Method Article
Collection, Processing, and Storage Consideration for Urinary Biomarker Research
En este artículo
Resumen
This protocol aims to provide considerations for urine sample collection, processing, and storage for urine tract infection biomarker studies.
Resumen
There are several urinary proteins that show promise as novel markers of urinary tract infections. The identification of a novel biomarker that has greater predictive accuracy compared to current diagnostic methods has the potential to greatly improve the ability to manage patients with urinary tract infections. However, sample collection, processing, and storage can all potentially impact the results of biomarker research. Understanding the effects of each of these stages on biomarker studies is necessary to inform future, high-quality research in this area, as well as to critically review other studies in this area. Here, the study reviews the literature regarding the effects of each stage of urine sample processing and reports the effects of various conditions on urinary proteins. The protocol will focus on collection techniques, time and temperature of storage, processing techniques, use of reagents, and long-term freezing on biomarker stability. It will focus on proteins but will briefly discuss other materials that may be utilized in biomarker research. In doing so, this protocol will provide a guide to future researchers to assist in the design of urinary biomarkers studies.
Introducción
Urinary tract infections (UTI) are one of the most common bacterial infections in both children and adults1. While the diagnosis of UTI in some populations can be uncomplicated, it can be more complex in others, such as those with neuropathic bladders2. The ability to accurately diagnose UTIs will help improve antibiotic stewardship efforts by decreasing the use of unnecessary antibiotics and potentially aid in the earlier diagnosis of UTI, thus decreasing the risk of morbidity. Given the prevalence of UTIs, there is significant interest in improving the management of this common infection.
There is an increasing number of novel biomarkers within the literature that show promise in their ability to diagnose UTI3,4,5,6,7. However, there are several factors associated with the processing of urine samples that have the potential to alter results. These factors range from collection methods, temperature and duration of short and long-term storage, processing techniques, reagent use, and freeze-thaw cycles8. Understanding how changes in each of these can affect biomarker readings is necessary to both critically interpret research within the literature as well as design high-quality studies focused on urine biomarkers.
Here, a narrative review of the literature is provided on the effects of each factor, including collection techniques, short and long-term storage temperature and duration, reagent use, and the effect of freeze-thaw cycles, on proteins that may have utility as urine biomarkers and provide recommendations for optimal processing based on this review of the literature. This protocol will focus on protein biomarkers measured using western blots or ELISAs.
Protocolo
This protocol follows the guidelines of the institution's human research ethics committee. Ensure that the approval is obtained from the institutional review board (IRB) prior to the collection and utilization of biological specimens for research.
1. Collection
- Obtain urine sample in a sterile specimen cup. Decide the type of urine sample, as well as specific inclusion and exclusion criteria, based on the specific study design. For UTI studies, use either the clean-catch method or catheterization to avoid perineal contamination.
- To obtain a clean-catch urine sample, instruct the participants to wipe down the periurethral area with a towelette, void a small amount into the toilet, and then urinate into the specimen cup.
- Instruct women to use their fingers to spread the labia and men to retract their foreskin (if applicable) prior to urination to avoid contamination.
- Record the time of collection.
- Collect the relevant clinical data from each participant, as required by individual study design and research question.
- Consider performing a urinalysis or urine dipstick on each sample prior to processing and storage if this data is not reliably available from the electronic health record.
2. Sample processing and storage
- Process the samples immediately. If this is not possible, store the sample at 4 °C for up to 24 h.
- If samples cannot be stored at 4 °C or need to be stored at 4 °C longer than 24 h, add 0.2 M boric acid or 10 mM NaN3 to the samples. Check to ensure such reagents are compatible with planned downstream applications.
- Record the duration of time samples spent at 4 °C.
- Centrifuge the samples at 1000-1500 x g for 10-20 min. Centrifugation does not need to be at 4 °C.
- Collect the supernatant and aliquot it into separate microcentrifuge tubes.
- Label the tubes with multiple, clear, identifiers (such as the date and sample identification (ID)). Consider using computer-generated barcodes specifically designed for storage of biological samples at -80 °C. If unavailable, ensure that the pen used to label samples is water-resistant.
- Label each freezer box such that each location has a specific code. For this, number each column with a different letter and each row with a number. This will allow for the creation of maps or other guides for easy sample location.
- Freeze the samples immediately at -80 °C. Record the time of freezing.
- Thaw the samples in a 37 °C water bath on the day of measurement to minimize unnecessary storage at either room temperature or 4 °C.
- Record the times and number of additional freeze-thaw cycles for each aliquot.
3. Analysis
- When using commercially available ELISAs, follow the manufacturer's instructions.
- Run the samples in duplicate.
- Identify the expected concentration of the protein of interest to ensure that the protein levels in the samples fall within the range of the kit. If the expected level of protein exceeds the upper standard, dilute the samples.
- After data is obtained from the plate reader (ELISA) or western blot, determine the concentration of each biomarker in the sample manually (not recommended) or using any software.
- Analyze the results. Data analysis depends on the individual study design.
- Consider adjusting biomarker values to account for the urine concentration.
NOTE: Traditionally, biomarker researchers have used urine creatinine as a method of normalization, especially in participants with normal renal function, to account for urine concentration. However, others report that normalization does not make a difference in the results4. To overcome this hurdle, some researchers report both normalized and non-normalized results. - Recommend reporting ranges of times from collection to freezing, as well as the duration of time at 4 °C prior to processing in published manuscripts to allow for interpretation of results in the context of sample processing (Figure 1)
4. Effect of various storage conditions on neutrophil gelatinase-associated lipocalin (NGAL).
- Spike fresh urine with 2 ng/mL of recombinant NGAL.
- Aliquot the urine and subject it to different processing and storage conditions.
- Centrifuge the urine at 1000-1500 x g for 10-20 min. Centrifugation does not need to be at 4 °C. Store at different conditions (20 °C, 4 °C, -20 °C) for 24 h, 48 h, or 72 h.
- Store aliquot of the sample at -80 °C for comparison.
- After maintaining the samples in the different conditions as mentioned in step 4.2.1, measure the levels of NGAL in the samples using a commercially available ELISA kit which includes the controls as per the manufacturer's instructions.
Resultados
Centrifugation had a small impact on NGAL levels. Centrifuged samples stored at -80 °C had lower levels of NGAL than non-centrifuged samples (2.17 ng/mL ± 0.32 ng/mL, 2.77 ng/mL ± 0.21 ng/mL). Freeze cycles also had an impact on NGAL levels after the third freeze-thaw cycle. (Figure 2). Of the conditions studied (centrifugation, freeze-thaw cycles, and storage temperature), storage temperature had the greatest impact on NGAL levels. Storage at -80 °C immediately after sample collection was the most stable method of sample preservation. Variation was seen in NGAL levels with each of the other storage conditions, with samples stored at room temperature (20 °C) with the largest change in levels. (Figure 3)
Taken together, these results indicate how measured protein levels can vary based on the sample processing method, underscoring not only the importance of a consistent and reliable protocol for reproducible results but also the necessity to evaluate the parameters analyzed here for individual biomarkers.
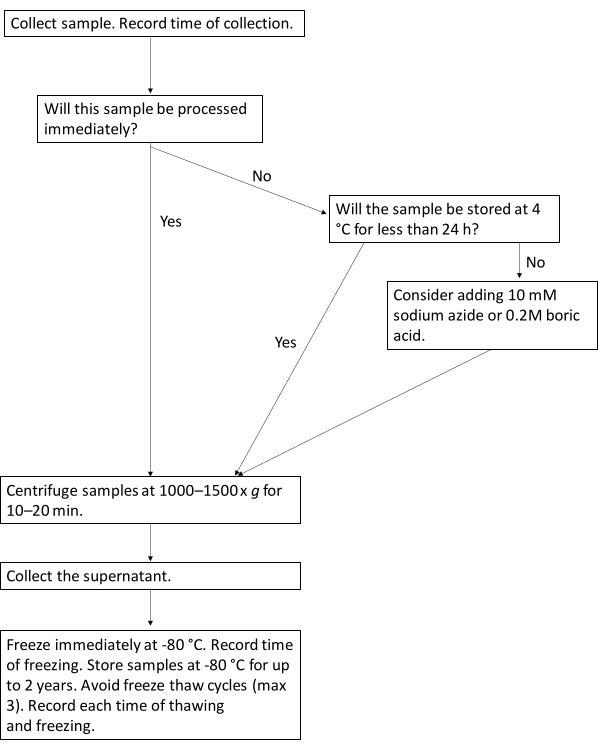
Figure 1: Protocol workflow for optimal processing of urine biomarkers. This flowchart illustrates the suggested workflow for processing urine samples for urine biomarkers. Please click here to view a larger version of this figure.

Figure 2: Urine NGAL concentrations in samples subjected to freeze-thaw cycles. Urine NGAL concentrations decrease after the third freeze-thaw cycle. Error bars represent standard deviation. A total of 8 samples were analyzed, run in duplicate for all 4 freeze-thaw cycle conditions. Please click here to view a larger version of this figure.

Figure 3: Urine NGAL concentrations in various storage conditions. Urine samples spiked with recombinant NGAL were stored at 20 °C, 4 °C, -20 °C for 24 h, 48 h, or 72 h. Samples were also immediately frozen at -80 °C for comparison. Concentrations of urine NGAL have a small degree of variation based on freezing temperature and duration. Error bars represent standard deviation. A total of 20 samples were analyzed, run in duplicate for all 10 time and temperature conditions. Please click here to view a larger version of this figure.
Discusión
The importance of producing consistent and reproducible results is not limited to the success of individual studies but will also enable a better comparison of results within the literature9. Variation between studies in key procedural steps can introduce irreversible bias that may affect biomarker signals and their interpretation, which may be responsible for discrepancies among several studies10. This demonstrates the need to establish a more standardized approach to processing urine samples for biomarker studies.
The study design of most urinary biomarker studies will dictate the time and method of urine collection. However, when measuring biomarkers in a non-time-sensitive method, we recommend avoidance of a first-morning sample. While first-morning urine samples are considered to supply the most information-rich urinary profile due to their concentrated nature, these samples are not ideal for measuring urine biomarkers as they are susceptible to bacterial and epithelial cell contamination. This is particularly the case for female urine samples: The comparison of female first-void urine to midstream samples demonstrates significantly different proportions of proteins11. Thus, a second morning or random midstream urine sample is better suited for studying protein biomarkers12. The method of urine collection is also usually dictated by study design. We recommend the use of either clean catch urine or catheterized samples. Residual urine initially collected for routine clinical purposes is often used in UTI studies and is an accepted method of sample collection. However, while the clean catch is a simple procedure, urethral catheterization is invasive. Catheterized urine collection should only be performed by those who have been appropriately trained to do so and done with the approval of the local IRB. We recommend collaborating with clinicians with appropriate training to obtain catheterized urine samples or utilization of residual urine initially collected for routine clinical care.
Samples should be processed right away to preserve the integrity of any markers of interest. However, this is often not possible; therefore, it becomes necessary to examine the effect of storage conditions, including duration and temperature, on sample stability. In general, storage at room temperature should be minimized as it has been well-founded that prolonged sample storage at room temperature for biomarkers such as NGAL can result in degradation13. While some studies have found that 24 h of storage at room temperature (25 °C) has minimal impact on concentration for biomarkers including NGAL and KIM-114,15, others have determined that significant degradation does indeed begin to occur within this time frame8. The urinary biomarker IL-18 has been found to be particularly sensitive to short-term storage at 25 °C14,15. When possible, samples should be stored short-term at 4 °C instead. At 4 °C, NGAL and KIM-1, IL-18, L-FABP, and cystatin C exhibit high stability for 24 h8 and up to 48 h15. However, after 48 h at 4 °C, KIM-1 begins to degrade significantly16. Finally, if short-term storage of samples at room temperature exceeds 8 h, or if storage at 4 °C exceeds 16 h, sodium azide or boric acid should be considered as a preservative to inhibit bacterial overgrowth and contamination12.
Duration of long-term storage is another important consideration. Storage of samples in -80 °C leads to significantly improved stability of urinary proteins compared to storage at -20 °C, which results in significant variability of degradation in proteins such as NGAL14,13. At -80 °C, samples can be reliably stored for at least 6 months16 and up to 2 years10. One study found that protein levels had a small but significant decrease after 5 years at -80 °C8, suggesting that samples should be analyzed prior to 5 years. During long-term storage of samples, freeze-thaw cycles should be minimized to three or fewer10,11. Exceeding three freeze-thaw cycles has been found to alter the urine sample17, which is also confirmed in the data presented here. Of note, for biomarkers without documented stability within the literature, we recommend the researchers to consider performing a pilot stability study prior to planning storage of samples at any temperature other than -80 °C.
There is a lack of consensus around the use of reagents for protein stability. Protease inhibitors are not necessary for urine samples given a relative lack of proteases in urine. This is supported by data demonstrating no difference in the types or amounts of proteins between urine samples stored both with and without protease inhibitors.18 Further, the addition of a protease inhibitor did not make a difference in urine levels of NGAL, KIM-1, or NAG10. While some studies have used protease inhibitors, their use is not standardized throughout the wider urine biomarker literature. Other reagents include those to prevent bacterial overgrowths, such as sodium azide and boric acid. While not traditionally used within the UTI biomarker literature, consideration should be given on the basis of the mechanism of the biomarker of interest. For biomarkers whose levels are associated with the degree of bacteriuria, the use of these reagents may be preferable. The other situation in which these reagents may be considered is when urine cannot be stored at 4 °C for less than 24 h, or must be stored at room temperature for a prolonged period of time prior to processing. Although not commonly used within the UTI biomarker literature, if bacterial overgrowth is a concern and optimal storage conditions are not possible, sodium azide or boric acid can be added to samples.19
Centrifugation is an important step to minimize contamination of the urine sample by proteins from other cells (e.g., red blood cells, white blood cells, epithelial cells). Samples should be centrifuged or filtered rapidly following sample collection. The literature suggests that cells within the urine can begin to lyse within 20-30 min after sample collection, potentially confounding protein measurement12. Although rapid centrifugation is frequently not possible, researchers must acknowledge the potential for alternative sources of urinary proteins. Given that pyuria is common in UTIs, this is an important consideration for researchers focused on urine biomarkers in UTI. Samples should not be frozen prior to centrifugation. Indeed, freezing the sample, even with prior centrifugation, may result in the formation of a precipitate of primarily calcium oxalate dehydrate and amorphous calcium crystals upon thawing. In samples kept frozen at -20 °C overnight, Saetun et al. demonstrated that these precipitates might cause an associated decrease in urinary protein levels20. Another consideration supporting the recommendation not to freeze non-centrifuged urine is that potential lysis of cells in a non-centrifuged sample may further confound results.
Following centrifugation, the next step is to aliquot the samples. The appropriate number of aliquots of urine to be frozen depends on the individual study design. Aliquoting into several tubes allows for the minimization of freeze-thaw cycles at a later point. The volume of the aliquots depends on the intended downstream applications. Most ELISAs or western blots use a small amount of urine, and therefore a minimum of 0.5 mL is usually sufficient for each aliquot. However, much larger volumes of urine are needed for unbiased methodologies, such as mass spectrometry. We recommend identifying the minimum amount of urine needed for the planned experiments to ensure sufficient volumes.
In this protocol, specific details around the measurement of specific urinary biomarkers were left out as this is highly dependent on individual study design. However, the majority of these biomarkers can be measured using ELISA. The first general step of an ELISA includes coating a 96-well plate with an antibody. Many kits will come with this step already completed, but others require that researchers perform this step. Following coating the plate with antibodies, the next step is to add the standards, samples, and controls. Most kits will provide standards for use in the ELISA. It is important to follow the kit's instructions for standard preparation to ensure the creation of the correct standard curve. Use of negative control is recommended. The next step is to add the detection antibody, followed by the addition of the enzyme conjugate. The final step is to add substrate, often followed by a stop buffer. At this point, the ELISA is ready to be read by a 96-well plate reader. Follow the manufacturer's instructions about how to appropriately configure the plate reader for the ELISA in question. This is especially relevant when interpreting the final results. It is important to note the distribution of the expected standard curve from the manufacturer's instructions appropriately, as this will affect both the fit of the curve and the accuracy of the results. Note the R2 of the standard curve. This denotes how well the curve fits the standards. A low R2, below 0.90, suggested inaccurate pipetting, which calls into question the accuracy of the results of the ELISA. The standard curve can then be used to determine the biomarker concentration based on the absorbance generated from the plate reader.
Our protocol is similar to others within the literature that focuses on urinary proteins,21 with many published studies of urinary biomarkers using similar protocols6,23,24,25,26. Researchers have varied this protocol using a protease inhibitor26 or filtrating the urine specimen rather than centrifugation to adapt the protocol to settings without such equipment4,28. A major limitation to creating a single, standard protocol is that various biomarkers have differing properties, which may affect processing considerations. Additionally, downstream applications differ based on study design, which may also affect processing. We have highlighted steps in this protocol to signal researchers of places where consideration may be needed based on specifics of their research design. Finally, this protocol is written with a focus on urine protein biomarkers often investigated in UTI studies. Samples collected for unbiased methodologies, such as mass spectrometry, may require additional considerations28. Further, other novel biomarkers, such as RNA markers, will require a distinct protocol for optimal processing and storage. Protocols are elsewhere regarding sample considerations for urobiome studies29.
Divulgaciones
None of the authors have any conflicts of interest to disclose.
Agradecimientos
No external funding was obtained for this work. Institutional funds were used to obtain the data in this work.
Materiales
| Name | Company | Catalog Number | Comments |
| Boric acid | Sigma-Aldrich | B6768 | To be considered for samples that cannot be rapidly processed and frozen |
| Freezer boxes | Fisher Scientific | 03-395-464 | |
| Microcentrifuge tubes | Thomas scientific | 1149X93 | |
| NGAL ELISA Kit | R&D Systems | DLCN20 | Used to create representative results |
| Pipette and tips | Dependent on pipette size and volume of fluid. | ||
| Sodium azide | Sigma-Aldrich | S2002 | To be considered for samples that cannot be rapidly processed and frozen |
| Urine collection cups | Thermo Scientific | 3122B03ORG | Sterile cups not required unless needed for other studies |
Referencias
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. American Journal of Medicine. 113, 5-13 (2002).
- Forster, C. S., Pohl, H. Diagnosis of urinary tract infection in the neuropathic bladder: Changing the paradigm to include the microbiome. Topics in Spinal Cord Injury Rehabilitation. 25 (3), (2019).
- Gadalla, A. A. H., et al. Identification of clinical and urine biomarkers for uncomplicated urinary tract infection using machine learning algorithms. Scientific Reports. 9 (1), (2019).
- Shaikh, N., et al. Biomarkers that differentiate false positive urinalyses from true urinary tract infection. Pediatric Nephrology. 35 (2), 321-329 (2020).
- Renata, Y., Jassar, H., Katz, R., Hochberg, A., Nir, R. -. R., Klein-Kremer, A. Urinary concentration of cytokines in children with acute pyelonephritis. European journal of Pediatrics. 172 (6), 769-774 (2013).
- Forster, C. S., Haffey, W. D., Bennett, M., Greis, K. D., Devarajan, P. Identification of urinary CD44 and Prosaposin as specific biomarkers of urinary tract infections in children with neurogenic bladders. Biomarker Insights. 14, (2019).
- Bitsori, M., et al. Urine IL-8 concentrations in infectious and non-infectious urinary tract conditions. Pediatric Nephrology. 26 (11), 2003-2007 (2011).
- Schuh, M. P., et al. Long-term Stability of urinary biomarkers of acute kidney injury in children. American Journal of Kidney Diseases. 67 (1), 56-61 (2016).
- Hepburn, S., et al. An analysis of the impact of pre-analytical factors on the urine proteome: Sample processing time, temperature, and proteolysis. Proteomics - Clinical Applications. 9 (5-6), 507-521 (2015).
- Han, W. K., Wagener, G., Zhu, Y., Wang, S., Lee, H. T. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clinical Journal of the American Society of Nephrology. 4 (5), 873-882 (2009).
- Schaub, S., et al. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney International. 65 (1), 323-332 (2004).
- Thongboonkerd, V. Practical points in urinary proteomics. Journal of Proteome Research. 6 (10), 3881-3890 (2007).
- Grenier, F. C., et al. Evaluation of the ARCHITECT urine NGAL assay: Assay performance, specimen handling requirements and biological variability. Clinical Biochemistry. 43 (6), 615-620 (2010).
- Liu, K. D., et al. Storage time and urine biomarker levels in the ASSESS-AKI study. PLoS ONE. 11 (10), 1-9 (2016).
- Parikh, C. R., et al. Urine stability studies for novel biomarkers of acute kidney injury. American Journal of Kidney Diseases. 63 (4), 567-572 (2014).
- Van De Vrie, M., Deegens, J. K., Van Der Vlag, J., Hilbrands, L. B. Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL). American Journal of Kidney Diseases. 63 (4), 573-576 (2014).
- Hubel, A., Aksan, A., Skubitz, A. P. N., Wendt, C., Zhong, X. State of the art in preservation of fluid biospecimens. Biopreservation and Biobanking. 9 (3), 237-244 (2011).
- Havanapan, P. O., Thongboonkerd, V. Are protease inhibitors required for gel-based proteomics of kidney and urine. Journal of Proteome Research. 8 (6), 3109-3117 (2009).
- Thongboonkerd, V., Saetun, P. Bacterial overgrowth affects urinary proteome analysis: Recommendation for centrifugation, temperature, duration, and the use of preservatives during sample collection. Journal of Proteome Research. 6 (11), 4173-4181 (2007).
- Saetun, P., Semangoen, T., Thongboonkerd, V. Characterizations of urinary sediments precipitated after freezing and their effects on urinary protein and chemical analyses. American Journal of Physiology - Renal Physiology. 296 (6), 1346-1354 (2009).
- Project, H. K. . Standard Protocol for Urine Collection and Storage. , (2021).
- Nickolas, T. L., et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. Journal of the American College of Cardiology. 59 (3), 246-255 (2012).
- Forster, C. S., Loechtenfeldt, A. M., Shah, S. S., Goldstein, S. Urine neutrophil gelatinase-associated lipocalin in girls with recurrent urinary tract infections. Pediatric Nephrology. , 1-8 (2020).
- Forster, C. S., et al. Predictive ability of NGAL in identifying urinary tract infection in children with neurogenic bladders. Pediatric Nephrology. 33 (8), 1365-1374 (2018).
- Forster, C., et al. Urinary NGAL deficiency in children with recurrent urinary tract infections. Journal of Pediatric Urology. 32, 1077-1080 (2017).
- Gupta, S., Preece, J., Haynes, A., Becknell, B., Ching, C. Differentiating asymptomatic bacteriuria from urinary tract infection in the pediatric neurogenic bladder population: NGAL as a promising biomarker. Topics in Spinal Cord Injury Rehabilitation. 25 (3), 214-221 (2019).
- Shaikh, N., et al. Host and bacterial markers that differ in children with cystitis and pyelonephritis. Journal of Pediatrics. 209, 146-153 (2019).
- Harpole, M., Davis, J., Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert Review of Proteomics. 13 (6), 609-626 (2016).
- Jung, C. E., et al. Benchmarking urine storage and collection conditions for evaluating the female urinary microbiome. Scientific Reports. 9 (1), 13409 (2019).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados