Method Article
Rapid and Specific Detection of Acinetobacter baumannii Infections Using a Recombinase Polymerase Amplification/Cas12a-based System
In This Article
Summary
To facilitate the rapid and precise detection of Acinetobacter baumannii, we present a protocol that employs Recombinase Polymerase Amplification (RPA) in conjunction with LbaCas12a endonuclease for identifying A. baumannii infections.
Abstract
Acinetobacter baumannii, a gram-negative bacterium, is notorious for causing severe infections with high mortality rates. Rapid and accurate detection of A. baumannii is crucial for prompt treatment, effective infection control, and curbing antibiotic resistance. However, there is no suitable method for rapid and easy on-site detection of A. baumannii. The DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR) system offers a rapid, precise, and sensitive approach to A. baumannii detection by integrating the target-specific recognition capabilities of Cas12a with the isothermal amplification efficiency of Recombinase Polymerase Amplification (RPA). This protocol details the detection of A. baumannii using RPA combined with LbaCas12a endonuclease. The following steps are described in this article: extraction of DNA, selection of a specific DNA sequence, design of primer and CRISPR RNA (crRNA), construction of positive recombinant plasmid, setup of Cas12a-RPA assay, optimization of the RPA amplification system, visualization of the RPA-CRISPR/Cas12a assay using a fluorescence detection tool such as a real-time PCR instrument, and evaluation of sensitivity and specificity evaluation.
Introduction
In clinical microbiology, detecting Acinetobacter baumannii infections presents a significant challenge. This gram-negative bacterium can cause infections with severe clinical symptoms, even with high mortality rates, particularly among immunocompromised patients1. Traditional culture-based detection methods for pathogen infections are time-consuming and may lack sensitivity, which may delay antibiotic treatment and compromise patient outcomes. Rapid and accurate identification of A. baumannii is crucial for effective treatment and outbreak control. Molecular techniques employing nucleic acid amplification have been explored to meet this need. However, these approaches often need sophisticated thermal cycling equipment and may be limited by well-trained technicians and well-established laboratories. To overcome these challenges, research is increasingly focused on developing isothermal amplification methods2,3,4.
Recombinase Polymerase Amplification (RPA) is a method established by Piepenburg et al 5 and used to amplify DNA, similar to polymerase chain reaction (PCR) but without the need for temperature cycling. This method includes 50 mM Tris (pH 7.5), 100 mM potassium acetate, 14 mM magnesium acetate, 2 mM DTT, 5% PEG20000 (a high molecular weight polyethylene glycol), 200 µM dNTPs,3 mM ATP, 50 mM phosphocreatine, 100 µg/mL creatine kinase, 120 µg/mL UvsX, 30 µg/mL UvsY, 900 µg/mL Gp32, 30 µg/mL Bsu LF, 450 nM primers, and DNA templates. The amplification process begins with the recombinase protein UvsX binding to primers in the presence of 3 mM ATP and 5% PEG20000 forming a recombinase-primer complex. This complex then facilitates the combination of primers with the homologous sequences on the double-stranded DNA.
With the help of UvsY, the recombinase UvsX facilitates the exchange between the primer and the template strand, resulting in the displacement of one strand of the target DNA. Gp32 helps maintain the single-stranded DNA structure. Finally, the recombinase dissociates, and a DNA polymerase (Bsu LF) capable of displacing DNA strands binds to the 3' end of the primer, elongating it in the presence of deoxyribonucleoside triphosphates (dNTPs). This process is repeated cyclically, achieving exponential amplification. All amplification processes can be completed within 20-40 min and at relatively constant temperatures between 37 °C and 42 °C. This temperature range is approximately identical to physiological temperatures, which permits RPA to be conducted in a minimalist environment, making RPA a versatile and efficient tool for DNA detection and analysis.
The clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (CRISPR-Cas) system functions as an adaptive immune mechanism in bacteria and archaea, encompassing Class I and Class II systems. Class II includes proteins such as Cas12 (a, b, f), Cas13 (a, b), and Cas14, which identify and cleave target DNA or RNA guided by CRISPR RNA (crRNA)6,7,8 . LbaCas12a (or Cpf1) is a crRNA-guided DNA endonuclease. The crRNA serves as a guiding RNA within the CRISPR-Cas system, where it complexes with Cas proteins. It leverages its spacer region to pair with the target DNA, effectively steering the protein complex to the target sequences. The sequence 5'-UAAUUUCUACUAAGUGUAGAU-3' serves as the conserved crRNA repeat, a constant element in all LbaCas12a crRNAs. Following this repeat is a target-specific segment that differs based on the intended DNA target. This targeting sequence ranges from 18 to 24 nucleotides in length.
The PAM (Protospacer-Adjacent Motif) sequence (TTTV, where V can be A, C, or G) is located at the 5' end of the non-complementary strand of the DNA target. The Cas12a cuts target double-stranded DNA at the PAM sequence. Subsequently, the activated Cas proteins execute non-specific trans-cleavage of single-stranded DNA (ssDNA)9,10,11,12. Thus, ssDNA labeled with a fluorophore and quencher undergoes cleavage, resulting in the emission of a fluorescent signal following collateral cleavage8,13. The fluorescence intensity can be quantified using a fluorescence reader for precise nucleic acid detection14.Notably, Cas12a is extensively utilized for DNA analysis, with its binding and cleavage of target sequences contingent upon the recognition of the Protospacer-Adjacent Motif (PAM), while Cas13a operates independently of such a requirement.
CRISPR-Cas systems15,16,17 facilitate cleavage within a single reaction18,19,20,21. Combining the above two can create a robust tool known as A. baumannii-DETECTR (LbaCas12a-Enabled Detection of Targeted A. baumannii) for nucleic acid detection22,23,24.This protocol demonstrates the use of RPA in conjunction with the DNase activity of Cas12a to specifically target and cleave a crRNA-guided sequence within the 16s rDNA gene of A. baumannii, thereby enabling sensitive and precise detection of the pathogen.
The A. baumannii-DETECTR method presents several advantages over conventional detection methods25,26. Firstly, the isothermal characteristic of RPA streamlines operational procedures and reduces costs through its thermal cycler-independent amplification mechanism5. Secondly, the Cas12a endonuclease increases the assay's specificity by means of crRNA-mediated targeting and Watson-Crick base pairing7. Lastly, using the one-tube method of A. baumannii-DETECTR not only saves time but also significantly reduces the risk of amplicon contamination19.
The protocol outlines steps for the extraction of DNA extraction, the selection of target DNA sequences, the design of primers and crRNAs, the construction of positive recombinant plasmids, the establishment of setting up the Cas12a-RPA assay, the optimization of optimizing RPA amplification, and the visualization of visualizing results with using fluorescence detection instruments tools such as a Real-time PCR machine, complete with sensitivity and specificity evaluations.
The A. baumannii-DETECTR method holds promise in enhancing patient outcomes by facilitating timely and effective treatment, robust infection control, and the containment of antibiotic resistance spread. This protocol can serve as a comprehensive guideline for the implementation of Cas12a-RPA technology, enhancing its utility in various healthcare settings. However, it is crucial to note that every step should be optimized based on specific lab conditions and the variety of samples to ensure consistent and reliable results.
Protocol
1. Construction of the A. baumannii -DETECTR
NOTE: The construction of A. baumannii-DETECTR is a four-step process involving the design of primer and crRNA, the construction of positive recombinant plasmid construction, the preparation of reaction solutions, isothermal DNA amplification by RPA, and the visualization of the RPA-CRISPR/Cas12a assay. The schematic of the DETECTR assay is illustrated in Figure 1.
- Design of primer and crRNA (see Supplemental File 1)
- Design 12 pairs of primers using design toolson the basis of Primer design principles. Obtain four forward primers (F0-F3) and three reverse primers (R1-R3) to compose twelve pairs of primers (F0R1, F0R2, F0R3, F1R1, F1R2, F1R3, F2R1, F2R2, F2R3, F3R1, F3R2, F3R3).
- Ensure that the primer's 5' end contains C or T bases within the first 3-5 nucleotides instead of a continuous G stretch. Additionally, make sure the last three nucleotides at the 3' end are ideally G and C bases to enhance amplification.
- Design primers without self-complementary sequences to avoid hairpin formation and with no more than four complementary or homologous bases between them. Steer clear of 3' end complementarity to avoid primer dimerization.
- Evaluate each primer pair for specificity using the Primer-BLAST tool of National Center for Biotechnology Information (NCBI) to assess the efficacy and specificity of the primers.
NOTE: Primer-BLAST can utilize the NCBI database to find sequences that match the designed primers, helping users assess the efficacy and specificity of the primers. - Following the primer screening, design the CRISPR RNA (crRNA) or guide RNA (gRNA) using the LbaCas12a crRNA scaffold sequence (5'-UAAUUUCUACUAAGUGUAGAU-3') as the fixed sequence. Incorporate a target-specific segment (5'-GUUAAUACCUAGAGAUAGUG-3'), ensuring that the PAM motif is located at the 5' end of the non-complementary strand.
NOTE: The sequences of all oligonucleotides and crRNA are presented in Table 1.
- Design 12 pairs of primers using design toolson the basis of Primer design principles. Obtain four forward primers (F0-F3) and three reverse primers (R1-R3) to compose twelve pairs of primers (F0R1, F0R2, F0R3, F1R1, F1R2, F1R3, F2R1, F2R2, F2R3, F3R1, F3R2, F3R3).
- Positive recombinant plasmid construction
- Amplify the 16s rDNA segment using the primer set A. baumannii 16s rDNA-F (forward primer) and A. baumannii 16s rDNA-R (reverse primer).
- Add 1 µL of 10 µM F and 10 µM R, 10 µL of 5x FastpFu Buffer, 1 µL of FastpFu Polymerase, 4 µL of 2.5 mM dNTPs, 1 µL of 10 ng/µL A. baumannii genomic DNA, and 32 µL of Nuclease-free Water to the 50 µL PCR system.
- Set the PCR cycling conditions as follows: initial denaturation at 95 °C for 5 min, followed by 33 cycles of denaturation at 95 °C for 30 s, annealing at 55°C for 30 s, and extension at 72 °C for 1 min and 20 s. Finally, perform 10 min at 72°C for the final extension.
- To purify the positive PCR product, use a DNA Purification Kit according to the manufacturer's instructions. Thereafter, clone the purified DNA into the pUC57 vector, which has been digested with BamHI and SalI enzymes.
NOTE: This process will result in the creation of a positive recombinant plasmid containing a conserved region. - Dilute the positive recombinant plasmid serially in tenfold increments with sterilized ddH2O to achieve a concentration of one copy/µL to 107 copies/µL. Store the diluted plasmid at -20 °C for subsequent experiments.
- Amplify the 16s rDNA segment using the primer set A. baumannii 16s rDNA-F (forward primer) and A. baumannii 16s rDNA-R (reverse primer).
- Solution preparation
- Prepare Solution A as follows: for each reaction, add 29.5 µL of Primer Free Rehydration buffer, 2.4 µL of 10 µM A. baumannii-F and 10 µM A. baumannii-R, and 12.2µL of ddH2O to achieve a final volume of 46.5 µL. Vortex and spin briefly. Next, add the reaction mixture to the enzyme reaction tubes from the RPA Kit. Pipette to mix.
NOTE: The primers of A. baumannii-F and R are used in the assay that targets the A. baumannii gene. Please refer to Table 1 for the sequence. - Prepare Solution B as follows: for each reaction, add 0.24 µL of 10 µM ssDNA reporter, 2 µL of 10x Cas12a Reaction Buffer, 1 µL of 1 µM crRNA, 1 µL of 1 µM Cas12a, and 10.76µL of ddH2O to achieve a final volume of 15 µL.
NOTE: The probes (ssDNA reporter) were purchased and consisted of a single-stranded DNA reporter that was tagged with a 5-Carboxyfluorescein (FAM) fluorophore at the 5' end and a Black Hole Quencher 1 (BHQ1) quencher at the 3' end. In the case of numerous samples needing detection, prepare a substantial amount of solution B, and then divide the samples into aliquots later.
- Prepare Solution A as follows: for each reaction, add 29.5 µL of Primer Free Rehydration buffer, 2.4 µL of 10 µM A. baumannii-F and 10 µM A. baumannii-R, and 12.2µL of ddH2O to achieve a final volume of 46.5 µL. Vortex and spin briefly. Next, add the reaction mixture to the enzyme reaction tubes from the RPA Kit. Pipette to mix.
- Isothermal DNA amplification (RPA)
- Add 1 µL of the positive recombinant plasmid of 107 copies/µL to solution A with different primer pairs; mix thoroughly. Then, add 2.5 µL of 280 mM Magnesium Acetate (MgOAc) to the solution and mix well.
NOTE: RPA reactions start as soon as MgOAc is added. - Incubate at 39 °C for 20 min. After 20 min, purify the products amplified with different primers using RPA with a purification kit, and then run the clean amplicons on a 1% agarose gel at 120 V for 10 min to select the brightest and broadest band as the best primer pair.
NOTE: For low template copy number, after 4 min, remove the EP tube, vortex, and spin briefly; then replace it in the heating device for another 16 min. After amplification, tubes should be opened with extreme caution to avoid contaminating work surfaces with amplicons. This precaution will reduce the risk of inducing false positive results in subsequent experimental procedures.
- Add 1 µL of the positive recombinant plasmid of 107 copies/µL to solution A with different primer pairs; mix thoroughly. Then, add 2.5 µL of 280 mM Magnesium Acetate (MgOAc) to the solution and mix well.
- Optimization of RPA amplification system
NOTE: The optimal primer pair F3R3 selected in the previous step was set to different final concentrations for RPA amplification reaction to screen out the optimal primer concentration and time. NC is a blank control for the corresponding primer concentration.- Maintain the concentrations of the other components constant and the total volume of46.5 µL in Solution A. Adjust the volume of RNase-free ddH2O accordingly to achieve the final primer concentrations of 10 µM F3 and 10 µM R3 at 0.40 µM (2.0 µL), 0.44 µM (2.2 µL), 0.48 µM (2.4 µL), and 0.52 µM (2.6 µL), respectively.
- Incubate at 39 °C for different times:10 min, 20 min, 30 min.
- Clean the RPA products using a DNA Purification Kit and perform electrophoresis on a 1% agarose gel at 120 V for 10 min to identify the optimal primer concentration and incubation times by analyzing the characteristics of the bands.
- Visualization of the RPA-CRISPR/Cas12a assay
- Add 5 µL of the RPA product into solution B and mix thoroughly until homogeneous.
- Place the samples in the fluorescence quantitative PCR instrument, set the FAM channel, and read the values every minute for 60 min at 37 °C, 60x for a total of 60 min.
NOTE: Green fluorescence signal can last several days. To make sure the system works effectively, it is better to perform a DNA and RNase free test.
2. Specificity evaluation of the RPA-CRISPR/Cas12a-based detection platform
NOTE: To test the specificity of A. baumannii-DETECTR, the nucleic acids of A. baumannii, Streptococcus pneumoniae, Staphylococcus aureus, Rickettsia mooseri, Enterobacter, Escherichia coli, Pseudomonas aeruginosa, Klebsiella, Chlamydia psittaci, Legionella, Cockerella beckeri, Serratia, and human samples were subjected to the DETECTR test.
- Use DNA templates obtained from various species by using the referenced DNA Kit or isolating them by boiling the bacteria in the water. Ensure that all templates have equal concentrations. Use water as a negative control.
- Add 10 ng or 1 µL of DNA templates to Solution A containing F3R3 primers. Add 2.5 µL of MgOAc, mix thoroughly, and incubate the mixture at 39 °C for 20 min.
- After 20 min, add 5 µL of the product from the previous step to solution B and mix.
- Place in the fluorescence quantitative PCR instrument, set the channel to FAM, and read the value once every minute at 37 °C, for a total of 60 x for 60 min.
3. Sensitivity evaluation of the RPA-CRISPR/Cas12a-based detection platform
- Use a positive recombinant plasmid, which has been prepared to a concentration of 1-107 copies/µL, as the template for the reaction. Use water for the negative control.
- Perform the method by following steps 2.2-2.4.
NOTE: The primer set RPA-F3 and RPA-R3 is designed to amplify a shorter fragment of 174 base pairs from the positive recombinant plasmid.
4. Preparations while the sample is under UV light and LFS detection
- Take out the required number of test strips and label them appropriately.
- Transfer 10 µL of the amplified products from steps 2 and 3 into separate PCR tubes, respectively. Then, add 40 µL of ddH2O to each tube.
NOTE: If using a self-prepared reaction system, it is recommended to explore the optimal dilution ratio. - Perform the Test.
- Subject the PCR tube containing the amplified products to a transilluminator to assess the presence of fluorescence.
- Insert the test strips into the PCR tubes with the sample pad end facing downwards.
- Let it stand at room temperature for 5-10 min to read the result. After this time, ensure the liquid level does not exceed the maximum line before proceeding further.
- Interpret the results as negative if the T line does not show color. Interpret the results as positive if the T line is visible to the naked eye, indicating that the nucleic acid probe has been cleaved by the Cas enzyme, and the Cas enzyme has been activated. Consider the result to be invalid if neither the C line nor the T line shows color.
Results
In this study, we introduce a novel, portable diagnostic platform named A. baumannii-DETECTR, which integrates the isothermal amplification efficiency of RPA and CRISPR-Cas12a system for rapid and reliable field identification of A. baumannii. The schematic of the DETECTR assay is illustrated in Figure 1.
Twelve pairs of primers were designed using design toolson the basis of primer design principles. Four forward primers (F0-F3) and three reverse primers (R1-R3) were obtained, resulting in 12 pairs of primers (F0R1, F0R2, F0R3, F1R1, F1R2, F1R3, F2R1, F2R2, F2R3, F3R1, F3R2, F3R3). Following RPA amplification at 39 °C for 20 min, the RPA PCR products were analyzed on a 1% agarose gel at 120 V for 10 min to determine the optimal primer combination. The primer pair F3R3 emerged as the most effective, displaying the brightest and densest bands (Figure 2).
Having identified the optimal primer pair F3R3, we next optimized the final primer concentrations and reaction times for the RPA amplification, maintaining all other components constant. Our findings revealed that the amplification efficiency peaked at a primer concentration of 0.44 µM and a reaction time of 20 min (Figure 3). Using a positive recombinant plasmid at a concentration of 107 copies/µL as the template, we observed a time-dependent increase in fluorescence intensity for crRNA-1, while crRNA-2 showed no such increase over time. Consequently, crRNA-1 was selected as the crRNA for the detection of A. baumannii (Figure 4).
The specificity of A. baumannii-DETECTR was evaluated using nucleic acids isolated from a diverse array of bacterial strains. During the PCR assays, a significant fluorescence signal was only observed when the genome of A. baumannii was targeted, while no fluorescence detected when using genomic DNA from other bacterial sources (Figure 5). These results confirm that the A. baumannii-F3 and R3 primer pair exhibits specificity for A. baumannii in the DETECTR assay, indicating that A. baumannii-DETECTR can reliably detect this pathogen.
To assess the sensitivity of the A. baumannii-DETECTR assay, we performed a series of dilutions with A. baumannii DNA. The copy number was calculated using the following formula: copy number (copies/µL) = 6.02 × 1023 x P (ng/µL) x 10-9 / (number of plasmid bases x 660) (where P represents the plasmid concentration). The assay demonstrated the capability to detect down to 1 copy/µL, indicating its potential as an exquisitely sensitive diagnostic tool, well-suited for on-site applications (Figure 6). Thus, the A. baumannii-DETECTR exhibits remarkable sensitivity.
In this protocol, we also integrated the CRISPR/Cas12a-mediated DETECTR detection platform with lateral flow strip (LFS) technology to facilitate the identification of target DNA sequences. We evaluated the sensitivity and specificity of the LFS-based assay. A specificity assay conducted using LFS revealed no signs of cross-reactivity with other bacterial species, demonstrating the method's high specificity (Figure 7A). Furthermore, we utilized LFS to detect A. baumannii across a range of dilutions from 107 copies/µL down to a single copy/µL. Notably, all tested samples yielded positive results (Figure 7B), which were in alignment with the outcomes under UV light (Figure 7C, D). Collectively, these results underscore that our platform serves as a rapid, robust, and sensitive diagnostic instrument for A. baumannii in field settings.

Figure 1: A. baumannii-DETECTR workflow. Abbreviations: RPA =R ecombinase Polymerase Amplification; DETECTR = DNA Endonuclease Targeted CRISPR Trans Reporter. Please click here to view a larger version of this figure.

Figure 2: Design and selection of primers. The optimal combination of primers for the RPA reaction was identified based on the results obtained from agarose gel electrophoresis. The primer pair F3R3 performed best with bright and dense bands. Please click here to view a larger version of this figure.

Figure 3: The optimization of primer concentration and amplification time for RPA. 1-4: The primer concentrations 0.40 µM, 0.44 µM, 0.48 µM, 0.52 µM. The amplification times are respectively 10 min, 20 min, and 30 min. The amplification effect reaches its highest when the concentration of primer is 0.44 µM and the reaction time is 20 min. Abbreviations: M = 8000 DNA Marker; RPA = Recombinase Polymerase Amplification; NC = blank control. Please click here to view a larger version of this figure.
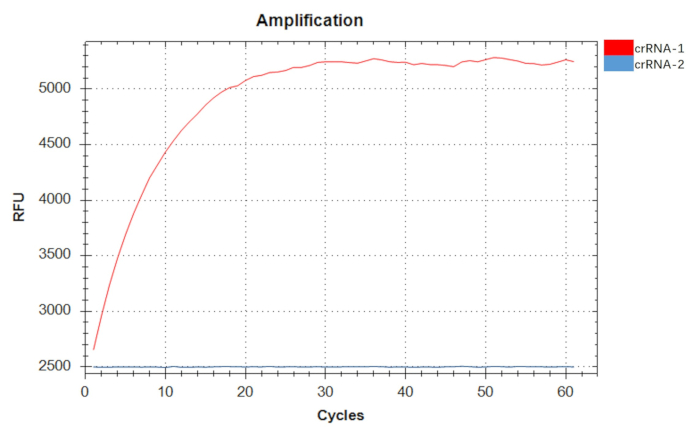
Figure 4: Screening of two crRNAs (crRNA-1 and crRNA-2). The fluorescence intensity of crRNA-1 detection increased with time, while that of crRNA-2 detection did not increase with time, showing no significant detection activity. crRNA-1 was used as the crRNA for the detection of A. baumannii. Please click here to view a larger version of this figure.
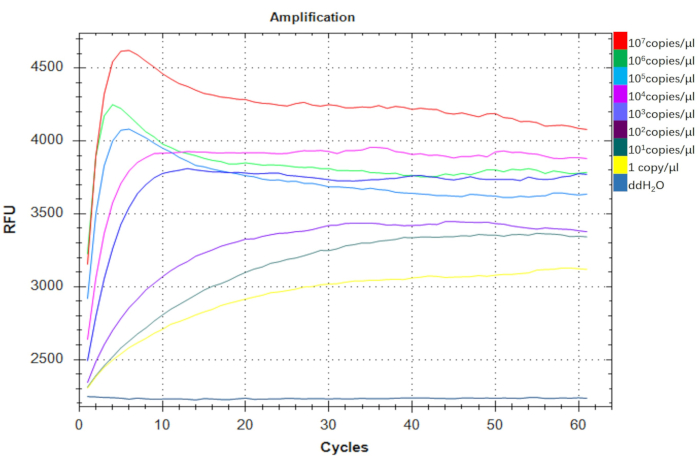
Figure 5: Testing the specificity of the A. baumannii-DETECTR. The amplification of A. baumannii-DETECTR was achieved using the primer pair A. baumannii-F3 and R3, utilizing genomic DNA extracted from A. baumannii as well as other bacterial species. Abbreviation: DETECTR = DNA Endonuclease Targeted CRISPR Trans Reporter. Please click here to view a larger version of this figure.

Figure 6: Testing the sensitivity of the A. baumannii-DETECTR. The pUC57-16s rDNA positive recombinant plasmids served as targets to evaluate the limit of detection for A. baumannii using the visual fluorescence-based RPA-CRISPR/Cas12a assay. The assay successfully detected a range of one copy to 107 copies/µL using consistent primers. The fluorescence intensity in the one-copy group was significantly elevated compared to the ddH2O control group. Abbreviations: DETECTR = DNA Endonuclease Targeted CRISPR Trans Reporter; RPA = Recombinase Polymerase Amplification. Please click here to view a larger version of this figure.
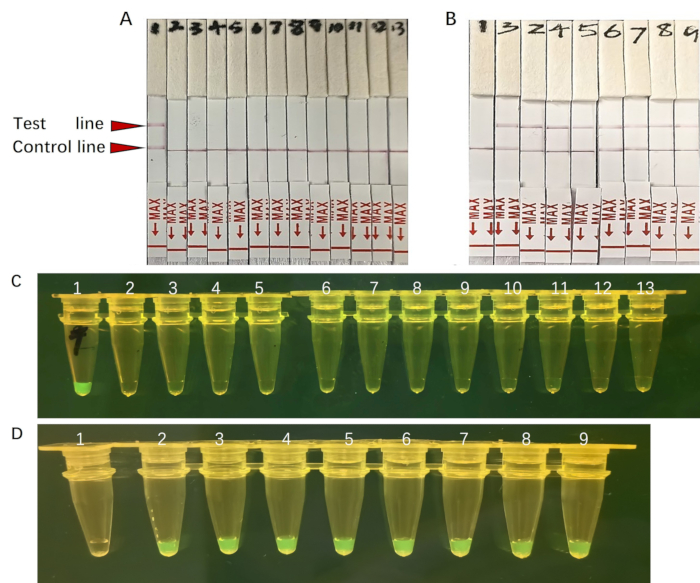
Figure 7: The fluorescence signal of the sample under UV light and LFS detection. A. baumannii-DETECTR reaction with plasmid standards. (A-C) Different pathogens were detected by A. baumannii-DETECTR. Only A. baumannii was successfully detected. 1-13 represent A. baumannii, Streptococcus pneumoniae, Staphylococcus aureus, Rickettsia mooseri, Enterobacter, Escherichia coli, Pseudomonas aeruginosa, Klebsiella, Chlamydia psittaci, Legionella, Coxiella burnetii, Serratia, Human peripheral. (B-D) The positive recombinant plasmid with serially diluted in tenfold increments detected by the A. baumannii-DETECTR.All were detected except for the negative control. 1-9 represent negative control, 1 copy/µL, 101 copies/µL, 102 copies/µL, 103 copies/µL, 104 copies/µL, 105 copies/µL, 106 copies/µL, 107 copies/µL. Please click here to view a larger version of this figure.
| No | Name | Sequence(5‘-3‘) | |||
| 1 | crRNA-1 | uaauuucuacuaaguguagauGUUAAUACCUAGAGAUAGUG | |||
| 2 | crRNA-2 | uaauuucuacuaaguguagauGUUGGUAUUCCGGAAGAAGU | |||
| 3 | crRNA-1-DNA | GTTAATACCTAGAGATAGTG | |||
| 4 | crRNA-2-DNA | GTTGGTATTCCGGAAGAAGT | |||
| 5 | Targed sequence | TAACTGAAGAGTTTGATCATGGCTCAGATTGAACGCTGG CGGCAGGCTTAACACATGCAAGTCGAGCGGGGGAAGGT AGCTTGCTACTGGACCTAGCGGCGGACGGGTGAGTAATG CTTAGGAATCTGCCTATTAGTGGGGGACAACATCTCGAAA GGGATGCTAATACCGCATACGTCCTACGGGAGAAAGCAG GGGATCTTCGGACCTTGCGCTAATAGATGAGCCTAAGTCG GATTAGCTAGTTGGTGGGGTAAAGGCCTACCAAGGCGAC GATCTGTAGCGGGTCTGAGAGGATGATCCGCCACACTGG GACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGT GGGGAATATTGGACAATGGGGGGAACCCTGATCCAGCCAT GCCGCGTGTGTGAAGAAGGCCTTATGGTTGTAAAGCACTT TAAGCGAGGAGGAGGCTACTTTAGTTAATACCTAGAGATAG TGGACGTTACTCGCAGAATAAGCACCGGCTAACTCTGTGC CAGCAGCCGCGGTAATACAGAGGGTGCGAGCGTTAATCGG ATTTACTGGGCGTAAAGCGTGCGTAGGCGGCTTATTAAGTC GGATGTGAAATCCCCGAGCTTAACTTGGGAATTGCATTCGAT ACTGGTGAGCTAGAGTATGGGAGAGGATGGTAGAATTCCAG GTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGAT GGCGAAGGCAGCCATCTGGCCTAATACTGACGCTGAGGTAC GAAAGCATGGGGAGCAAACAGGATTAGATACCCTGGTAGTC CATGCCGTAAACGATGTCTACTAGCCGTTGGGGCCTTTGAGG CTTTAGTGGCGCAGCTAACGCGATAAGTAGACCGCCTGGGGA GTACGGTCGCAAGACTAAAACTCAAATGAATTGACGGGGGCC CGCACAAGCGGTGGAGCATGTGGTTTAATTCGATGCAACGC GAAGAACCTTACCTGGCCTTGACATACTAGAAACTTTCCAGA GATGGATTGGTGCCTTCGGGAATCTAGATACAGGTGCTGCAT GGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCC CGCAACGAGCGCAACCCTTTTCCTTACTTGCCAGCATTTCG GATGGGAACTTTAAGGATACTGCCAGTGACAAACTGGAGGAA GGCGGGGACGACGTCAAGTCATCATGGCCCTTACGGCCAGG GCTACACACGTGCTACAATGGTCGGTACAAAGGGTTGCTACA CAGCGATGTGATGCTAATCTCAAAAAGCCGATCGTAGTCCGG ATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAG TAATCGCGGATCAGAATGCCGCGGTGAATACGTTCCCGGGCC TTGTACACACCGCCCGTCACACCATGGGAGTTTGTTGCACCA GAAGTAGCTAGCCTAACTGCAAAGAGGGCGGTTACCACGGTG TGGCCGATGACTGGGGTGAAGTCGTAACAAGGTAGCCGTAGG GGAACCTGCGGCTGGATCACCTCCTTA | |||
| 6 | A.baumannii-F0 | CGGGAGGCAGCAGTGGGGAATATTGGACAAT | |||
| 7 | A.baumannii-F1 | AAGAAGGCCTTATGGTTGTAAAGCACTTTAA | |||
| 8 | A.baumannii -F2 | GGTTGTAAAGCACTTTAAGCGAGGAGGAGGCTA | |||
| 9 | A.baumannii -F3 | AAGGCCTTATGGTTGTAAAGCACTTTAAGCGA | |||
| 10 | A.baumannii-R1 | ACAGAGTTAGCCGGTGCTTATTCTGCGAGTAA | |||
| 11 | A.baumannii-R2 | AATCCGATTAACGCTCGCACCCTCTGTATTA | |||
| 12 | A.baumannii-R3 | AGTTAGCCGGTGCTTATTCTGCGAGTAACGTC | |||
| 13 | ssDNA-FQ | ssDNA-FQ FAM-TTTATTT-BHQ1 | |||
| 14 | A.baumannii-16s rDNA-F | 5′-CGCGGATCCTAACTGAAGAGTTTGATCATGG-3′ | |||
| 15 | A.baumannii-16s rDNA-R | 5′-ACGCGTCGACTAAGGAGGTGATCCAGC-3′. | |||
Table 1: Nucleic acids used in this study. The nucleic acid used here is ssDNA-FQ, which is composed of single-stranded DNA conjugated with a 5-Carboxyfluorescein at the 5' end and the fluorescence quencher Black Hole Quencher 1 at the 3'end. Abbreviations: F = fluorophore; Q = quencher.
Supplemental File 1: Design of primers and crRNA.Please click here to view a larger version of this figure.
Discussion
Traditional diagnostic methods for A. baumannii infections have various restrictions that make them less accessible and feasible for point-of-care testing27. For example, PCR has good sensitivity and specificity but necessitates specialized thermal cycling equipment and complex workflows that must be operated by professionals. The novel method presented here, which couples RPA and CRISPR-LbaCas12a genome editing with recombineering, known as the A. baumannii-DETECTR assay, allows efficient genetic manipulation. It provides a rapid, sensitive, and specific detection method for Acinetobacter baumannii infections, as well as for the diagnosis of other bacterial pathogens. The workflow is illustrated in Figure 1.
Our method is particularly advantageous in diagnosing and managing infections, which should facilitate patient recovery and mitigate the spread of A. baumannii by overcoming the shortcomings of existing diagnostic techniques. The A. baumannii-DETECTR system was found to identify A. baumannii with high specificity and sensitivity (Figure 5 and Figure 6). Our findings revealed that only the A. baumannii genome exhibited a positive fluorescence fold change during the real-time assessment, while the genomes of all other species showed negative results (Figure 5).
To assess the sensitivity of the RPA-CRISPR/Cas12a detection platform, recombinant plasmids pUC57-16s rDNA, which are positive for A. baumannii, were employed as targets to determine the limit of detection (LOD) of the system. In the fluorescence-based RPA-CRISPR/Cas12a assay, detection ranged from one copy per microliter to 107 copies per microliter, with a notable enhancement in fluorescence intensity observed in the 1 copy/µL group compared to the negative control group (Figure 6). Importantly, for real-time diagnosis, the outcomes of the detection can be visualized directly via a handheld portable device, independent of laboratory-based equipment, which is essential for real-time diagnosis.
Several critical factors must be considered regarding the protocol26. First, it is essential to prevent cross-contamination during the sample collection process,given the high sensitivity of the A. baumannii-DETECTR assay. Additionally, the reaction reagents should be shielded from direct sunlight exposure. Adherence to all procedural steps is crucial for attaining the best possible results. A positive control, consisting of a plasmid that harbors A. baumannii-specific gene fragments, should be incorporated to verify the proper functionality of the A. baumannii-DETECTR system in practical applications.
The protocol shows that parameters can be optimized for specific laboratory conditions and sample types, such as primer concentration, reaction time, and other variables. In this experiment, the optimal primer combination, primer concentrations, and reaction times for RPA amplification were selected to produce the highest amplification efficiency and specificity. This was determined by agarose gel electrophoresis (Figure 2 and Figure 3). Additionally, a positive recombinant plasmid, containing a conserved region of the A. baumannii genome was used as a control to ensure accuracy in the protocol. It is worth noting that commercial bacterial genomic DNA extraction kits provide a standardized and reliable method for DNA extraction in this protocol. However, in future studies, different sample types, such as clinical or environmental samples, may require specific extraction methods28 to effectively isolate A. baumannii DNA.
In the absence of detectable fluorescence signals from the positive control, it is likely that inhibitors are present in the reaction mixture, necessitating a change of reagents. Conversely, if the fluorescence intensity is insufficient for clear differentiation, extending the incubation duration may be beneficial. However, prolonged incubation could also result in false positives26.
Current detection methodologies for A. baumannii necessitate the use of costly PCR equipment, fixed operational locations, and specialized personnel. In contrast, the method proposed herein facilitates accurate and sensitive diagnosis of A. baumannii in field settings, making it accessible to a broader demographic.
The characteristics of isothermal amplification and data collected through fluorescence make A. baumannii-DETCTER workflow simple and low equipment requirements as well as making it possible to perform rapid pathogen detection in remote areas or during outbreaks. RPA-LFS29,30,31,32, a novel isothermal nucleic acid amplification technique, has gained significant traction in the detection of viral, bacterial, fungal, and parasitic pathogens owing to its operational simplicity and reduced temporal demands. The protocol details the fluorescence signal of the sample under ultraviolet (UV) light or Transilluminator and lateral flow strips (LFS) detection. It can avoid dependence on high-accuracy instruments and specialized technicians, as visual inspection results in a short time (Figure 7), thereby eliminating the need for expensive and complex laboratory equipment and the operational population. Moreover, the one-tube method is not only time-saving but also decreases the risk of amplicon contamination33.
Future studies should aim to improve the method to make it workable for various types of samples and different environmental conditions, such as exploring more efficient and lower-cost DNA extraction techniques. The use of A. baumannii-DETECTR is not only for clinical diagnosis but also can be used for environmental assessment, monitoring and management of outbreaks, enabling communities and hospitals to quickly identify A. baumannii. Furthermore, by modifying the crRNA and primers, this molecular diagnostic approach can be adapted for the detection of various respiratory bacterial infections, such as Streptococcus pneumoniae, Staphylococcus aureus, and Enterobacter.
In summary, A. baumannii-DETECTR can identify A. baumannii as quickly and accurately as possible due to its high sensitivity and specificity, user-friendly design, and portability. This method can enhance patient health outcomes by facilitating timely and effective treatment, infection control, and mitigating the proliferation of antibiotic resistance, while also tackling the increasing threat of A. baumannii infections.
Disclosures
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by Science and Technology Development Plan Project of Jilin Province, China (20240305027YY) and Jilin Provincial Department of Finance (JLSWSRCZX2023-55, JLSWSRCZX2021-041).
Materials
| Name | Company | Catalog Number | Comments |
| -20 °C Freezer | Haier | HYCD-290 | China |
| Agarose Basic | BioFroxx | 1110GR100 | China |
| All oligonucleotides and crRNA were synthesized by company | www.comatebio.com | ||
| Cas12a cutting substrate - ssDNA - fluorescent type | EZassay Biotech. Co. Ltd. | DNA-FAM-BHQ | China |
| Cas12a cutting substrate - ssDNA - test paper type | EZassay Biotech. Co. Ltd. | DNA-FAM-BIO | China |
| Electrophoresis apparatus | BIO-RAD | POWER PAC1000 | USA |
| Fluorescence quantitative PCR instrument | BIO-RAD | CFX Connect | USA |
| Gel Imaging System | BIO-RAD | Gel Doc 2000 | USA |
| https://ezassay.com/rna | |||
| https://www.ezassay.com/primer | |||
| https://www.ncbi.nlm.nih.gov/tools/primer-blast | |||
| Lateral flow paper strip (Biotin/FAM) | EZassay Biotech. Co. Ltd. | HD-FMBO | China |
| LbaCas12a (Cpf1) enhanced protein | EZassay Biotech. Co. Ltd. | CAS-12E-001 | China |
| LED Transilluminator | LABGIC | BL-20 | China |
| Magnesium acetate, MgOAc | TwistDx | TABAS03KIT | UK |
| Microcentrifuge | allsheng | Mini-6k | China |
| PCR strip tubes | PCR strip tubes | PST-0208-FT-C | China |
| TGrade Dry Bath Incubator | Tiangen biochemical technology | OSE-DB-01 | China |
| Tianamp Bacteria DNA Kit | Tiangen biochemical technology | DP302-02 | China |
| TIANamp Bacteria DNA Kit | TIANGEN BIOTECH (BEIJING) CO.; LTD. | DP302 | China |
| TransStart FastPfu DNA Polymerase | TransGen Biotech. Co. Ltd. | AP221 | China |
| TwistAmp Basic Kit | TwistDX | TABAS03KIT | UK |
| Universal DNA Purification Kit | Tiangen biochemical technology | DP214-03 | China |
References
- Lenie, D., Alexandr, N., Harald, S. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 5 (12), 939-951 (2007).
- Li, P., et al. Rapid detection of Acinetobacter baumannii and molecular epidemiology of carbapenem-resistant A. baumannii in two comprehensive hospitals of Beijing, China. Front Microbiol. 6, 997 (2015).
- Wu, X., et al. A diagnostic test that uses isothermal amplification and lateral flow detection sdaA can detect tuberculosis in 60 min. J Appl Microbiol. 130 (6), 2102-2110 (2020).
- Huang, B., et al. A cas12a-based fluorescent microfluidic system for rapid on-site human papillomavirus diagnostics. Appl Microbiol Biotechnol. 107 (20), 6287-6297 (2023).
- Piepenburg, O., Williams, C. H., Stemple, D. L., Armes, N. A. DNA detection using recombination proteins. Plos Biology. 4 (7), 1115-1121 (2006).
- Abudayyeh, O. O., et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 353 (6299), aaf5573 (2016).
- Chen, J. S., et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 360 (6387), eaar6245 (2018).
- Xiong, D., et al. Rapid detection of SARS-CoV-2 with CRISPR-Cas12a. PLoS Biol. 18 (12), e3000978 (2020).
- Gootenberg, J. S., et al. Nucleic acid detection with crispr-Cas13a/C2C2. Science. 356 (6336), 438-442 (2017).
- Harrington, L. B., et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 362 (6416), 839-842 (2018).
- Li, S. Y., et al. CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 28 (4), 491-493 (2018).
- Wang, B., et al. Cas12aVDet: A CRISPR/Cas12a-based platform for rapid and visual nucleic acid detection. Anal Chem. 91 (19), 12156-12161 (2019).
- Huang, Z., et al. Ultra-sensitive and high-throughput crispr-p owered COVID-19 diagnosis. Biosens Bioelectron. 164, 112316 (2020).
- Zhang, W. S., et al. Reverse transcription recombinase polymerase amplification coupled with CRISPR-Cas12a for facile and highly sensitive colorimetric SARS-CoV-2 detection. Anal Chem. 93 (8), 4126-4133 (2021).
- Marraffini Luciano, A. CRISPR-Cas immunity in prokaryotes. Nature. 526 (7571), 55-61 (2015).
- Mohanraju, P., et al. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 353 (6299), aad5147 (2016).
- Makarova, K. S., et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 13 (11), 722-736 (2015).
- Li, S. Y., et al. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discovery. 4 (1), 20 (2018).
- Li, L., Li, S., Wu, N., Wu, J., Wang, J. Holmesv2: A CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth Biol. 8 (10), 2228-2237 (2019).
- Liang, M., Li, Z., Wang, W., Liu, J., Zhang, L. X. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat Communications. 10 (1), 3672 (2019).
- Aquino-Jarquin, G. CRISPR-Cas14 is now part of the artillery for gene editing and molecular diagnostic. Nanomedicine. 18, 428-431 (2019).
- Zetsche, B., et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 163 (3), 759-771 (2015).
- Fonfara, I., Richter, H., Bratovic, M., Le Rhun, A., Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 532 (7600), 517-521 (2016).
- Swarts, D. C., John, V. D. O., Jinek, M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol Cell. 66 (2), 221-233 (2017).
- Jiang, Y., et al. CRISPR-Cpf1 assisted genome editing of Corynebacterium glutamicum. Nat Commun. 8, 15179 (2017).
- Yan, M. Y., et al. CRISPR-Cas12a-assisted recombineering in bacteria. Appl Environ Microbiol. 83 (17), e00947-e01017 (2017).
- Ashraf, A., et al. A novel multiplex pcr assay for simultaneous detection of nine clinically significant bacterial pathogens associated with bovine mastitis. Mol Cell Probes. 33, 57-64 (2017).
- Zhu, L., et al. A rapid on-site visualization platform based on RPA coupled with CRISPR-Cas12a for the detection of genetically modified papaya 'huanong no.1'. Talanta. 277, 126437 (2024).
- Zheng, C., et al. Rapid developments in lateral flow immunoassay for nucleic acid detection. Analyst. 146 (5), 1514-1528 (2021).
- Wang, Y., et al. Establishment and clinical application of a RPA-LFS assay for detection of capsulated and non-capsulated Haemophilus influenzae. Front Cell Infect Microbiol. 12, 878813 (2022).
- Wang, F., et al. Rapid and sensitive recombinase polymerase amplification combined with lateral flow strips for detecting Candida albicans. Anal Biochem. 633, 114428 (2021).
- Ma, B., et al. A simple and efficient method for potential point-of-care diagnosis of human papillomavirus genotypes: Combination of isothermal recombinase polymerase amplification with lateral flow dipstick and reverse dot blot. Anal Bioanal Chem. 411 (28), 7451-7460 (2019).
- Sun, Y., Yu, L., Liu, C., Ye, S., Huang, W. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a. J Transl Med. 19 (1), 74 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved