Method Article
In vivo and In vitro Infection of Potato Roots with Plant Parasitic Nematodes for the Assessment of Induced Structural Changes
In This Article
Summary
The protocol describes infection of Solanum tuberosum roots with plant parasitic nematodes under in vivo greenhouse conditions and potato in vitro transgenic roots for histochemical analysis of root structure through optical microscopy.
Abstract
Soil-dwelling plant parasitic nematodes (PPNs) are important potato pests that cause lesions and/or change plant roots structure, leading to reduced crop fitness and productivity. Research on the cellular and subcellular mechanisms of PPNs infection and development can resort to field plants or seedlings under greenhouse conditions. Field studies are more representative of natural environments but are subjected to the unpredictability of environmental conditions that can heavily influence research outcomes. Greenhouse studies allow higher control over environmental variables and higher safety against contaminants or pathogens. However, in some hosts, genetic diversity becomes an important factor of variability and influences the host-parasite complex response. We have developed in vitro co-cultures of transgenic roots with PPNs as a reliable alternative that occupies less space, requires less time to obtain, and is free from contamination or from host genetic variability. Co-cultures are obtained by introducing aseptic PPNs to host in vitro transgenic roots. They can be maintained indefinitely, which makes them excellent support for keeping collections of reference PPNs. In the present work, a protocol is detailed for the controlled infection of in vivo potato roots with the root lesion nematode and for establishing in vitro co-cultures of potato transgenic roots with the root-knot nematode. The in vitro co-cultures provided a laboratory proxy for the natural potato infection condition and produced nematode life stages irrespective of season or climate conditions. Additionally, the methodology used for structural analysis is detailed using histochemistry and optical microscopy. The acid fuchsin dye is used to follow nematode attack sites on roots, while differential staining with Periodic acid-Schiff (PAS) and toluidine blue O highlights nematode structures in potato internal root tissue.
Introduction
Root and tuber crops rank 4th among the world's most important staple foods. Potato (Solanum tuberosum L.) is one of the most important cultivated tubers. It had its origin in the Andes mountains of South America, but after being introduced to Europe in the 16th century quickly became the most common food source for the population with a lower income. Today, potatoes make up 1.7% of the world's caloric intake1. Crop production is heavily affected by plant pests and pathogens, of which plant parasitic nematodes (PPNs) can cause average yield losses that rise up to 12%2. Plant parasitic nematodes are responsible for some of the most damaging diseases to crops in modern agriculture. Soil-dwelling PPNs impose heavy losses to farmers because they affect plant roots and interfere with crop productivity by reducing production and/or injuring products, turning them unmarketable3. These dangerous phytoparasites use their stylet (a needle-like mouthpart) to puncture root cells and feed on cell content. Some PPNs feed from outside the roots, others enter the root and cause tissue damage (migratory), while others enter the roots and become sedentary, heavily changing root structure to facilitate feeding4. The main PPNs affecting potato are the potato cyst nematodes, Globodera spp., root-knot nematodes (RKN), Meloidogyne spp., root lesion nematodes, Pratylenchus spp., the false root-knot nematode Nacobbus aberrans, and the potato rot nematode Ditylenchus destructor. For these PPNs, different feeding habits induce different structural changes in host root tissues5,6. Research on the mechanisms of PPN infection and host response is often performed through field or greenhouse trials to maintain reference PPN culture collections or to perform large scale experiments7,8. Testing under natural conditions is strongly influenced by environmental variation and biotic or abiotic stress factors. Greenhouse bioassays are a closer alternative to a natural condition while allowing a relative control of environmental variation and limiting the influence of abiotic and biotic stress. However, host genetic diversity can still be a challenge for trials that require a finer control of biological variability. These limitations can be overcome by resorting to in vitro plant tissue cultures. These are versatile laboratory systems with many advantages for PPNs disease research. For soil-dwelling PPNs, in vitro cultures of transgenic roots are a useful tool for research in laboratory conditions9,10.
Transgenic roots, or hairy roots (HR), are obtained after infection of plant material with Rhizobium rhizogenes (Riker et al. 1930) Young et al. 200111. This gram-negative bacterium induces the transfection of its Ri plasmid into the host genome and changes the regulation of plant hormone biosynthesis, promoting the formation of root tissue12. Transgenic roots can be maintained indefinitely under asepsis in a culture medium. The advantages of using HR for studying PPNs are a high growth rate in the absence of plant growth regulators that influence nematode infection and development, a high ratio of biomass production per unit time, and cellular integrity and longevity, which determine a higher genetic and biochemical stability6. By resorting to in vitro transgenic roots, PPNs genotypes can be maintained indefinitely under laboratory conditions, infection and PPNs development can be easily followed, host genetic variability can be reduced, manipulation of host molecular makeup can be directly linked to nematode response, and host and parasite structural changes can be more accurately followed6,13. For studies on PPN diseases of potato, in vitro transgenic root co-cultures allow carrying out experiments independently of season or potato tuber dormancy.
In this protocol, the traditional methodology of PPNs maintenance and in vivo infection of potato plants are detailed. For the structural analysis of infected roots, an improved methodology based on the establishment of in vitro co-cultures of transgenic potato roots with PPNs is also detailed as an alternative that allows a higher control of environmental and host genetic variability. To follow PPNs infection and development in the root tissue, histochemistry is employed to aid in PPNs observation under optical microscopy. The overall aim of this protocol is to optimize the study of PPN-host interactions, ensuring more controlled and reproducible conditions for experimentation while facilitating detailed structural and developmental analyses of nematodes in the root tissue.
Protocol
1. Infection of greenhouse-grown potato plants
NOTE: Greenhouse trials are performed with suspensions of PPNs in mixed life stages or second-stage juveniles (J2), depending on the specific life cycle of the PPN pest. For this protocol, suspensions of mixed life stages of the root lesion nematode (RLN) Pratylenchus penetrans were used. PPNs can either be reared in the lab or requested from certified reference laboratories.
- Multiplication and maintenance of root lesion nematodes
NOTE: Sterilized carrot disks are used for the multiplication and maintenance of RLNs14. Use commercially acquired carrots (var. Nice) with no visible damage to reduce microbial contamination. Preferably, they should be free from pesticides to avoid hindering RLN development.- Wash the carrot in running tap water to remove larger debris, and afterward, with a common detergent solution (1 drop per 40 mL of water) to remove finer debris. Dry with laboratory paper towels.
- Under asepsis, in a vertical flow hood, insert a sterilized metal skewer on the top of the carrot (1 to 2 cm inwards) so it can be more easily held.
- With the help of a wash bottle with a nozzle, wet the carrot with 96% (v/v) ethanol. Blot the bottom tip of the carrot in a sterilized filter paper and carefully take it to the flame.
CAUTION: Be aware that ethanol strongly ignites, so stand at a distance. - Peel the carrot from the top down using a sterile peeler and then repeat the previous step. Discard the top and bottom sections (2 cm inwards) and set the middle section of the carrot in a sterile Petri dish (150 mm in diameter). Using a sterile blade and tweezers, carefully cut 1 cm thick sections from the approximately 2 cm diameter portion of the carrot (Figure 1).
- Transfer the sections to sterile Petri dishes (60 mm diameter) and seal the border with transparent film. Using a UV light, sterilize the surface of the carrot disks for 60 min on each side.
- Keep at 25 °C for 1-2 weeks in darkness and discard any carrot disks that begin to show signs of microbial contamination15.
NOTE: Visible signs of contamination are excessive browning (rotting), liquid accumulation in the lower border of the carrot disk, or fungal mycelium growth on the surface. - The remaining carrot disks are ready to be infected with RLN suspensions. Begin by making an X-shaped incision at the center of the carrot disk using a sterile blade. Be sure to cut only halfway deep.
- Inoculate the RLN by pipetting 50 µL of a suspension containing at least 50 mixed life stages in the center of the X-shaped wound. Close the Petri dish and seal the border with transparent film to avoid desiccation.
- Determine the average number of nematodes in the suspension by counting five 50 µL aliquots under a binocular stereomicroscope (40x) at room temperature in a concave slide. Set the suspension of mixed life-stage RLNs to 1000 per mL by adding water to the suspension or waiting for nematodes to settle (approximately 60 min) and lowering the volume by decanting the surface water.
- Keep the carrot disks at 25 °C, in darkness, for up to 3 months and follow weekly under a binocular stereomicroscope for signs of necrotic lesions, resultant of RLN population growth.
NOTE: Successfully parasitized carrot discs can be stored at 11 °C for later use for up to 2 months but check regularly for microbial contamination. Parasitized carrot discs that show signs of microbial infection must be decontaminated by autoclaving before being discarded. - Under the flow hood, extract RLNs by transferring carrot disks with visible tissue necrosis at the inoculation site (Figure 1) to an 8 cm diameter and 75 µm mesh sieve set in a sterile glass bowl. Leave a small 1 cm gap between the bottom of the sieve and the bowl concavity to collect the RLNs.
NOTE: In the lack of a commercially acquired sieve, one can be crafted from an 8 cm diameter plastic tube/a sturdy plastic cup, and a tight mesh gauze. Use rubber bands to secure the gauze to the plastic tube or cup. - Pour an antibiotic solution into the sieve until the carrot disks are covered and keep for 12 h (overnight) in darkness. The RLNs egress from the carrot disks and settle at the bottom of the bowl. The antibiotics solution should be prepared extemporaneously with the extraction by adding 50 µg/mL each of kanamycin and carbenicillin in sterilized distilled water16.
NOTE: Antibiotics stock solutions are prepared at 50 mg/mL by dissolving 0.5 g kanamycin monosulfate or 0.5 g carbenicillin disodium each in 10 mL of sterilized distilled water. Stock solutions are filtered (0.22 µm mesh) in the flow hood and can be kept at -20 °C for up to 1 year. - Remove the sieve, use a sterilized glass pipette to draw the RLNs from the bottom of the bowl into a sterilized glass staining block (4 cm x 4 cm x 1 cm), and wash by pipetting 1 mL of antibiotic solution. Wait 30 to 40 min for the nematodes to settle before collecting the used antibiotic solution. Repeat this wash 4x-5x.
- Use the aqueous suspension with the RLNs immediately or keep it at 11 °C for a longer storage period (up to 2 months).
- In vivo infection of potato plants with PPNs
NOTE: To grow susceptible potato plants (S. tuberosum var. Désirée), certified seed potatoes should be acquired from agro-dealers between January and March. Choose certified seed potatoes because they are sold with a phytosanitary passport, ensuring they are uncontaminated with quarantine phytoparasites. As a precaution, an initial step of disinfection with a 10% bleach solution followed by washing in running tap water can be performed to ensure disinfection of the potato tuber surface. Common commercialized potatoes are not recommended since the treatments imposed to reduce sprouting and vigor may interfere with potato growth and response to infection.- Select same-sized potato tubers and discard those with holes, bruises, or softer sections. Gently remove all grown sprouts (1 mm) before sowing to synchronize sprouting.
NOTE: If needed, store the seed potatoes in a well-ventilated, dry, and dark place before sowing. - Fill 5 L pots (22 cm x 18 cm) with a 1:1 mixture of autoclaved soil and fine coarse sand mixed with 22.5 g of slow-release NPK fertilizer (12-12-12) and sow the potatoes at 9 cm below the soil surface.
NOTE: Soil and sand should be sieved to remove debris larger than 2 mm, autoclaved 2x at 121 °C for 15 min, and dried at 100 °C for 1 to 2 days, with frequent mixing. Aerate the next 7 – 10 days by mixing frequently, before use. - Keep the pots in a greenhouse under humid conditions (50%-70% humidity) and water frequently (keep the soil at 70% maximum water holding capacity), avoiding temperature extremes, until potato plant shoots begin emerging at the soil surface.
- After plant emergence, use freshly extracted RLN suspensions to infect the potato roots. Begin by creating evenly distributed 4 to 6 holes (1 cm wide) around the plant to seed depth.
- Evenly pipette an 8 mL suspension of 30,000 living mixed life stage RLNs into the holes, so that the inoculum is at a ratio of 4 live RLNs per g of soil mixture, and cover with soil mixture. For the pots with RLNs, withhold watering on the day of inoculation.
NOTE: RLNs are counted under a stereomicroscope (40x). Dead nematodes are nonmotile and have an extended shape, while live nematodes are generally moving (non-extended shape). Physical prodding is used to ascertain mortality. - Keep the pots for 2 months under the conditions described above (Figure 2). Afterward, uproot the potato plants and weigh the shoots and roots separately.
- Carefully wash the root system before checking the location of RLN attack sites through staining techniques5.
- Select same-sized potato tubers and discard those with holes, bruises, or softer sections. Gently remove all grown sprouts (1 mm) before sowing to synchronize sprouting.
2. Establishment of in vitro co-cultures of potato transgenic roots with PPNs
- Establishing in vitro potato transgenic roots
NOTE: For this protocol, we used Rhizobium rhizogenes carrying the gus reporter gene co-integrated in the Ri plasmid and driven by a double 35S promoter (A4pRiA4::70GUS)17. Bacteria can be acquired from commercial sources or requested from certified reference laboratories.- To obtain bacteria at the exponential growth phase, spread plate R. rhizogenes in a Luria-Bertani (LB)18 solid medium plate and keep overnight at 26 °C.
NOTE: LB medium can be acquired commercially or prepared in the laboratory by adding 10 g/L peptone, 5 g/L yeast extract, 10 g/L NaCl, and 15 g/L agar and then steam sterilizing for 15 min at 121 °C. - With an inoculation loop, pick a colony and inoculate 10 mL of liquid LB broth (LB medium without agar) in a sterile 50 mL flask. Keep overnight in the dark at 26 °C under agitation (180 rpm).
- Measure the absorbance of the liquid culture until A600 reaches 0.6. At this stage, bacteria are at the exponential growth phase and are used for inoculating the plant material.
- Inoculation is performed in aseptic fresh potato tubers. To sterilize the surface of potato tubers, begin by washing in running tap water to remove larger debris, and then with a common detergent solution (1 drop per 40 mL of water), with vigorous agitation, to remove the finer debris.
NOTE: Select a potato variety with known susceptibility to the PPNs in use. For this protocol, we used S. tuberosum var. Désirée. - Place the tubers in a container, cover with a commercial bleach solution (1:4, commercial bleach in tap water) and close. Mix for 15 min, dispose of the bleach solution and rinse 3x with sterilized tap water.
- In a flow hood, immerse the tubers in an ethanol solution (80%, v/v) for 15 min with vigorous agitation, dispose of the ethanol and rinse 3x with sterilized tap water.
- Using a sterile scalpel, remove the peripheral portions of the tubers (approximately 50% of the tuber from the surface inwards), and section the inner central piece into 0.5 cm thick segments19. Inoculate immediately with the bacterial suspension prepared in step 2.1.3.
- For inoculation, dilute the bacterial suspension by adding 1 mL of bacterial suspension to 9 mL of Schenk and Hildebrandt20 (SH) medium supplemented with 30 g/L sucrose at pH = 5.6. Dip the tip of a sterile scalpel in the diluted suspension and wound the surface of the potato segment. Repeat this step 5x for each potato segment.
- Dry the segments of excess humidity in sterile filter paper for 1 min, place them in semi-solid SH medium (SH medium with 30 g/L sucrose, 8 g/L agar, pH = 5.6) and keep in darkness at 25 °C for plasmid transfection to occur.
- After 3 days, transfer the infected segments to plates of semi-solid SH medium supplemented with 150 µg/mL of each of the antibiotic's cefotaxime and carbenicillin. Keep for over 3 months with weekly medium renewal to ensure elimination of the bacteria.
NOTE: Cefotaxime stock solution can be prepared at 100 mg/mL by dissolving 1 g cefotaxime sodium in 10 mL of sterile demineralized water and filtering (0.22 µm mesh) under the flow hood. Antibiotics can be kept at -20 °C for up to 1 year. - After 3 months, transgenic root growth is extensive. Transfer the roots to a fresh, semi-solid SH medium without antibiotics by gathering a 1 g root clump with the tips of a sterile tweezers and placing it at the center of the culture medium in a new plate (Figure 3).
NOTE: Approximately 1 month after infection, small masses of cell growth appear in the surface of the potato segment, from where the transgenic roots start developing6. Be sure to keep them in contact with the culture medium otherwise they might desiccate. - To ensure genetic and metabolic stability, keep transgenic roots under a monthly sub-culture routine (as described in step 2.1.11.) at 25 °C in darkness for over 1 year before infecting with PPNs.
NOTE: Ensure that at least six replicates are kept at every step of the protocol since unwanted microbial contamination often occurs. Once established, a single co-culture plate can be used as an inoculum for several new co-cultures; however, be sure to keep at least six replicate plates.
- To obtain bacteria at the exponential growth phase, spread plate R. rhizogenes in a Luria-Bertani (LB)18 solid medium plate and keep overnight at 26 °C.
- Establishing in vitro co-cultures of transgenic potato roots with PPNs
NOTE: To obtain co-cultures of transgenic roots with PPNs, the nematode sterilization process is critical. For the present protocol we used second stage juveniles of the quarantine root-knot nematode Meloidogyne chitwoodi. Nematode inoculum can be obtained from certified reference laboratories in the form of root galls.- Under a binocular stereomicroscope (20x), isolate nematode egg masses from the root galls with sterile ultra-fine point tweezers. Place egg masses in a covered Petri dish with 5 mL of sterile tap water and let the eggs hatch for 48 h. Set the J2 suspension to 100 nematodes per mL.
- In a flow hood, pipette 5 mL of a suspension containing 500 J2 into a sterile 20 µm mesh sterile sieve and wash with sterile tap water.
- Immerse the bottom half of the sieve containing the J2s in a 20 % hydrogen peroxide (H2O2) solution, and mix manually in a circular motion for 15 min.
- Wash the sterile J2s by dispensing sterile tap water through the sieve. Repeat this step 3x. In the final wash, tilt the sieve so that the nematodes collect at the sieve border. Recover the sterile nematode suspension by pipetting 1 mL of sterile ultrapure water in the sieve border, and store at 11 °C or use immediately.
NOTE: Success of sterilization can be assessed by plating a 100 µL aliquot of the nematode suspension in SH medium and regularly monitoring, for 1 week, for contamination. - In the flow hood, subculture a 1 g clump of potato transgenic roots (as described in step 2.1.11.) onto SH plates with 100 sterile nematodes (100 µL of a suspension with 1000 J2s per mL). After 2 to 3 weeks, small galls begin to appear in the new roots.
- Follow the co-culture regularly under an inverted microscope (100x), and when egg masses begin to be noticeable, subculture to a new semi-solid SH medium plate, making sure galls are taken with the root clump (Figure 4). Keep co-cultures under a monthly sub-culture routine at 25 °C in darkness.
3. Structural analysis of PPNs infection
NOTE: To follow PPNs induced changes in root tissue structure, histochemical staining techniques are used to contrast tissues with different chemical compositions. Differential staining is performed in roots masses or in thin sections of fixed root material, where specific dyes react with the target tissue according to their chemical affinity21. For the present protocol, we used acid fuchsin, or periodic acid-Schiff's reagent (PAS) combined with toluidine blue O dyes for differential staining.
- Plant parasitic nematodes distribution in roots stained with acid fuchsin
NOTE: To follow the distribution of PPNs throughout the root system, acid fuchsin is used to stain the nematode muscle tissue in a red hue5.- Begin by washing the root system under running tap water for 5 min to remove any soil debris (in vivo plant roots) or residues of the culture medium (in vitro transgenic roots). Use your fingers in a circular motion to aid in detaching the soil from the root system.
- Cut the root system into 1 to 2 cm long sections and place it inside a 150 mL beaker. Dispense 70 mL of a 1.5 % sodium hypochlorite (NaOCl) solution and mix vigorously for 4 min to clear root tissues. Afterward, dispose of the NaOCl solution, rinse the roots in running tap water, and soak for 15 min in demineralized water to remove residual NaOCl22.
NOTE: Chlorine bleach contains a minimum of 5.25% NaOCl, so add 20 mL of chlorine bleach to 50 mL of water to obtain a 1.5% NaOCl solution. For softer material (e.g., softer tissue of young roots or of in vitro grown transgenic roots) use a 0.9% NaOCl solution, while for harder material (older lignified roots) a 2.0% NaOCl solution is used. - Drain the cleared roots and set them on a borosilicate glass beaker with 30 mL of demineralized water. Pipette 1 mL of acid fuchsin stain solution, mix manually and boil for 30 s on a hot plate. Let the glass beaker cool down, drain the solution and wash the stained roots in running tap water.
NOTE: The acid fuchsin stain solution is made by dissolving 3.5 g of acid fuchsin dye powder in 250 mL of acetic acid and adding 750 mL of demineralized water. Staining is always followed by a destaining step to remove unused excess stains and enhance specimen contrast. - Destain by adding 10-30 mL of glycerin acidified with a few drops of HCl (5N)13.
- Observe under the stereomicroscope or inverted microscope to roughly assess where in the root structure the PPNs are preferentially attacking (Figure 5 and Figure 6).
- Assessment of root cell morphology with periodic acid-Schiff (PAS)/toluidine blue O
NOTE: The influence of PPNs infection or damage to root cell morphology can be assessed under the microscope by first fixating, sectioning, and differentially staining infected potato roots.- In a closed sample vial, fix fresh root material with 2.5% glutaraldehyde, prepared in 0.1 M sodium phosphate buffer, at pH 7.2, for 24-48 h, at room temperature6.
CAUTION: Glutaraldehyde is toxic. Avoid inhalation and contact. Use protective lab coat and gloves and work in a fume hood. Sample vials should be closed unless in rinsing or in vacuum procedures. Dispose of glutaraldehyde following Hazardous Waste Procedures rules. Steps 3.2.1 to 3.2.5 are performed in a fume hood to avoid reagents inhalation.
NOTE: To prepare 1 L of sodium phosphate buffer, pH 7.2, add 68.4 mL of 1M Na2HPO4 (141.96 g in 1 L solution) to 31.6 mL of 1M NaH2PO4 (119.98 g in 1 L solution) and fill up to 1 L by adding 900 mL of demineralized water. To help infiltration of the fixative, place the uncapped sample vials with root material under a low vacuum (26 mm Hg) for 2 min in a desiccator connected to a vacuum pump. - With a glass Pasteur pipette, discard the fixative solution and wash fixated roots with sodium phosphate buffer (3x).
- Begin gradually dehydrating the fixated root tissue by replacing the buffer with a 10 % ethanol solution (v/v) for 15 min. Afterward, exchange with a 20% ethanol solution using a glass pipette, and keep the roots imbedded for 15 min. Continue with the graded succession of increasing ethanol concentrations (30%, 40%, 50%, 60%, 70%, 80%, and 90% for 15 min each) until the pure ethanol step, where the roots should be kept for 1 h.
- Embed the dehydrated roots gradually in resin (2-hydroxyethyl methacrylate). With a glass pipette, replace the pure ethanol with a 3:1 (v/v) ethanol/resin solution and keep for 24 h at 4 °C. Follow this with 1:1 and 1:3 (v/v) ethanol/resin solutions, each with a 24 h incubation period at 4 °C. Afterwards replace the 1:3 (v/v) solution with pure resin supplemented with dibenzoyl peroxide (1%) as a polymerization initiator.
- Set the samples in a resin mold tray, add a resin:dimethyl sulfoxide 15:1 (v/v) mixture, and keep at 60 °C over a hot plate for 48 h for the resin to harden.
- Set the impregnated samples in a rotary microtome equipped with a tungsten knife and slice 2-5 µm sections onto glass slides, according to the manufacturer's instructions.
- Begin the differential staining by immersing the slides for 10 min in a glass staining jar with a 15% 2,4-dinitrophenylhydrazine solution in acetic acid, at room temperature. Afterwards wash carefully under running tap water for 15 min and dry in an oven at 60 °C (15 min).
- Following, immerse the slides in periodic acid (1%) for 10 min, and then wash under running tap water for 5 min and leave to dry in an oven at 60 °C (15 min).
- Immerse the slides in Schiff′s Reagent (composed of 1% pararosaniline and 4% sodium metabisulfite, in 0.25 M hydrochloric acid), for 30 min. Afterwards, wash with a sodium metabisulfite solution (0.5%) in hydrochloric acid (0.05 M), for 2 min. Repeat 3x. Finally wash in running tap water for 5 min, and dry at room temperature.
- For contrast, stain by immersing the slides in 0.05% toluidine blue O for 15 min, washing in running tap water for 15 min and drying in an oven at 60 °C (15 min).
- Observe under a microscope (100x) equipped with image capture hardware (Figure 7).
- In a closed sample vial, fix fresh root material with 2.5% glutaraldehyde, prepared in 0.1 M sodium phosphate buffer, at pH 7.2, for 24-48 h, at room temperature6.
Results
Carrot disks can be used to multiply and maintain several types of migratory PPNs23. For the RLN, this technique is generally used to maintain reference collections of nematode species or isolates. Using carrot disks, an average 100x increase in nematode populations can be obtained in a period of 3 months (Figure 1). However, nematode numbers vary widely (between 30x and 200x), mainly owing to nematode genetic diversity and/or variation in nutritional contents of carrots. Also, despite several prevention measures used to reduce microbial contamination, 20% to 30% of carrot disks can contaminate, so be sure to prepare more plates than the required.
In potato plants, the presence of RLNs does not always induce visible symptoms since they depend greatly on the parasite population numbers attacking the root system (Figure 2). For P. penetrans, an initial inoculum of 4 RLNs/g of soil can build up to 2000 RLNs/g of root and 750 eggs/g of root after 2 months, inducing an almost 30% decrease in root weight5.
Potato hairy root cultures are a high throughput system for studying root-related diseases in a laboratory context. The potato transgenic roots established have a specific growth rate of 300 mg root fresh weight per L of SH medium per day and a doubling time of 2.6 days6 (Figure 3). When co-cultured with M. chitwoodi, these parameters are slightly affected since the nematodes feed on the root tissue and cause a sink for energy. For the co-cultures, a specific growth rate of 200 mg root fresh weight per L of SH medium per day and a doubling time of 3 days can be attained6. The number of nematodes developed can rise up to 1200 J2s per g of potato transgenic root fresh weight, and the amount of eggs can be 4x higher6,13 (Figure 4). When compared to tests in soil from greenhouse-grown RKN-infected tomato plants, the highest yield for nematode populations was only half that of transgenic roots per g of root material (unpublished data). However, RKN population growth is known to depend heavily on host plant species and even variety. Nevertheless, morphologically, the nematodes reared in transgenic roots show no substantial difference from the ones retrieved from field or greenhouse infections6,13.
The use of acid fuchsin is helpful in tracing nematode attacks on root tissue. Motile stages of the RLN are known to be found inside roots as soon as 1 day after inoculation5. This initial stage is believed to not depend on host susceptibility. However, after penetration, RLNs either remain feeding on the epidermal and cortex cells of the root, reproduce and induce the formation of necrosis in susceptible plants, or egress from the roots back into the soil5. These mechanisms are easily followed by staining with acid fuchsin (Figure 5). The co-cultures of potato hairy roots with RKNs provide a powerful laboratory tool to analyze the mechanisms of J2 penetration and decision-making in the establishment of the feeding site for the sedentary adult female. Every stage after infection can be followed, including the formation of the gelatinous matrix and egg release (Figure 6).
Tissue structure can be detailed using histochemistry and optical microscopy. Using differential staining techniques, the life cycle stages of the phytoparasite can be followed along with the changes induced in the surrounding root tissue. Meloidogyne chitwoodi, as well as other root-knot nematodes, promote the formation of a feeding site composed of a specific cell type called giant cells. These multinucleate cells are induced by secretions from the nematode J2s and become metabolically overactive, producing food for the stationary adult female (Figure 7). Following the formation of this specialized structure provides important information on nematode feeding mechanisms and allows the identification of specific steps that can be targeted for disruption of its life cycle. Also, the feeding site structure can be specific to the RKN species, contributing to its identification.

Figure 1: Root lesion nematode maintenance in carrot disks. (A) Disks of carrot roots are sterilized and infected with Pratylenchus penetrans causing (B) characteristic necrotic lesions (dark sections) due to (C) population growth. Bar=1 cm (A and B), 100 µm (C). Please click here to view a larger version of this figure.

Figure 2: Infection of roots of Solanum tuberosum grown in greenhouse conditions. Control potato plants (pots in the back) and plants infected with the root lesion nematode (pots in the front) show no visible symptoms of infection in the shoots after 30 days of infection. Bar = 10 cm. Please click here to view a larger version of this figure.

Figure 3: Development of Solanum tuberosum transgenic roots. (A) Small cell growth masses begin appearing along the scalpel wounding area in the potato tuber section (arrow), (B) followed by the emergence of the first transgenic roots (right side insert), (C) that quickly grow and sustain in the culture medium. (D) A root clump can be transferred to a fresh culture medium plate for continuous growth. Bar = 1 cm. Please click here to view a larger version of this figure.

Figure 4: Co-cultures of Solanum tuberosum with the plant parasitic nematode Meloidogyne chitwoodi. (A) In vitro, potato transgenic root cultures can be infected with (B) Columbia root-knot nematode aseptic second-stage juveniles (J2) to establish a plant/nematode co-culture. (C) Root galls can be obtained with adult females bearing egg masses. Bar = 1 cm (A, B) and 200 µm (C). Please click here to view a larger version of this figure.
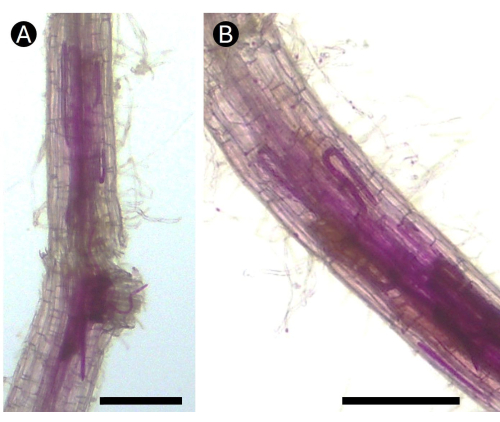
Figure 5: Acid fuchsin-stained roots of potatoes infected by the root lesion nematode Pratylenchus penetrans under greenhouse conditions. (A) Several life stages of the nematode can be seen in the cortex area of (B) the root causing necrotic lesions. Bar = 100 µm. Please click here to view a larger version of this figure.

Figure 6: Acid fuchsin stained root galls from transgenic roots infected with Meloidogyne chitwoodi. (A) The gall tissue can be seen (B) encasing part of the adult female M. chitwoodi (C) that has already produced the egg mass (D) with the eggs. Bar = 500 µm (A), 100 µm (B, C) and 20 µm (D). Please click here to view a larger version of this figure.

Figure 7: Cellular structure of a transgenic root gall formed by Meloidogyne chitwoodi. (A) Ultrathin sections of a transgenic root gall stained with periodic acid-Schiff (PAS) and toluidine blue O, showing the adult female feeding site and (B) the giant cells induced by the nematode encased by root gall tissue. Bar = 100 µm. Please click here to view a larger version of this figure.
Discussion
The study of the mechanisms of infection and disease development in plants attacked by soil-dwelling PPNs is difficult because these phytoparasites generally infect the inner tissues of the root system and induce unspecific symptoms in the shoots. Despite the controlled environmental conditions of the greenhouse, sprouting potato tubers and the growth of potato plants are still favored in the spring and summer months, reducing the experimental period available to one season per year. Also, a substantial number of pots have no emergence of potato plants. The life cycle of the RLN is relatively long and around 2 to 3 months are needed to reach population peaks capable of inducing disease symptomatology. The main shortcomings of resorting to greenhouse bioassays for PPNs research are that a) pot tests often present a high variability when it comes to susceptibility to PPNs diseases; b) although infections are performed with pure populations of the PPNs, there is a risk of unsuccessful PPNs establishment and/or cross-contamination with different PPNs species; c) even though preventative measures are enforced, there is always the risk of contaminations by microbial pathogens; and d) depending on the available funds and size of the greenhouse, the number of replicates is often too reduced for an adequate sampling scheme, for statistical validity.
Potato transgenic roots are a versatile laboratory tool that does not require large spaces for maintenance; can be obtained in less amount of time; are free from contamination or host genetic variability; and, more importantly, allow controlling single environmental or nutritional variables, which ensures that root response is directly resultant from the treatment imposed12. For studies on PPNs infection and development, using HRs is an improvement when compared to greenhouse bioassays. Firstly, the need for previously growing large amounts of PPNs before each experiment is unnecessary since potato HR/PPNs co-cultures are a continuous system that provides all stages of nematode development irrespective of season or climate conditions. Also, genetic variability is very limited because HRs are clonal somatic tissue, so changes in PPNs response are directly dependent on the conditions imposed. Potato HR/PPNs co-cultures occupy a small space; a temperature-controlled growth chamber can house many Petri dishes, so experimentation is seldom limited by the number of replicates. Lastly, the technique is expandable to other potato PPNs, e.g., Globodera spp., being mainly limited by the nematode sterilization step, which can become challenging depending on the PPNs species and life stage selected for decontamination19,24. Despite its many advantages, research using co-cultures is limited to studies on the tissue level, e.g., ultrastructural morphology or on biochemical regulation mechanisms, under biotic or abiotic stress6,25, and is inadequate for studies that require, for example, determining tuber yield or damage phenotype. Also, plant-nematode interactions in natural conditions are influenced by many variables so caution is advised for direct comparisons with results obtained from studies with co-cultures.
Histochemistry combines histology with chemical aspects, enabling the determination of the nature of substances present in tissues and their location21. Differential staining techniques are widely used for the distinction of specific chemical and morphological changes. The acid fuchsin dye stains nematode tissues by penetrating the impermeable cuticle during the boiling step. Later, destaining the root system with acidified glycerin allows identifying the sites where nematodes are attacking, since they will contrast with the root tissue. However, if kept in acidified glycerol for longer than 1 to 2 months, the stain will decrease in intensity, and the contrast between the nematodes and plant roots will reduce.
Periodic acid-Schiff (PAS) and toluidine blue O are employed for a double staining technique commonly used to stain fresh or resin-embedded tissue. This is an easy-to-apply technique but with low specificity and sensitivity. The sequential application of two dyes allows simultaneous staining of multiple cellular targets with different chemical properties. Periodic acid with Schiff reagent will stain polysaccharides with a pink hue, mainly starch, cell wall polysaccharides, and some phenols, but not cellulose or callose. Toluidine blue O dye highlights PAS staining and will stain xylem and sclerenchyma cell walls green or blue-green, collenchyma and parenchyma cell walls in purple-red, and phloem walls and the middle lamella of cell walls in red. Callose and starch will not get stained21.
The protocol described offers several promising future applications in plant science, agriculture, and biotechnology. It enables detailed studies into the molecular and cellular mechanisms of parasitism, providing insights into how PPNs infect and manipulate hosts. It supports the breeding of resistant crops by aiding the screening of potato cultivars or transgenic lines for resistance to PPNs, as well as the identification of key genes involved in resistance or susceptibility. Furthermore, in vitro co-cultures can serve as a powerful tool for the high-throughput screening of nematicides or biological control agents (e.g., microbes or natural products), allowing researchers to evaluate their efficacy in controlling PPN diseases.
Disclosures
We have nothing to disclose.
Acknowledgements
This research was partly funded by Fundação para a Ciência e a Tecnologia (FCT), through grants NemACT, DOI: 10.54499/2022.00359.CEECIND/CP1737/CT0002 (JMSF), CEECIND/00040/2018, DOI: 10.54499/CEECIND/00040/2018/CP1560/CT0001 (CSLV) and SFRH/BD/134201/2017 (PB); project PratyOmics, DOI: 10.54499/PTDC/ASP-PLA/0197/2020; and structural funding UIDB/00329/2020 | cE3c (DOI: 10.54499/UIDB/00329/2020) + LA/P/0121/2020 |CHANGE (DOI: 10.54499/LA/P/0121/2020), and GreenIT (DOI: 10.54499/UIDB/04551/2020 and DOI: 10.54499/UIDP/04551/2020)..
Materials
| Name | Company | Catalog Number | Comments |
| 2,4-Dinitrophenylhydrazine | Sigma-Aldrich | D199303 | |
| 2-Hydroxyethyl methacrylate | Sigma-Aldrich | 17348 | |
| Acetic acid | Sigma-Aldrich | 695092 | |
| Acid Fuchsin | Sigma-Aldrich | F8129 | |
| Benzoyl peroxide | Sigma-Aldrich | B5907 | |
| borosilicate glass beaker | Sigma-Aldrich | Z231827 | |
| Carbenicillin disodium salt | Sigma-Aldrich | C3416 | |
| Cefotaxime sodium salt | Sigma-Aldrich | C7039 | |
| Dimethyl sulfoxide | Sigma-Aldrich | 472301 | |
| Ethanol | Supelco | 1.00983 | |
| Fertilizer | Compo Expert | ||
| Flower pot 5 L | VWR | 470049-676 | |
| Glutaraldehyde | Sigma-Aldrich | 354400 | |
| Glycerol | Sigma-Aldrich | G7893 | |
| Hydrochloric acid | Sigma-Aldrich | 258148 | |
| Kanamycin monosulfate | Sigma-Aldrich | BP861 | |
| LB Broth with agar | Sigma-Aldrich | L3147 | |
| MCE syringe filter | Millipore | SLGSR33SS | |
| PARAFILM M sealing film | BRAND | HS234526B-1EA | |
| Pararosaniline hydrochloride | Sigma-Aldrich | P3750 | |
| Periodic acid | Sigma-Aldrich | P0430 | |
| Phyto agar | Duchefa Biochemie | P1003 | |
| Scalpel blade no. 24 | Romed Holland | BLADE24 | |
| Schenk & Hildebrandt Basal salt medium | Duchefa Biochemie | S0225 | |
| Schenk & Hildebrandt vitamin mixture | Duchefa Biochemie | S0411 | |
| Schiff′s reagent | Sigma-Aldrich | 1.09033 | |
| Sodium metabisulfite | Sigma-Aldrich | 161519 | |
| Sodium phosphate dibasic | Sigma-Aldrich | S9763 | |
| Sodium phosphate monobasic | Sigma-Aldrich | S5011 | |
| Soil / Substrate | Compo Sana | ||
| Stainless Steel Tweezers | Sigma-Aldrich | 22435-U | |
| Sucrose | Duchefa Biochemie | S0809 | |
| Toluidine Blue O | Sigma-Aldrich | 198161 |
References
- Çalışkan, M. E., Yousaf, M. F., Yavuz, C., Zia, M. A. B., Çalışkan, S. History, production, current trends, and future prospects. Potato Production Worldwide. , 1-18 (2022).
- Barker, K. R., Koenning, S. R. Developing sustainable systems for nematode management. Ann Rev Phytopathol. 36 (1), 165-205 (1998).
- Jones, J. T., et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 14 (9), 946-961 (2013).
- Davis, E. L., Hussey, R. S., Baum, T. J. Getting to the roots of parasitism by nematodes. Trend Parasitol. 20 (3), 134-141 (2004).
- Figueiredo, J., Vieira, P., Abrantes, I., Esteves, I. Commercial potato cultivars exhibit distinct susceptibility to the root lesion nematode Pratylenchus penetrans. Horticulturae. 8 (3), 244 (2022).
- Faria, J. M. S., et al. In vitro co-culture of Solanum tuberosum hairy roots with Meloidogyne chitwoodi: Structure, growth and production of volatiles. Plant Cell Tissue Organ Culture. 118 (3), 519-530 (2014).
- Wesemael, W. M. L., Moens, M., Viaene, N., Taning, L. M. Life cycle and damage of the root-knot nematode Meloidogyne minor on potato, Solanum tuberosum. Nematology. 16 (2), 185-192 (2014).
- Subramanian, P., et al. Differential metabolic profiles during the developmental stages of plant-parasitic nematode Meloidogyne incognita. Int J Mol Sci. 18 (7), 1351 (2017).
- Gutierrez-Valdes, N., et al. Hairy root cultures-A versatile tool with multiple applications. Front Plant Sci. 11, 33 (2020).
- Cho, H. J., Farrand, S. K., Noel, G. R., Widholm, J. M. High-efficiency induction of soybean hairy roots and propagation of the soybean cyst nematode. Planta. 210 (2), 195-204 (2000).
- Young, J. M., Kuykendall, L. D., Martínez-Romero, E., Kerr, A., Sawada, H., et al. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol. 51 (1), 89-103 (2001).
- Giri, A., Narasu, M. L. Transgenic hairy roots. Biotechnol Adv. 18 (1), 1-22 (2000).
- Faria, J. M. S., Rusinque, L., Cavaco, T., Nunes, J. C., Inácio, M. L. Essential oil volatiles as sustainable antagonists for the root-knot nematode Meloidogyne ethiopica. Sustainability. 15 (14), 11421 (2023).
- European Mediterranean Plant Protection Organization (EPPO). PM 7/148 (1) Guidelines for the management of nematode collections used for the production and maintenance of reference material. EPPO Bulletin. 51 (3), 507-548 (2021).
- Boisseau, M., Sarah, J. L. In vitro rearing of Pratylenchidae nematodes on carrot discs. Fruits. 63 (5), 307-310 (2008).
- Barbosa, P., et al. Nematicidal activity of phytochemicals against the root-lesion nematode Pratylenchus penetrans. Plants. 13 (5), 726 (2024).
- Santos, P. M., et al. Essential oils from hairy root cultures and from fruits and roots of Pimpinella anisum. Phytochemistry. 48 (3), 455-460 (1998).
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 62 (3), 293-300 (1951).
- Kumar, A., Forrest, J. M. Reproduction of Globodera rostochiensis on transformed roots of Solanum tuberosum cv. Desiree. J Nematol. 22 (3), 395-398 (1990).
- Schenk, R. U., Hildebrandt, A. C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian J Botany. 50 (1), 199-204 (1972).
- Figueiredo, A. C. S., Barroso, J. M. G., Pedro, L. M. G., Ascensão, L. . Histoquímica e citoquímica em plantas: princípios e protocolos. Universidade de Lisboa. , (2007).
- Bybd, D. W., Kirkpatrick, T., Barker, K. R. An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol. 15 (1), 142-143 (1983).
- Coyne, D., Adewuyi, O., Mbiru, E. . Protocol for in vitro culturing of lesion nematodes: Radopholus similis and Pratylenchus spp. on carrot disc. , (2014).
- Faria, J. M. S., Vicente, C. S. L., Rusinque, L., Camacho, M. J., Inácio, M. L. Plant-Nematode co-cultures in the screening of sustainable nematicides against soil-dweling parasitic nematodes of plants. Revista de Ciências Agrárias. 45 (4), 436-439 (2022).
- Faria, J. M. S., et al. In vitro co-cultures of Pinus pinaster with Bursaphelenchus xylophilus: a biotechnological approach to study pine wilt disease. Planta. 241 (6), 1325-1336 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved