Method Article
A Porcine Corneal Endothelial Organ Culture Model Using Split Corneal Buttons
W tym Artykule
Podsumowanie
Here, a step-by-step protocol for the preparation and cultivation of porcine split corneal buttons is presented. As this organo-typically cultivated organ culture model shows cell death rates within 15 days, comparable to human donor corneas, it represents the first model allowing long-term cultivation of non-human corneas without adding toxic dextran.
Streszczenie
Experimental research on corneal endothelial cells is associated with several difficulties. Human donor corneas are scarce and rarely available for experimental investigations as they are normally needed for transplantation. Endothelial cell cultures often do not translate well to in vivo situations. Due to the biostructural characteristics of non-human corneas, stromal swelling during cultivation induces substantial corneal endothelial cell loss, which makes it difficult to perform cultivation for an extended period of time. Deswelling agents such as dextran are used to counteract this response. However, they also cause significant endothelial cell loss. Therefore, an ex vivo organ culture model not requiring deswelling agents was established. Pig eyes from a local slaughterhouse were used to prepare split corneal buttons. After partial corneal trephination, the outer layers of the cornea (epithelium, bowman layer, parts of the stroma) were removed. This significantly reduces corneal endothelial cell loss induced by massive stromal swelling and Descemet's membrane folding throughout longer cultivation periods and improves general preservation of the endothelial cell layer. Subsequent complete corneal trephination was followed by the removal of the split corneal button from the remaining eye bulb and cultivation. Endothelial cell density was assessed at follow-up times of up to 15 days after preparation (i.e., days 1, 8, 15) using light microscopy. The preparation technique used allows a better preservation of the endothelial cell layer enabled by less stromal tissue swelling, which results in slow and linear decline rates in split corneal buttons comparable to human donor corneas. As this standardized organo-typically cultivated research model for the first time allows a stable cultivation for at least two weeks, it is a valuable alternative to human donor corneas for future investigations of various external factors with regards to their effects on the corneal endothelium.
Wprowadzenie
Corneal transplantation procedures are among the most commonly performed transplantations worldwide1. As there is a severe shortage of human donor corneas, experimental research addressing corneal endothelial cells in human corneas is difficult to perform1. However, the introduction of irrigation solutions and other substances used within the eye, ophthalmic viscoelastic devices, as well as surgical instruments and techniques (e.g., phacoemulsification instruments and techniques, ultrasound energy) requires valid and extensive investigations regarding their effects on the corneal endothelium before clinical use.
Few alternatives to human donor corneas exist for research. Animal research models are very valuable but at the same time very resource consuming and increasingly questioned ethically. A major drawback of in vitro cell cultures is their limited translation to the human eye. Results obtained from cell cultures can be incongruous to in vivo conditions, because cells may undergo endothelial mesenchymal transition (EMT), resulting in fibroblast-like morphology caused by the loss of cell polarity and changes in cell shape and gene expression2.
Whereas previous ex vivo models reported cultivation periods of up to only 120 h, a novel preparation technique to establish a porcine corneal endothelial organ culture model by culturing fresh pig corneas for at least 15 days was recently introduced3,4,5,6. If the corneal epithelium and parts of the stroma are removed (approximately 300 µm in total) from the cornea prior to cultivation, swelling of the stroma is reduced in split corneal buttons resulting in less endothelial cell loss and a well maintained endothelial cell layer after up to 15 days, whereas non-split corneal buttons show significant endothelial cell loss due to uneven stromal swelling and formation of Descemet's folds. Eye banks usually use osmotic deswelling agents such as dextran to reduce swelling of corneas prior to transplantation. However, these agents were shown to induce increased endothelial cell loss7,8,9.
This article aims to visualize this standardized ex vivo research model in a detailed step-by-step protocol in order to enable future investigators to perform research on the corneal endothelium using split corneal buttons. This model represents a straightforward method to test substances and techniques used within the eye, such as ophthalmic viscoelastic devices, irrigation solutions, and ultrasound energy, or other procedures where the corneal endothelium is of interest.
Protokół
This protocol follows the ethical guidelines of our institution. In accordance with the statutes of our institution's ethical review committee no ethical approval had to be obtained prior to the experiments, as all porcine corneas were obtained from the local slaughterhouse.
1. Organ culture
- Prepare pig eyes.
- From the local slaughterhouse, obtain pig eyes that were removed shortly postmortem but before thermal treatment. Transport the eyes to the lab and process them within a few hours. During transport, keep the eyes at room temperature (approximately 21 °C) before processing.
- Remove all orbital adnexes (eye muscles, conjunctiva, adipose tissue) prior to disinfection using eye scissors and colibri forceps. Separate and discard all eyes with obvious trauma, external damage (e.g., knife cut), or visible corneal opacities.
- Prepare a 5% iodine-PBS-solution (1:20) in a sterile cup by adding 3 mL of 7.5% povidone iodine to 57 mL of a phosphate buffered saline (PBS) solution. Also prepare a separate sterile cup containing 60 mL pure PBS.
NOTE: The total amount of used iodine-PBS-solution and the size of the cup depends on the number of corneas to be dissected. Five to six eyes require approximately 60 mL of the 5%-iodine-PBS-solution to be disinfected properly. - Put five to six eyes into the 5%-iodine-PBS solution for a total of 5 min to properly disinfect the eyes' surface. Carefully stir every minute to ensure the eyes' surface is fully submerged and disinfected completely in the iodine solution.
- After 5 min, transfer the disinfected eyes to the prepared cup filled with pure PBS. Again, carefully stir to ensure the iodine-PBS-solution is washed away from the eyes' surface.
NOTE: Perform this step on a clean bench to prevent contamination of the disinfected eyes.
- Prepare cell culture plates.
NOTE: Perform the following steps on a clean bench with constant laminar air flow.- Thaw 2 mL of fetal calf serum (FCS). Fill a syringe (5 mL) with the FCS using a blunt cannula. Empty the syringe through a syringe filter (0.22 µm) to prevent possible bacterial contaminationof the FCS into 80 mL of dextran-free culture medium I (minimum essential medium [MEM] with Earle's salts, penicillin/streptomycin, L-glutamine [200 mM], amphotericin B [250 µg/mL], Hepes buffer [1 M] [50x], NaHCO3, and distilled water). Agitate the mixture to ensure even substrate distribution.
- Fill each well of a 12 well cell culture plate with 3 mL of the substrate mixture.
- Perform dissection and incubation.
NOTE: Perform this step on a clean bench. Do not touch the corneal endothelium with any instruments.- Transfer an eye bulb from the cup with PBS into the eye bulb holder with the cornea facing up and place it beneath an ophthalmic surgical microscope. Use a syringe filled with 0.9% NaCl to slightly apply some suction to the eye over the eye bulb holder to fixate the eye in position for dissection.
- Use a trephine (ø 7.5 mm) containing a standardized inlay, which ensures the trephined depth will not exceed 300 µm, to cut superficially into the central cornea (Figure 1A).
- Use colibri forceps and a single-use scalpel with a triangular blade to cut and remove the partially trephined part of the cornea horizontally through the stroma. Discard the separated corneal part consisting of the corneal epithelium, the bowman layer, and a part of the stroma.
NOTE: Strictly maintain the horizontal cutting direction to obtain an even thickness of the remaining stroma. - Superficially place a 10-0 suture (10-0 polyamide 6) into the stroma to be able to differentiate the endothelial from the stromal side during the experiments (Figure 1B). Prevent penetration of the corneal endothelium. Discard the eye bulb if the endothelium is penetrated.
NOTE: Penetration of the corneal endothelium with the suturing needle will be visible, as fluid from the anterior eye chamber will leak through the suture channel. - Use the trephine without the inlay to advance the trephined cut to full depth until the anterior eye chamber is reached (Figure 1C). A distinct drop in resistance and fluid leaking from the anterior eye chamber can be perceived after complete penetration of the cornea.
NOTE: Use a hockey knife if the split corneal button remains attached on one side of the split corneal button after trephination. - Transfer the obtained split corneal button, now consisting of a part of the stroma (Figure 2), the Descemet's membrane, and the corneal endothelium, into the culture medium (culture medium I + FCS, see section 1.2) into the 12 well cell culture plate with the tagged side facing down, so that the corneal endothelium is facing up.
NOTE: If the endothelial side is facing down, the endothelium may get damaged. - Assign an individual number to every split corneal button for identification during the follow ups and label the wells on the cell culture plate accordingly.
- Incubate the filled cell culture plates in an incubator under standard conditions at a temperature of 37 °C, 5% CO2 and a relative air moisture of 95%. Change the culture medium (culture medium I + FCS) on day 8 if an incubation period of 7 days is exceeded.
NOTE: The incubation procedure using split corneal buttons has been validated for up to 15 days.

Figure 1: Dissection of the porcine cornea to obtain split corneal buttons. (A) After trephination of the cornea using a trephine with an inlay to cut into a depth of 300 µm and removal of the epithelium and parts of the stromal tissue, (B) a suture is placed superficially into the stroma without penetration of the corneal endothelium for later identification of the stromal side. (C) Full trephination of the remaining cornea is followed by (D) the removal of the obtained split corneal button from the eye bulb. Please click here to view a larger version of this figure.
2. Microscopy and examination of the endothelium
- Perform unstained examination.
NOTE: Unstained examination can be performed multiple times (e.g., at weekly follow ups on day 1, 8, and 15). However, unstained counting only allows the assessment of endothelial cell density, not morphological parameters.- Fill 3 mL of hypotonic balanced salt solution (hBSS, see composition in Table of Materials) in each well of a 12 well cell culture plate. Carefully place a single split corneal button in hBSS to induce swelling of the corneal endothelial cells and improve visibility of the cells for cell counting.
NOTE: Make sure that the endothelial side is facing the direction of the microscope. If an inverted phase contrast microscope is used, the endothelial side needs to be facing downwards. While in place, the culture plate should not be moved to prevent endothelial cell damage. - Let the cells swell for 1-2 min before taking a picture of the endothelium with the camera attached to the microscope. Take at least three photographs of at least three different areas in order to obtain a representative impression of the actual condition of the corneal endothelium. Add a scale bar with the true to scale length of 100 µm for later analysis of the corneal endothelial cell density and morphological parameters.
- To prevent osmotic damage, remove the split corneal button from the hBSS after a maximum of 5 min and transfer it back into the culture medium3.
- Fill 3 mL of hypotonic balanced salt solution (hBSS, see composition in Table of Materials) in each well of a 12 well cell culture plate. Carefully place a single split corneal button in hBSS to induce swelling of the corneal endothelial cells and improve visibility of the cells for cell counting.
- Perform stained examination.
NOTE: Staining terminates the experiments, as the staining substances used are cytotoxic. Therefore, staining may only be performed at the end of the observation period for the assessment of morphological parameters (reformation figures, rosette formations, alizarin red stained cells).- Prepare a 0.25% trypan blue solution and 0.2% alizarin red S solution for the staining procedure.
- For the 0.25% trypan blue solution, dilute the 0.4% trypan blue solution with a 0.9% NaCl solution.
NOTE: For example, to obtain 20 mL of a 0.25% trypan blue solution, dilute 12.5 mL of the 0.4% trypan blue solution with 7.5 mL of the 0.9% NaCl solution. The trypan blue solution may be stored at room temperature for several weeks or months. - To obtain a 0.2% alizarin red S solution dissolve 100 mg of the alizarin red S powder in 50 mL of a 0.9% NaCl solution under constant stirring and heating to 50 °C on a magnet stirring plate with heating function. To remove possible precipitates, filter the obtained solution. Adjust the pH of alizarin red S solution to pH 4.2 by adding sodium hydroxide or hydrochloric acid accordingly.
NOTE: Before each application of the alizarin red S solution make sure the pH is corrected to 4.2 and that there are no precipitates in the solution. The solution may be stored at room temperature. Do not store the solution for longer than 4 weeks.
- For the 0.25% trypan blue solution, dilute the 0.4% trypan blue solution with a 0.9% NaCl solution.
- Place the split corneal buttons in Petri dishes with the endothelial side facing upwards in order to stain the endothelial cells. Use a pipette to slowly drip the 0.25% trypan blue solution drop by drop onto the corneal endothelium for 90 s.
NOTE: Trypan blue allows identification of damaged corneal cells with a permeable membrane because it stains their nuclei. - Carefully rinse the split corneal button 3x in 0.9% NaCl in a small glass beaker. Again, use a pipette to slowly drip the 0.2% alizarin red S solution drop by drop onto the corneal endothelium for 90 s.
NOTE: Alizarin red S stains the Descemet's membrane, which helps to highlight cell borders in undamaged corneal endothelial cells and to highlight destroyed cells when the underlying Descemet's membrane becomes visible. Also, it enables easy identification of larger destroyed areas. - For examination of the stained corneal endothelium follow the steps explained for unstained counting in section 2.1.
- Prepare a 0.25% trypan blue solution and 0.2% alizarin red S solution for the staining procedure.
3. Analysis of the corneal endothelial cell density and morphological parameters
- Project counting squares on the pictures using a graphics editing software (Table of Materials).
- Open the software and open an image of the corneal endothelium by selecting File | Open | Select.
- Project a square with a true to scale side length of 100 µm on the image.
NOTE: The following steps of section 3.1.2 can be ignored if the viewing software allows for the projecting of counting squares onto the picture. The side length of the square depends on the resolution of the images taken with the microscope's camera and can be calculated with the scale bar.- Crop the scale bar from the photograph taken with the microscope: select the Rectangular Marquee Tool on the tool bar or press M, then border the scale bar, then select Edit | Crop or press Ctrl + X.
- Click File | New (or press Ctrl + N). On the upcoming window select Clipboard in the Document Type drop-down list. The number of pixels shown is the side length of the counting square. Note the corresponding pixels for the other pictures and click OK.
- Select File | New (or Ctrl + N). Insert width and length (in pixels) of the counting square in the upcoming window according to the length of the scale bar. Select background color White, then press OK.
- Click Select | Select all (or Ctrl + A). Select Edit | Copy (or Ctrl + C).
- Select the image of the corneal endothelium opened in step 3.1.1. Select Edit | Paste (or Ctrl + V) to insert the square on the photo of the corneal endothelium.
- Select the square's layer and adjust the transparency. Select Layer | Layer Style | Blending Options | Set Opacity to 30% | OK.
- Save the image with the projected square with a true to scale side length of 100 µm. Repeat with the other pictures needed for analysis.
- Assess the endothelial cell density and morphological parameters.
- Open the images with the projected squares in ImageJ (Version 1.50i) and use the CellCounter PlugIn to count the cells within the square. Open ImageJ, select File | Open (or Ctrl + O) | Select Image.
NOTE: ImageJ and the CellCounter PlugIn are freeware available for download online. - Select Plugins | Cell_Counter | Cell Counter | Initialize. Count the endothelial cells and record the result. On two sides of the square, count the cells that are cut by the square's edge. Do not count cut cells on the other two sides.
- Analyze at least six squares per cornea from different areas (e.g., three pictures, two squares per picture) and determine the average corneal endothelial cell density per square (100 µm2). Extrapolate the endothelial cell density per 100 µm2 to 1 mm2 by multiplying by 100.
- Open the images with the projected squares in ImageJ (Version 1.50i) and use the CellCounter PlugIn to count the cells within the square. Open ImageJ, select File | Open (or Ctrl + O) | Select Image.
- For the assessment of morphological changes, count reformation figures (joint meeting of ≥4 cells/cell borders instead of three), rosette formations (characteristic rosette-shaped appearance, five or more radially arranged cells surrounding a destroyed cell), or alizarin red areas (destroyed cells) in the projected squares.
NOTE: Alternatively, it may be useful to use larger squares (e.g., 200 x 200 µm2) for morphological analysis in well-preserved samples to obtain more representative results.
Wyniki
The presented dissection technique implies partial removal of stromal tissue, resulting in a thinner cornea sample and thus less stromal swelling (Figure 1 and Figure 2). Less stromal swelling induces less shear and pinch forces that have a negative impact on the corneal endothelium, thus causing lower endothelial cell loss rates6. Split corneal buttons show a significantly better-preserved endothelial cell layer after 15 days of cultivation compared to non-split corneal buttons and whole corneoscleral samples, which reflects less endothelial cell loss and a lower number of reformation figures (≥ 4 cells/cell borders conjoined instead of three), rosette formations (five or more radially arranged cells meeting in a single spot), and alizarin red (destroyed) cells after 15 days6.
Over a period of 15 days, split corneal buttons show a steady decline in endothelial cell density with an average weekly percental endothelial cell loss of 4.90% (n = 40, Figure 3). Starting off on day 1 with an endothelial cell density of 4,033 ± 146/163 cells/mm2 (median ± 25%/75% quartiles), cell density decreased to 3,850 ± 167/233 cells/mm2 on day 8, and 3,650 ± 200/233 cells/mm2 on day 15. Determined cell losses were similar for the first and second week. On days 1−8, 4.00 ± 2.17/1.93% (median ± 25%/75% quartiles); days 8−15, 4.88 ± 5.52/4.42%; days 1−15, 8.64 ± 4.32/2.71%). Thus, the given decline rate is similar to the rate reported in human donor corneas during cultivation in previous studies10,11,12.
Morphological parameters were assessed after staining of the endothelial cell layer on day 15 (n = 28, Figure 4). Reformation figures made up 7.18 ± 2.36/2.90% (median ± 25%/75% quartiles) of the merging cell borders. A median of 1.11 ± 1.11/15.56 rosette formations/mm2 (median ± 25%/75% quartiles) were present in the investigated samples, whereas 13.33 ± 4.44/11.67 alizarin red stained cells/mm2 (median ± 25%/75% quartiles) marked punctual cell losses.
Figure 5 provides representative images of the corneal endothelium during microscopic evaluation in hBSS (Figure 5A) and after staining with trypan blue and alizarin red S (Figure 5B). The assessment of the endothelial cell density in hBSS can be performed multiple times (e.g., on days 1, 8, and 15), whereas stained samples can be used for the assessment of morphological parameters (reformation figures, rosette formations, alizarin red stained cells) or determination of endothelial cell density at the end of the experiments due to the cytotoxic properties of most staining substances.
If the split corneal buttons are placed and cultivated with the endothelial side facing down by accident, extensive endothelial cell damage is to be expected (Figure 6A). Non-split corneal buttons suffer significantly increased endothelial cell loss due to stromal swelling, causing Descemet's membrane folding over 15 days of cultivation (Figure 6B), whereas split corneal buttons show a largely preserved corneal endothelium after 15 days of cultivation, indicated by scattered punctual alizarin red stained areas indicating single destroyed cells (Figure 6C).
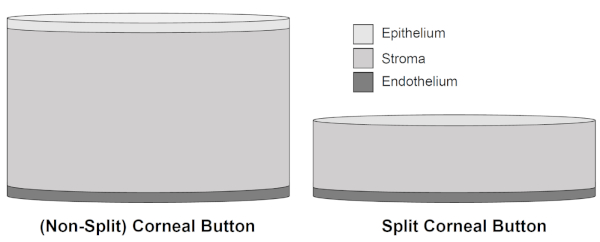
Figure 2: Schematic illustration of non-split corneal buttons and split corneal buttons. After removal of 300 µm of the porcine cornea, including epithelium and major parts of the stromal tissue, the thickness of split corneal buttons is reduced in favor of better conservation of the corneal endothelium due to decreased stromal swelling throughout cultivation of up to 15 days. Please click here to view a larger version of this figure.
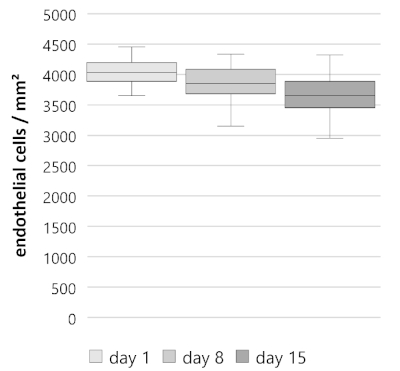
Figure 3: Endothelial cell density (ECD) of split corneal buttons over 15 days. Split corneal buttons (n = 40) showed a steady decline. ECD on day 1, 4,033 ± 146/163 cells/mm2 (median ± 25%/75% quartiles); day 8, 3,850 ± 167/233 cells/mm2; day 15, 3,650 ± 200/233 cells/mm2. Data is depicted as median ± 25%/75% quartiles, whiskers represent either minimum and maximum or 1.5 of the interquartile range (IQR). Please click here to view a larger version of this figure.
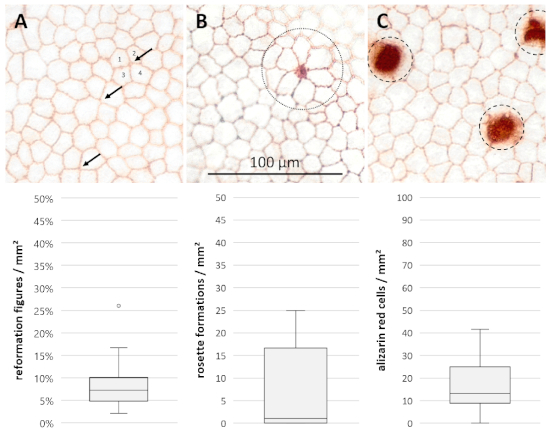
Figure 4: Morphological parameters for additional evaluation of cell damage and rearrangement processes. Microscopic photographs of the corneal endothelium in 400x magnification after 15 days of cultivation and staining with trypan blue and alizarin red S showing (A) reformation figures (arrows, joint meeting of four or more cells/cell borders instead of three), (B) rosette formations (dotted circle, central diminishing cell with characteristic rosette formations of five or more adjacent cells) and (C) alizarin red stained cells (dashed circles, destroyed cells). Data (n = 28) are depicted as median ± 25%/75% quartiles. Whiskers represent either minimum and maximum or 1.5 of the interquartile range (IQR). Circles represent outliers not within the IQR. Please click here to view a larger version of this figure.

Figure 5: Unstained and stained corneal endothelium. The endothelial cell layer as seen during microscopic evaluation (400x magnification) in (A) hypotonic balanced salt solution (hBSS) causing endothelial cell swelling and thus better cell visibility and (B) after staining with trypan blue and alizarin red S. Please click here to view a larger version of this figure.
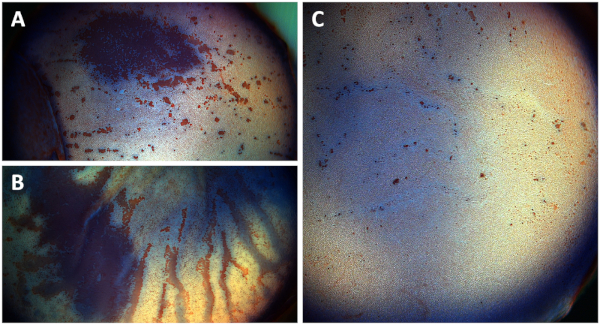
Figure 6: Overview photographs of the corneal endothelium of (A) a split corneal button cultivated upside down, (B) a non-split corneal button, and (C) a split corneal button after staining. Photographs show the corneal endothelium after staining with trypan blue and alizarin red S. (A) Extensive corneal endothelial cell damage (red area) is observed after split corneal buttons are cultivated with the endothelial side facing down after one week of cultivation. (B) Significantly increased endothelial cell damage in non-split corneal buttons due to Descemet's membrane folding and stromal swelling after 15 days of cultivation (red streaks). (C) Split corneal buttons show a well-preserved endothelial cell layer after 15 days of cultivation only showing punctual destroyed cells seen in alizarin red stained areas. Please click here to view a larger version of this figure.
Dyskusje
This protocol provides a method for the preparation of porcine split corneal buttons, which represents a standardized and low-cost ex vivo corneal endothelial organ culture model for research purposes6. Porcine split corneal buttons showed a decrease of the endothelial cell density comparable to endothelial cell losses observed in human donor corneas cultivated in eye banks over a two-week period6,10,11,12.
The superiority over non-split corneal buttons as well as whole porcine corneoscleral samples was shown previously6. In that study, three groups were compared on days 1, 8, and 15. The corneal endothelium of all groups (corneoscleral buttons, non-split corneal buttons, split corneal buttons) was in good condition on day 1, represented by a well-preserved endothelial cell layer6. However, due to stromal swelling, folding of the Descemet's membrane destroyed large areas of the endothelium of whole corneoscleral samples, so that a representative assessment of the corneal endothelial cell density on days 8 and 15 could not be performed. Although the endothelium of the non-split corneal buttons was well preserved until day 15, some Descemet's membrane folds were also present. Although compared to the corneoscleral samples the endothelial cell layer of non-split corneal buttons was in better condition, the endothelial cell layer of the split corneal buttons was in far better condition. This can be seen in quantitative and qualitative parameters, as the endothelial cell loss within 15 days of cultivation was significantly higher (p = 0.041) in non-split corneal buttons (-575 ± 25/250 cells/mm2) compared to split corneal buttons (-417 ± 138/179 cells/mm2), which is congruent to the determined morphological characteristics evident in a more regular hexagon cell pattern, fewer reformation figures and rosette formations, as well as fewer destroyed cells (alizarin red areas) in split corneal buttons6. Percental endothelial cell losses confirm these findings, as the percental cell loss within 15 days in non-split and split corneal buttons is 14.89% and 10.2% (p = 0.032) respectively6. As this method was validated for a period of up to 15 days, it allows longer observation periods than published studies thus far (72 to 120 h)3,4,5.
The improvements in the preservation of the endothelial cell layer, applying common cultivating protocols also used in eye banks, can solely be attributed to the reduced swelling of the corneal stroma, since a major portion (300 µm) of the stroma is removed prior to cultivation6,13. Normally the stroma tends to swell enormously during cultivation due to its hydrophilic properties and molecules embedded within the stromal tissue14,15. Swelling, which induces shear and pinch forces and Descemet's membrane folding, which also causes mechanical strain on the corneal endothelium and endothelial cell loss, are reduced after partial removal of the stroma6,10. As opposed to eye banks, which often use osmotic agents such as dextran to deswell human donor corneas before transplantation, split corneal buttons do not require osmotic deswelling16,17. As culture medium supplemented with dextran is known to be absorbed by corneal endothelial cells and to induce increased cell loss7,8,9,18,19,20, the cultivation of split corneal buttons without dextran (or other osmotic agents) eliminates as many negative toxic factors as possible6. As the culture medium is not supplemented with any additives in the presented method, no toxic influences caused by any added substance are expected, which makes this model valuable for investigations of biocompatibility tests of new substances.
Although the corneal endothelium is a very delicate cell layer and the preparation of split corneal buttons requires moderate surgical skills and gentle handling, this technique can be a standardized method to work with and to obtain reliable results regarding the effects of various factors on the corneal endothelium within a very reasonable time frame. Nonetheless, there are a few steps in this protocol where the corneal endothelium is at risk. Obviously damaged or opaque eyes need to be carefully identified and discarded in the beginning to prevent possible biases. Also, the corneal endothelium must always be left untouched during trephination, dissection, extraction, and handling (e.g., transferring from eye to culture plate, from culture plate to culture plate, etc.) of split corneal buttons in order to prevent any mechanical damage. When placing the suture superficially in the stroma, the endothelium may potentially be penetrated if the needle is accidentally inserted too far in depth. If so, noticeable fluid from the anterior eye chamber will be passing through the suture channel and the corresponding eye needs to be discarded. To prevent this, the needle should be kept superficially within the stroma. Furthermore, care must be taken to strictly split the corneal button horizontally using the scalpel. Uneven cutting will result in uneven swelling during cultivation, possibly causing increased endothelial cell loss.
The examination of unstained corneal endothelial cells in hBSS is commonly performed in human donor corneas. There is no evidence of significant cell damage caused by osmotic swelling of the endothelial cells for the chosen examination time of split corneal buttons in hBSS3. Although staining enhances the visibility of cell borders, unstained counting does not result in significantly different results of the endothelial cell density compared to stained counting21. The clear benefit of unstained counting is that it allows multiple follow up examinations throughout the course of the experiments, whereas staining substances are usually cytotoxic and terminate the observation period. Stained counting, however, remains important to assess the morphological characteristics of the endothelium. Trypan blue highlights the nuclei of damaged cells that often seem undamaged in unstained counting. Alizarin red S clearly enhances the visibility of the cell borders and damaged cells by staining the Descemet's membrane, which facilitates the assessment of the endothelial cell density and allows the analysis of morphological features of the corneal endothelium, such as reformation figures, rosette formations, and alizarin red stained cells (Figure 4).
A major limitation of split corneal buttons as an ex vivo model is that, just like in vitro models, they are only suitable for investigating external influences on corneal endothelial cells. Therefore, in vivo models are irreplaceable for research on systemic diseases and conditions with an impact on the eye and the corneal endothelium. Regardless, this preparation technique can generate valid data for testing the effects of various external factors on the corneal endothelium (e.g., in biocompatibility testing of new substances)22. Following the 3R-principle (replacement, reduction, refinement) to reduce the number of live animal experiments, this method provides an adequate research model to further close the gap between in vitro cell cultures, where results are often incongruous to the in vivo situation in humans, and animal research, which requires substantial efforts and increasingly raises ethical concerns23.
Due to their properties and availability, pig eyes seem to be the only adequate substitute for human eyes for research purposes. Corneas from non-human primates are not a good alternative due to ethical reasons and availability, although these animals are the closest species to humans. On the other hand, the eyes of smaller animals are simply too small to allow efficient cornea removal. Pig eyes are comparable to human eyes in size and show similar properties of the corneal endothelial cells, which is also reflected in the research dealing with possible future xenotransplantation of genetically modified porcine corneas24,25,26. Also, being a by-product of slaughterhouses, they are easy to obtain.
In conclusion, the presented method using porcine corneas offers a highly reproducible organo-typically cultivated research model enabling cost-efficient research on corneal endothelial cells. Future investigators may use split corneal buttons to analyze the effects of various factors such as new substances, surgical techniques, equipment, and other possible external influences where the corneal endothelium is of great interest.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The establishment of the presented research model was supported by KMU-innovativ (FKZ: 13GW0037F) of the Federal Ministry of Education and Research Germany.
Materiały
| Name | Company | Catalog Number | Comments |
| Subject | |||
| Pig eyes | local abbatoir | ||
| Substances | |||
| Alizarin red S | Sigma-Aldrich, USA | ||
| Culture Medium 1, #F9016 | Biochrom GmbH, Germany | ||
| Dulbecco's PBS (1x) | Gibco, USA | ||
| Fetal calf serum | Biochrom GmbH, Germany | ||
| Hydrochloric acid (HCl) solution | own production | ||
| Hypotonic balanced salt solution | own production | per 1 L of H2O: NaCl 4.9 g; KCl 0.75 g; CaCl x H2O 0.49 g; MgCl2 x H2O 0.3 g; Sodium Acetate x 3 H2O 3.9 g; Sodium Citrate x 2 H2O 1.7 g | |
| Povidon iodine 7.5%, Braunol | B. Braun Melsungen AG, Germany | ||
| Sodium chloride (NaCl) 0.9% | B. Braun Melsungen AG, Germany | ||
| Sodium hydroxide (NaOH) solution | own production | ||
| Trypan blue 0.4% | Sigma-Aldrich, USA | ||
| Materials & Instruments | |||
| Accu-jet pro | Brand GmbH, Germany | ||
| Beaker Glass 50 mL | Schott AG, Germany | ||
| Blunt cannula incl. Filter (5 µm) 18G | Becton Dickinson, USA | ||
| Cell culture plate (12 well) | Corning Inc., USA | ||
| Colibri forceps | Geuder AG, Germany | ||
| Corneal scissors | Geuder AG, Germany | ||
| Eppendorf pipette | Eppendorf AG, Germany | ||
| Eye Bulb Holder | L. Klein, Germany | ||
| Eye scissors | Geuder AG, Germany | ||
| Folded Filter ø 185 mm | Whatman, USA | ||
| Hockey knife | Geuder AG, Germany | ||
| Laboratory Glass Bottle with cap 100 mL | Schott AG, Germany | ||
| Magnetic stir bar | Carl Roth GmbH & Co. KG, Germany | ||
| MillexGV Filter (5 µm) | Merck Millopore Ltd., USA | ||
| Needler holder | Geuder AG, Germany | ||
| Petri dishes | VWR International, USA | ||
| Pipette tips | Sarstedt AG & Co., Germany | ||
| Scalpel (single use), triangular blade | Aesculap AG & Co. KG, Germany | ||
| Serological pipette 10 mL | Sarstedt AG & Co., Germany | ||
| Serological pipette 5 mL | Sarstedt AG & Co., Germany | ||
| Sterile cups | Greiner Bio-One, Österreich | ||
| Sterile gloves | Paul Hartmann AG, Germany | ||
| Sterile surgical drape | Paul Hartmann AG, Germany | ||
| Stitch scissors | Geuder AG, Germany | ||
| Suture Ethilon 10-0 Polyamid 6 | Ethicon Inc., USA | ||
| Syringe (5 mL) | Becton Dickinson, USA | ||
| trephine ø 7.5 mm | own production | ||
| Tying forceps | Geuder AG, Germany | ||
| Weighing paper | neoLab Migge GmbH, Germany | ||
| Equipment & Software | |||
| Binocular surgical microscope | Carl Zeiss AG, Germany | ||
| Camera mounted on microscope | Olympus, Japan | ||
| CellSens Entry (software) | Olympus, Japan | ||
| Cold-light source | Schott AG, Germany | ||
| Incubator | Heraeus GmbH, Germany | ||
| Inverted phase contrast microscope | Olympus GmbH, Germany | ||
| Magnetic stirrer with heating function | IKA-Werke GmbH & Co. KG, Germany | ||
| pH-meter pHenomenal | VWR International, USA | ||
| Photoshop CS2 | Adobe Systems, USA | ||
| Precision scale | Ohaus Europe GmbH, Switzerland |
Odniesienia
- Gain, P., et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmology. 134 (2), 167-173 (2016).
- Roy, O., et al. Understanding the process of corneal endothelial morphological change in vitro. Investigative Ophthalmology & Visual Science. 56 (2), 1228-1237 (2015).
- Meltendorf, C., Ohrloff, C., Rieck, P., Schroeter, J. Endothelial cell density in porcine corneas after exposure to hypotonic solutions. Graefe's Archive for Clinical and Experimental Ophthalmology. 245 (1), 143-147 (2007).
- Schroeter, J., Meltendorf, C., Ohrloff, C., Rieck, P. Influence of temporary hypothermia on corneal endothelial cell density during organ culture preservation. Graefe's Archive for Clinical and Experimental Ophthalmology. 246 (3), 369-372 (2008).
- Schroeter, J., Ruggeri, A., Thieme, H. Impact of temporary hyperthermia on corneal endothelial cell survival during organ culture preservation. Graefe's Archive for Clinical and Experimental Ophthalmology. 253 (5), 753-758 (2015).
- Kunzmann, B. C., et al. Establishment Of A Porcine Corneal Endothelial Organ Culture Model For Research Purposes. Cell and Tissue Banking. 19 (3), 269-276 (2018).
- Redbrake, C., et al. A histochemical study of the distribution of dextran 500 in human corneas during organ culture. Current Eye Research. 16 (5), 405-411 (1997).
- Zhao, M., et al. Poloxamines for Deswelling of Organ-Cultured Corneas. Ophthalmic Research. 48 (2), 124-133 (2012).
- Filev, F., et al. Semi-quantitative assessments of dextran toxicity on corneal endothelium: conceptual design of a predictive algorithm. Cell and Tissue Banking. 18 (1), 91-98 (2017).
- Pels, E., Schuchard, Y. Organ-culture preservation of human corneas. Documenta Ophthalmologica. 56 (1-2), 147-153 (1983).
- Borderie, V. M., Kantelip, B. M., Delbosc, B. Y., Oppermann, M. T., Laroche, L. Morphology, Histology and Ultrastructure of Human C31 Organ-Cultured Corneas. Cornea. 14 (3), 300-310 (1995).
- Linke, S. J., et al. Thirty years of cornea cultivation: long-term experience in a single eye bank. Acta Opthalmologica. 91 (6), 571-578 (2013).
- Schroeter, J., et al. Arbeitsrichtlinien - Gute Fachliche Praxis für Hornhautbanken [Procedural guidelines. Good tissue practice for cornea banks]. Ophthalmologe. 106 (3), 265-276 (2009).
- Dohlman, C. H., Hedbys, B. O., Mishima, S. The swelling pressure of the corneal stroma. Investigative Ophthalmology & Visual Science. 1, 158-162 (1962).
- Xuan, M., et al. Proteins of the corneal stroma: importance in visual function. Cell and Tissue Research. 364 (1), 9-16 (2016).
- Sperling, S. Human Corneal Endothelium in Organ Culture - The Influence of Temperature and Medium of Incubation. Acta Opthalmologica. 57 (2), 269-276 (1979).
- Schroeter, J. Endothelial Evaluation in the Cornea Bank. Developments in Ophthalmology. 43, 47-62 (2009).
- Pels, E., Schuchard, Y. The Effects of High Molecular Weight dextran on the Presevation of Human Corneas. Cornea. 3 (3), 219-227 (1985).
- van der Want, H. J. L., Pels, E., Schuchard, Y., Olesen, B., Sperling, S. Electron Microscopy of Cultured Human Corneas Osmotic Hydration and the Use of dextran Fraction (dextran T 500) in Organ Culture. Archives of Ophthalmology. 101 (12), 1920-1926 (1983).
- Thuret, G., Manissolle, C., Campos-Guyotat, L., Guyotat, D., Gain, P. Animal compound-free medium and poloxamer for human corneal organ culture and Deswelling. Investigative Ophthalmology & Visual Science. 46 (3), 816-822 (2005).
- Wenzel, D. A., Kunzmann, B. C., Spitzer, M. S., Schultheiss, M. Staining of endothelial cells does not change the result of cell density. Cell and Tissue Banking. 20 (2), 327-328 (2019).
- Wenzel, D. A., Kunzmann, B. C., Hellwinkel, O., Druchkiv, V., Spitzer, M. S., Schultheiss, M. Effects of perfluorobutylpentane (F4H5) on corneal endothelial cells. Current Eye Research. , (2019).
- Olsson, I. A. S., Franco, N. H., Weary, D. M., Sandøe, P. The 3Rs principle - mind the ethical gap!. ALTEX Proceedings, 1/12, Proceedings of WC8. , 333-336 (2012).
- Sanchez, I., Martin, R., Ussa, F., Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe's Archive for Clinical and Experimental Ophthalmology. 249 (4), 475-482 (2011).
- Kim, M. K., Hara, H. Current status of corneal xenotransplantation. International Journal of Surgery. 23 (Pt B), 255-260 (2015).
- Fujita, M., et al. Comparison of Proliferative Capacity of Genetically-Engineered Pig and Human. Ophthalmic Research. 49 (3), 127-138 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone