sample at each loading rate52. So, when attempting to compare the outcomes of various works, it is critical to take all of the indentation parameters into account. Overall, when measuring at the micrometer scale (as in this study with a 5 µm spherical cantilever tip), articular cartilage behaves as a nonstructured and uniform material, generating a cumulative elastic modulus that includes both elastic and viscous contributions to stiffness due to the poroviscoelastic nature of the tissue35.
Another assumption of the Hertzian model is that the indentation depth is lower than the radius of the spherical cantilever tip55. The indentation depth represents the maximum displacement of the cantilever tip after first contact with the sample. At maximum load, the maximum indentation depth is the overall displacement of the sample and the cantilever tip. Bueckle's guideline states a maximum indentation depth of 10% of the overall thickness of a sample with the same structure throughout56, else, the results vary according to the depth-to-thickness ratio. For a cantilever tip radius of 5 µm, the cartilage explants in this study were indented at 1.1 µm on average, with a few peaks of 3 µm in a few instances, particularly for the highly degenerated cartilage explants.In this case, a compromise was sought, as, in the experimental setting, relatively high forces associated with large indentations are required to neutralizethe surface irregularities of degenerated cartilage.A milder indentation would result in the examination of superficial fibrillation and collagen fissuring, both of which are common features of highly degenerated cartilage57.
Crucial for the Hertz fit model is also the correct identification of the point at which the cantilever tip comes into direct contact with the sample, generically termed the contact point. However, this might turn out to be problematic when indenting too sticky or too soft samples, as it may result in multiple probe-sample contact points58,59. In fact, as nicely emphasized by A-Hassan et al., for soft biological tissues, the accurate determination of the point of contact is one of the most vexing problems60. This effect was also observed in the native osteoarthritic cartilage explants, as, depending on the stage of degeneration, the tissue surface loses its native mechanical characteristics and is often uneven, presenting superficial fibrillation and clefting (Figure 3B,C). This phenomenon was particularly noted in the cartilage explants where the dominant cellular pattern was big clusters (Figure 2C). These inhomogeneities in the cartilage surface might lead to multiple probe-sample contact points and, thus, erroneous results. Vast deflections were observed in some cases, followed by a rapid recovery of the baseline before the final stretch of the force-distance curve (Figure 5A). This could be attributed to a large obstacle in the cantilever tip's path (e.g., advanced fibrillation areas with fraying and splitting cartilage). In other instances, the final slope of the force-distance curve was scattered with smaller irregularities (Figure 5B), indicating contact with successively smaller hindrances (e.g., micro-fibrillation of the tissue). In such cases, the measurement site must be remeasured or even changed to ensure data reliability and reproducibility. To this end, it is also important to carefully inspect the force-distance curve output of the AFM for the correct identification of the contact point. This is a crucial point to be aware of, as it has been shown that an incorrect identification of the contact point by 50 nm results in an incorrect estimation of the value of E by an order of magnitude61. Several studies have begun to use automated approaches to determine the contact point of force-distance curves, with the goal of bypassing subjective user input when estimating the contact point by visual inspection and improving accuracy. This becomes even more crucial when dealing with a large number of force-displacement curves, such as those generated in cell mechanics mesurements47,62. Although several strategies have been proposed to automate the contact point determination47,63,64,65, the optimal strategy is highly dependent on experimental conditions and factors such as the model used to analyze the data, the shape of the probe, the (non-)adhesive mechanical interaction between the cantilever tip and the sample, as well as the (non-)Hertzian behavior of the sample63.
Sample drift is another common issue that may cause artifacts and erroneous contact point determination (Figure 3E). It basically means that the sample is not properly mounted in the sample holder (Petri dish) and the sample is moving during the AFM measurements. The effect is particularly pronounced when moving the AFM cantilever to a new measurement site. This aspect can be easily observed during the actual measurements by a sudden change in the focal plane. The resulting force-distance curves typically have a biphasic extended slope, with a mild rise at first, corresponding to the narrowing of the empty space between the bottom of the disc and the Petri dish as the disc is pushed down by the cantilever (see Figure 3E), followed by a firmer inclination in the second section of the slope, indicating that the disc is being further indented now that it is in direct contact with the bottom of the Petri dish (Figure 5C,D). To overcome the distortions, one can try to better fix the samples by using an adequate sample adhesive (Figure 3D), keeping the temperature constant by turning off external sources of heat (lights) to avoid thermal drifting, and conducting fast scanning measurements.In the experiments here, we observed a cantilever deflection drift that occurred within the first 15 min of the cantilever's immersion in media (due to sudden changes in temperature). After this time lapse, the drift is usually negligible. As a result, we advise the experimenter to carefully examine the baseline after cantilever immersion and to begin measuring once it has stabilized.The duration of this process can vary greatly depending on the cantilever used.
Another critical parameter for any AFM measurement is the set point, which is, simplistically, a measure of the force applied by the cantilever to the sample. For the contact mode (as used in this study), the set point represents a certain deflection of the cantilever. When performing several scans or several site repetitions, like in the protocol here, the cantilever tip can adsorb particles from the sample surface, thus making it sometimes necessary to remove the cantilever, properly clean it66, and then recalibrate before proceeding with the measurements.
While AFM micro-indentations provide new and interesting data collection opportunities, in particular in the context of osteoarthritic cartilage, the consistency and reproducibility of the data produced are heavily dependent on several parameters, as outlined above.When using this approach to assess the mechanical changes caused by cartilage tissue degeneration, some pilot measurements on various spatial patterns must first be performed in order to scale up the results to the specific experimental design.Pilot AFM measurements should be performed with the most standardizable procedure taking enough samples (e.g., five discs) of the same pattern to provide an indication of the extent of data variability. This is particularly important when attempting to quantify and assess the earliest relevant OA stiffness changes (i.e., between single strings and double strings, Figure 4A). In fact, in a previous study, using a similar approach, we showed that a sample size of 30 human specimens was required to assess biomechanical changes in the matrix as a function of the spatial organization of the cells37.
Furthermore, many of the steps presented in this protocol are susceptible to human error and heavily rely on the operator's experience.Given all of the factors that can influence the actual AFM results, the absolute E values reported in this study are not generalizable and are rather specific to this experimental setup. However, the relationship presented here between the various Young's moduli and the cellular pattern-based cartilage explants (the more pathologic the spatial pattern, the lower the elastic modulus [EM] of the cartilage) is unaffected, as the findings are consistent with previous studies showing stiffness changes as a function of cellular pattern organization23,37.
Overall, this step-to-step protocol demonstrates the functionality of standardized 3D native articular cartilage explants, which are not only representative of OA-driven cellular reorganization events ranging from onset to advanced progression but are also associated with a gradual decrease in stiffness. The explants may reflect a reliable biomimetic model for studying OA onset and progression, allowing the testing and development of different treatment modalities ex vivo. The use of such a human explant model in combination with AFM-based biomechanical assessment could result in a paradigm shift for biomedical research and the pharmaceutical industry, paving the way for new ways to identify greatly needed effective OA drugs.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank the orthopedic surgeons from the Department of Orthopaedic Surgery of the University Hospital of Tuebingen for providing the tissue samples.
Materiały
| Name | Company | Catalog Number | Comments |
| Amphotericin B | Merck KGaA, Darmstadt, Germany | 1397-89-3 | |
| Atomic force microscop (AFM) head | CellHesion 200, Bruker Nano GmbH, Berlin, Germany | JPK00518 | |
| Biocompatible sample glue | Bruker Nano GmbH, Berlin, Germany | H000033 | |
| Calcein AM | Cayman, Ann Arbor, Michigan, USA | 14948 | Cell membrane permeable stain, used for cartilage disc sorting- top view imaging |
| Cantilever | Bruker Nano GmbH, Berlin, Germany | SAA-SPH-5UM | Frequency Nom: 30KHz, k: 0.2N/m, lenght nom: 115μm, width nom: 40μm, geometry: rectangular, cylindrical tip with a 5μm end radius |
| Cartilage ctting device | Self-made | n/a | Cutting plastic device containing predefined wholes of 4mmx1mm |
| CDD camera integrated in the AFM | The Imaging Source Europe GmbH, Bremen, Germany | DFK 31BF03 | |
| CDD camera integrated in the fluorescence microscope | Leica Biosystems, Wetzlar, Germany | DFC3000G | |
| Cryotome | Leica Biosystems, Wetzlar, Germany | CM3050S | |
| Data Processing Software for the AFM | Bruker Nano GmbH, Berlin, Germany | n/a | Version 5.0.86, can be downloaded for free from the following website https://customers.jpk.com |
| Dulbecco's modified Eagle's medium (DMEM) | Gibco, Life Technologies, Darmstadt, Germany | 41966052 | |
| Fluorescence Microscope (Leica DMi8) | Leica Biosystems, Wetzlar, Germany | 11889113 | |
| Glass block cantiliver holder | Bruker Nano GmbH, Berlin, Germany | SP-90-05 | Extra long glass block with angled faces, designed especially for the use with the JPK PetriDishHeaterTM (Bruker). |
| Inverted phase contrast microscope (integrated in the AFM) | AxioObserver D1, Carl Zeiss Microscopy, Jena, Germany | L201306_03 | |
| Leibovitz's L-15 medium without L-glutamine | Merck KGaA, Darmstadt, Germany | F1315 | |
| Microscope glass slides | Sigma-Aldrich, St. Louis, Missouri, USA | CLS294775X50 | |
| Mounting medium With DAPI | ibidi GmbH, Gräfelfing, Germany | 50011 | Mounting media with nuclear DAPI (4′,6-diamidino-2-phenylindole) counterstaining used for cartilage discs side view imaging |
| Penicillin-Streptomycin | Sigma-Aldrich, St. Louis, Missouri, USA | P4333 | |
| Petri dish heater associated with AFM (Petri Dish Heater) | Bruker Nano GmbH, Berlin, Germany | T-05-0117 | |
| Scalpel | Feather Medical Products, Osaka, Japan | 2023-01 | |
| Silicone Skirt | Bruker Nano GmbH, Berlin, Germany | n/a | Protective silicone membrane (D55x0.25) which is placed on the basis of the base of the glas block to prevent medium condensation in the AFM head. |
| Statistical program - SPSS | IBM, Armonk, New York, USA | SPSS Statistics 22 | Vesion 280.0.0.0 (190) |
| Tissue culture dishes | TPP Techno Plastic Products AG, Trasadingen, Switzerland | TPP93040 | |
| Tissue-tek O.C.T. Compound | Sakura Finetek, Alphen aan den Rijn, Netherlands | SA6255012 | Water-soluble embedding medium |
Odniesienia
- Allison, D. P., Mortensen, N. P., Sullivan, C. J., Doktycz, M. J. Atomic force microscopy of biological samples. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology. 2 (6), 618-634 (2010).
- Deng, X., et al. Application of atomic force microscopy in cancer research. Journal of Nanobiotechnology. 16 (1), 102 (2018).
- Radmacher, M. Studying the mechanics of cellular processes by atomic force microscopy. Methods in Cell Biology. 83, 347-372 (2007).
- Charras, G. T., Horton, M. A. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophysical Journal. 82 (6), 2970-2981 (2002).
- Rabinovich, Y., et al. Atomic force microscopy measurement of the elastic properties of the kidney epithelial cells. Journal of Colloid and Interface Science. 285 (1), 125-135 (2005).
- Dufrêne, Y. F. Using nanotechniques to explore microbial surfaces. Nature Reviews Microbiology. 2 (6), 451-460 (2004).
- Cykowska, A., Danalache, M., Bonnaire, F. C., Feierabend, M., Hofmann, U. K. Detecting early osteoarthritis through changes in biomechanical properties - A review of recent advances in indentation technologies in a clinical arthroscopic setup. Journal of Biomechanics. 132, 110955 (2022).
- Gavara, N. A beginner's guide to atomic force microscopy probing for cell mechanics. Microscopy Research and Technique. 80 (1), 75-84 (2017).
- Fuchs, J., Kuhnert, R., Scheidt-Nave, C. 12-Monats-Prävalenz von Arthrose in Deutschland. Journal of Health Monitoring. 2, 55-60 (2017).
- Felson, D. T. Osteoarthritis of the knee. New England Journal of Medicine. 354 (8), 841-848 (2006).
- Ganz, R., Leunig, M., Leunig-Ganz, K., Harris, W. H. The etiology of osteoarthritis of the hip. Clinical Orthopaedics and Related Research. 466 (2), 264-272 (2008).
- Saxby, D. J., Lloyd, D. G. Osteoarthritis year in review 2016: Mechanics. Osteoarthritis and Cartilage. 25 (2), 190-198 (2017).
- Buckwalter, J. A., Mankin, H. J. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional Course Lectures. 47, 487-504 (1998).
- Braun, H. J., Gold, G. E. Diagnosis of osteoarthritis: Imaging. Bone. 51 (2), 278-288 (2012).
- Guermazi, A., Roemer, F. W., Burstein, D., Hayashi, D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Research & Therapy. 13 (6), 247 (2011).
- Guermazi, A., et al. Different thresholds for detecting osteophytes and joint space narrowing exist between the site investigators and the centralized reader in a multicenter knee osteoarthritis study--Data from the Osteoarthritis Initiative. Skeletal Radiology. 41 (2), 179-186 (2012).
- Bedson, J., Croft, P. R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskeletal Disorders. 9 (1), 116 (2008).
- Dashefsky, J. H. Arthroscopic measurement of chondromalacia of patella cartilage using a microminiature pressure transducer. Arthroscopy. 3 (2), 80-85 (1987).
- Berkenblit, S. I., Frank, E. H., Salant, E. P., Grodzinsky, A. J. Nondestructive detection of cartilage degeneration using electromechanical surface spectroscopy. Journal of Biomechanical Engineering. 116 (4), 384-392 (1994).
- Appleyard, R. C., Swain, M. V., Khanna, S., Murrell, G. A. The accuracy and reliability of a novel handheld dynamic indentation probe for analysing articular cartilage. Physics in Medicine and Biology. 46 (2), 541-550 (2001).
- Hsieh, C. H., et al. Surface ultrastructure and mechanical property of human chondrocyte revealed by atomic force microscopy. Osteoarthritis and Cartilage. 16 (4), 480-488 (2008).
- Stolz, M., et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nature Nanotechnology. 4 (3), 186-192 (2009).
- Tschaikowsky, M., et al. Proof-of-concept for the detection of early osteoarthritis pathology by clinically applicable endomicroscopy and quantitative AI-supported optical biopsy. Osteoarthritis and Cartilage. 29 (2), 269-279 (2021).
- Tschaikowsky, M., et al. Hybrid fluorescence-AFM explores articular surface degeneration in early osteoarthritis across length scales. Acta Biomaterialia. 126, 315-325 (2021).
- Eaton, P., Batziou, K., Santos, N. C., Carvalho, F. A. Artifacts and Practical Issues in Atomic Force Microscopy. Atomic Force Microscopy: Methods and Protocols. , 3-28 (2019).
- Danalache, M., et al. Exploration of changes in spatial chondrocyte organisation in human osteoarthritic cartilage by means of 3D imaging. Scientific Reports. 11, 9783 (2021).
- Danalache, M., et al. Changes in stiffness and biochemical composition of the pericellular matrix as a function of spatial chondrocyte organisation in osteoarthritic cartilage. Osteoarthritis and Cartilage. 27 (5), 823-832 (2019).
- Danalache, M., Erler, A. L., Wolfgart, J. M., Schwitalle, M., Hofmann, U. K. Biochemical changes of the pericellular matrix and spatial chondrocyte organization-Two highly interconnected hallmarks of osteoarthritis. Journal of Orthopaedic Research. 38 (10), 2170-2180 (2020).
- Danalache, M., Tiwari, A., Sigwart, V., Hofmann, U. K. Application of atomic force microscopy to detect early osteoarthritis. Journal of Visualized Experiments. (159), e61041 (2020).
- Rolauffs, B., et al. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis and Rheumatism. 62 (2), 489-498 (2010).
- Wilusz, R. E., DeFrate, L. E., Guilak, F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. Journal of The Royal Society Interface. 9 (76), 2997-3007 (2012).
- Guilak, F., Ratcliffe, A., Lane, N., Rosenwasser, M. P., Mow, V. C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. Journal of Orthopaedic Research. 12 (4), 474-484 (1994).
- Billinghurst, R. C., et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. The Journal of Clinical Investigation. 99 (7), 1534-1545 (1997).
- Wu, P. J., et al. Detection of proteoglycan loss from articular cartilage using Brillouin microscopy, with applications to osteoarthritis. Biomedical Optics Express. 10 (5), 2457-2466 (2019).
- Loparic, M., et al. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: Validation with a gel-microfiber composite. Biophysical Journal. 98 (11), 2731-2740 (2010).
- Moshtagh, P. R., Pouran, B., Weinans, H., Zadpoor, A. The elastic modulus of articular cartilage at nano-scale and micro-scale measured using indentation type atomic force microscopy. Osteoarthritis and Cartilage. 22, 359-360 (2014).
- Danalache, M., Jacobi, L. F., Schwitalle, M., Hofmann, U. K. Assessment of biomechanical properties of the extracellular and pericellular matrix and their interconnection throughout the course of osteoarthritis. Journal of Biomechanics. 19, 109409 (2019).
- Houtman, E., et al. Human osteochondral explants: Reliable biomimetic models to investigate disease mechanisms and develop personalized treatments for osteoarthritis. Rheumatology and Therapy. 8 (1), 499-515 (2021).
- Anderson, J. R., Phelan, M. M., Foddy, L., Clegg, P. D., Peffers, M. J. Ex vivo equine cartilage explant osteoarthritis model: A metabolomics and proteomics study. Journal of Proteome Research. 19 (9), 3652-3667 (2020).
- Chen, C. T., Torzilli, P. A., Olson, S. A., Gauilak, F. In vitro cartilage explant injury models. Post-Traumatic Arthritis: Pathogenesis, Diagnosis and Management. , 29-40 (2015).
- Thudium, C. S., Engstrom, A., Groen, S. S., Karsdal, M. A., Bay-Jensen, A. -. C. An ex vivo tissue culture model of cartilage remodeling in bovine knee explants. Journal of Visualized Experiments. (153), e59467 (2019).
- Rolauffs, B., Williams, J., Grodzinsky, A., E Kuettner, K., Cole, A. Distinct horizontal patterns in the spatial organization of superficial zone chondrocytes of human joints. Journal of Structural Biology. 162 (2), 335-344 (2008).
- Deveza, L. A., Loeser, R. F. Is osteoarthritis one disease or a collection of many. Rheumatology. 57, 34-42 (2018).
- Stolz, M., et al. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophysical Journal. 86 (5), 3269-3283 (2004).
- Sicard, D., Fredenburgh, L. E., Tschumperlin, D. J. Measured pulmonary arterial tissue stiffness is highly sensitive to AFM indenter dimensions. Journal of the Mechanical Behavior of Biomedical Materials. 74, 118-127 (2017).
- Krieg, M., et al. Atomic force microscopy-based mechanobiology. Nature Reviews Physics. 1 (1), 41-57 (2019).
- Gavara, N. Combined strategies for optimal detection of the contact point in AFM force-indentation curves obtained on thin samples and adherent cells. Scientific Reports. 6, 21267 (2016).
- Mow, V. C., Kuei, S. C., Lai, W. M., Armstrong, C. G. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. Journal of Biomechanical Engineering. 102 (1), 73-84 (1980).
- Armstrong, C. G., Lai, W. M., Mow, V. C. An analysis of the unconfined compression of articular cartilage. Journal of Biomechanical Engineering. 106 (2), 165-173 (1984).
- Deng, L., et al. Fast and slow dynamics of the cytoskeleton. Nature Materials. 5 (8), 636-640 (2006).
- Fischer-Friedrich, E., et al. Rheology of the active cell cortex in mitosis. Biophysical Journal. 111 (3), 589-600 (2016).
- Gould, T. E., Jesunathadas, M., Nazarenko, S., Piland, S. G., Subic, A. Chapter 6 - Mouth Protection in Sports. Materials in Sports Equipment (Second Edition). , 199-231 (2019).
- Kontomaris, S. V., Malamou, A. Hertz model or Oliver & Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Materials Research Express. 7 (3), 033001 (2020).
- Guz, N., Dokukin, M., Kalaparthi, V., Sokolov, I. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophysical Journal. 107 (3), 564-575 (2014).
- Wu, C. -. E., Lin, K. -. H., Juang, J. -. Y. Hertzian load-displacement relation holds for spherical indentation on soft elastic solids undergoing large deformations. Tribology International. 97, 71-76 (2016).
- Westbrook, J. H., Conrad, H. . The Science of Hardness Testing and its Research Applications. , (1973).
- Pritzker, K. P. H., et al. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis and Cartilage. 14 (1), 13-29 (2006).
- Stylianou, A., Kontomaris, S. V., Grant, C., Alexandratou, E. Atomic force microscopy on biological materials related to pathological conditions. Scanning. 2019, 8452851 (2019).
- Sokolov, I. Atomic force microscopy in cancer cell research. Cancer Nanotechnology. 1, 1-17 (2007).
- Emad, A., et al. Relative microelastic mapping of living cells by atomic force microscopy. Biophysical Journal. 74 (3), 1564-1578 (1998).
- Crick, S. L., Yin, F. C. Assessing micromechanical properties of cells with atomic force microscopy: Importance of the contact point. Biomechanics and Modeling in Mechanobiology. 6 (3), 199-210 (2007).
- Shoelson, B., Dimitriadis, E. K., Cai, H., Kachar, B., Chadwick, R. S. Evidence and implications of inhomogeneity in tectorial membrane elasticity. Biophysical Journal. 87 (4), 2768-2777 (2004).
- Lin, D. C., Dimitriadis, E. K., Horkay, F. Robust strategies for automated AFM force curve analysis--I. Non-adhesive indentation of soft, inhomogeneous materials. Journal of Biomechanical Engineering. 129 (3), 430-440 (2007).
- Rudoy, D., Yuen, S. G., Howe, R. D., Wolfe, P. J. Bayesian change-point analysis for atomic force microscopy and soft material indentation. Journal of the Royal Statistical Society: Series C (Applied Statistics). 59 (4), 573-593 (2010).
- Benítez, R., Moreno-Flores, S., Bolós, V. J., Toca-Herrera, J. L. A new automatic contact point detection algorithm for AFM force curves. Microscopy Research and Technique. 76 (8), 870-876 (2013).
- Timashev, P. S., et al. Cleaning of cantilevers for atomic force microscopy in supercritical carbon dioxide. Russian Journal of Physical Chemistry B. 8 (8), 1081-1086 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone