Method Article
Transplantation of Bioengineered Lung Using Decellularized Mouse Lungs and Primary Human Endothelial Cells
In This Article
Summary
This paper describes how to create bioengineered mouse lungs using decellularization and recellularization methods. It also details subsequent orthotopic lung transplantation.
Abstract
Lung transplantation is a critical treatment for patients with end-stage lung diseases like idiopathic pulmonary fibrosis, but challenges such as donor shortages and posttransplant complications persist. Bioengineered lungs, integrating patient-specific cells into decellularized animal scaffolds, present a promising alternative. Despite progress in using bioengineered lungs in animal models, functionality and structure remain immature. This protocol addresses a critical barrier in organ bioengineering: the need for a cost-effective experimental platform. By using mouse models instead of larger animals like rats or swine, researchers can significantly reduce the resources required for each experiment, accelerating research progress.
The protocol outlines a detailed procedure for lung bioengineering using mouse heart-lung blocks and human primary cells, focusing on isolation strategy for the mouse heart-lung block, decellularization, bioreactor setup, perfusion-based organ culture, and orthotopic transplantation of bioengineered lungs. This mouse-scale platform not only reduces experimental costs but also provides a viable framework for optimizing cell types and numbers for recellularization, testing different cell types using histological and molecular methods, and ensuring blood flow post-transplantation. The method holds potential for broad applications, including studying cell interactions in three-dimensional culture conditions, cell-matrix interactions, and ex vivo cancer modeling, thereby advancing the field of organ bioengineering.
Introduction
Lung transplantation has been the decisive cure for patients having end-stage lung disease1 such as idiopathic pulmonary fibrosis, where drug treatment is ineffective to stop the deterioration of respiratory function. More eligible patients add up to the waiting list every year; however, the number of organ donations from deceased donors has been trailing the increasing number of waiting patients2,3. Even after undergoing lung transplantation, quite a few problems would degrade the function of transplanted lungs, including primary organ dysfunction, reactive allogenic syndrome, and infections, which significantly lower the 5-year survival of the lung transplantation recipients4.
Several options exist to counter the current problems in organ transplantation, including the utilization of marginal donors5, recovery of donor lungs in an ex vivo lung perfusion system6, and xenotransplantation using gene-edited swine7. These alternatives can expand the pool of donor organs; however, none can entirely address the donor organs' scarcity, immunogenicity, and functional heterogeneity.
It is far from reality but bioengineered artificial organs where patient-specific cells are integrated into the decellularized animal organ scaffold are a fascinating potential source of solid organ transplantation8. Several pioneering studies that demonstrated the potential utility of bioengineered lungs have been reported since 20109,10. In these studies, lungs from rats or swine were decellularized by detergents, animal or human cells were injected from the trachea or pulmonary vasculature to regenerate the lung tissue in the perfusion-based bioreactor, and some of them were transplanted orthotopically into the animal thoracic cavities11,12,13,14,15. However, the function and structure of the bioengineered lungs were premature, presumably because of the inadequate number of cells cultured in the bioreactor or less integrated intercellular junctions.
One obstacle to advancing research in organ bioengineering is the lack of a small-scale experimental platform. While rats or swine are the commonly used animals in this field, they require >108 lung cells per lung16, which is highly costly to academic labs. If mice are available for organ bioengineering research, we could dramatically reduce the cost of each experiment and speed up the research program. Although there exist anatomical differences between mouse and human lungs17, the basic architecture of the lung is similar across mammals18. Therefore, the results of mouse-scale experiments can apply to larger animals by simply multiplying the number according to the body size.
This protocol aims to describe the detailed experimental procedure of lung bioengineering using mouse heart-lung blocks and human primary cells19. We adopted previously reported and widely used mouse lung decellularization protocol for this study20,21,22. The challenging part of lung bioengineering is the recellularization of the decellularized capillary vasculature20; therefore, human umbilical cord vein endothelial cells will be used in this protocol.
Protocol
All experiments followed the Regulations for Animal Experiments and Related Activities at Tohoku University (15th edition), published by Tohoku University23. This study was approved by the Institutional Animal Care and Use Committee at Tohoku University (#2020AcA-041-01).
1. Preparation of materials for decellularization
- Preparation of decellularization solutions (1,000 mL format in a 1 L autoclavable glass bottle)
- Sterile deionized (DI) water: Add 1,000 mL of distilled or DI water to 1 L autoclavable glass bottles. Autoclave for 20 min at 121 °C.
- Triton X-100: Add 1 mL of Triton X-100 to 1,000 mL of sterile DI water in a 1 L format autoclavable glass bottle. (Optional) Add 10 mL of Penicillin and Streptomycin solution (final concentration, 500 units/mL of penicillin and 500 µg/mL of streptomycin).
NOTE: Do not autoclave. - Sodium deoxycholate: Add 20 g of sodium deoxycholate powder to 1,000 mL of sterile DI water in a 1 L format autoclavable glass bottle. Close the cap and flip the bottle to solubilize the powder. (Optional) Add 10 mL of penicillin and streptomycin solution.
NOTE: Do not autoclave. - 1 M NaCl: Add 58.44 g of NaCl to 1,000 mL of distilled or DI water in a 1 L format autoclavable glass bottle. Autoclave for 20 min at 121 °C.
- DNase I stock solution: Dilute at a 10 mg/mL concentration in Medium A.
- Medium A: Prepare 5 mM CaCl2 (5 mg of CaCl2 in 9 mL of sterile DI water) and 1:10 dilution with sterile DI water.
- DNase I working solution: Add 33 µL of DNase I stock solution to 10 mL of Medium B.
- Medium B: Prepare 10x Medium B (155 mg of MgSO4 + 220 mg of CaCl2 in 100 mL of sterile DI water) and 1:10 dilution with sterile DI water.
- Preparation of the catheters for the pulmonary artery (PA) and the trachea (sterile procedure)
- Cut the PA catheter (catheter for rat jugular vein) at a length of approximately 15 mm.
- Move the collar to the end of the catheter.
- Insert a catheter connector into the PA catheter and attach it to an injector. Prepared PA catheters are presented in Figure 1A.
- Store the PA catheter in 70% ethanol until use.
- Cut the 20 G i.v. catheter at a length of approximately 15 mm. This is used for a tracheal catheter.
- Mouse surgery for harvesting heart-lung block
- Euthanize a male mouse (C57BL/6, weight > 28 g) with an overdose of carbon dioxide or isoflurane.
- Place the mouse in a supine position on a surgical table and fix the limbs. Sterilize by spraying 70% ethanol on the surface of the chest and the abdomen.
- Open the abdominal cavity in the median line to the neck with stainless scissors and split the sternum with the scissors. Resect the diaphragm from the thoracic wall, cut the ventral chest wall to fully expose the thoracic cavities, and remove the thymus. Ligate the inferior vena cava and the right superior vena cava with a 4-0 silk to prevent the regurgitation of PBS in step 1.3.5, thereby enhancing the washout of the blood in the pulmonary vasculature.
- Cut the abdominal aorta with stainless scissors for drainage. If the abdominal aorta is indiscernible, cut the inferior vena cava and the abdominal aorta altogether.
- Inject 3 mL of sterile PBS from the right ventricle with a 5 mL sterile syringe with a 27 G needle by puncturing the right ventricle wall.
- Loop the main PA with a 4-0 silk using Dumont forceps.
NOTE: The main PA and the ascending aorta can be looped together. - Open a 2 mm window beneath the PA valves by cutting the right ventricle wall with spring scissors.
- Insert the PA catheter through the window and secure the previously looped 4-0 silk (Figure 1B).
NOTE: Avoid touching the PA during this procedure, which might damage the PA wall. The main PA and the ascending aorta can be ligated and secured together. - Inject 2 mL of PBS slowly through the PA catheter with a 5 mL sterile syringe. Make sure both lungs slightly expand as the PBS is being injected.
- Cannulate the trachea with a tracheal catheter and tie in place with 4-0 silk suture (Figure 1C).
- Inject 2 mL of air slowly through the tracheal catheter with an empty 5 mL sterile syringe and hold it for 10 s. Make sure there is no air leakage from the lungs.
- Remove the heart and lung en bloc. Grab the trachea with the Dumont forceps and with the tracheal catheter inside, cut the cervical esophagus, and dissect it from the vertebrae. Cut the bilateral subclavian veins and arteries. Finally, cut the esophagus and the infra vena cava at the level of the diaphragm.
NOTE: Do not touch the lung surface with any instruments. Any slight touch could result in air leakage.
- Decellularization of the mouse lungs (3 day procedure)
NOTE: All procedures in section 1.4 should be performed in a clean biosafety cabinet.- Day 1
- Transfer the resected heart-lung block to a 10 cm diameter plastic Petri dish and incubate the heart-lung blocks in sterile DI water for 1 h at 4 °C.
- Inject 2 mL of sterile DI water through the trachea catheter 3x and 2 mL of sterile water through the PA catheter with a 5 mL sterile syringe. Pause after each injection to allow the liquid to come out as the lung recoils (Figure 1C).
NOTE: Inject water at approximately 0.5 mL/s. - Inject 2 mL of 0.1% Triton X-100 solution into the tracheal catheter and 2 mL into the PA catheter.
- Place the heart-lung block in the Petri dish and statically incubate in Triton X-100 solution overnight at 4 °C.
- Day 2
- Remove the Triton X-100 solution from the lungs with sterile DI water as described in step 1.4.1.2.
- Inject 2 mL of 2% sodium deoxycholate solution into the tracheal catheter and 2 mL into the PA catheter. Incubate in deoxycholate solution for 24 h at 4 °C.
- Day 3
- Remove the sodium deoxycholate solution from the lungs with sterile DI water as described in step 1.4.1.2.
- Inject 2 mL of 1 M NaCl solution into the tracheal catheter and 2 mL into the PA catheter. Incubate in NaCl solution for 1 h at RT.
- Remove from NaCl solution with sterile DI water as described in step 1.4.1.2.
- Inject 2 mL of DNAse I working solution into the tracheal catheter and 2 mL into the PA catheter. Incubate in DNase working solution for 1 h at RT.
- Remove the DNase solution from the lungs as described in step 1.4.1.2 but with sterile PBS. Confirm that the lungs are white and transparent on the edge after the decellularization procedure.
NOTE: As the decellularization procedure progresses, the lung becomes more fragile. Always handle the heart-lung block with caution and avoid touching the lung surface. After decellularization, the heart-lung blocks can be stored in PBS/antibiotics at 4 °C for up to 3 weeks.
- Day 1
2. Culture of human primary cells
- Mix the Endothelial Cell Growth Medium-2 (EGM2) and Bullet Kit containing fetal bovine serum (final concentration, 2%), hydrocortisone (final concentration, 0.2 µg/mL), human basic fibroblast growth factor (final concentration, 4 ng/mL), vascular endothelial growth factor (2 ng/mL), R3-insulin-like growth factor-1 (final concentration, 5 ng/mL), ascorbic acid (final concentration, 75 µg/mL), human epidermal growth factor (final concentration, 10 ng/mL), gentamicin/amphotericin-1000 (final concentration, gentamicin: 30 µg/mL, amphotericin: 15 ng/mL), and heparin (final concentration, 1 ng/mL).
- Thaw 2 × 106 human umbilical cord vein endothelial cells (HUVECs) in frozen vials in a water bath at 37 °C.
- Mix the cells with EGM2 in a 15 mL conical tube and centrifuge at 500 × g for 5 min.
- Count the cells and subculture them at an appropriate cell density (2.0 x 104 cells/cm2 is recommended). Start from 6-well format plates and then transfer to T75 flasks.
- Passage the cells until the required number of cells is obtained.
NOTE: For complete pulmonary vascular coverage using HUVECs, 3 × 107 HUVECs will be required19.
3. Bioreactor setup and perfusion organ culture
- Preparation of an organ chamber and a cell reservoir
- Cut holes in a silicon stopper using a cork borer, as shown in Figure 2A. The hole sizes are 5 mm (i, ii, and iii), and 7 mm (iv and v). Each hole number (i-v) in Figure 2A corresponds to the hole numbers in Figure 2B.
- Insert the pump tubing through the silicon stopper as indicated in Figure 2B.
- Cut a 5 mm hole in a silicon septum of an open-top screw cap using a cork borer. Insert an L/S 14 platinum-cured tubing in the hole (Figure 2B,C).
- Autoclave the materials above, including the silicon stopper with tubing, the glass canister, the GL-45 screw cap with tubing, a 250 mL autoclavable glass bottle, and an L/S 14 tubing with lure fittings (Tubing B and Tubing C in Figure 3A) for 20 min at 121 °C. Select proper lure fittings to ensure tubing B and C make a loop.
NOTE: The glass canister is used for an organ chamber, and the 250 mL glass bottle is used for a cell reservoir.
- Assembly of perfusion-based bioreactor circuit
NOTE: The following procedures should be performed on a clean bench.- Add 70 mL of culture media to the glass canister and then, put the silicon stopper on top of the glass canister.
- Assemble a silicon stopper, a glass canister, a GL-45 screw cap with tubing, a 250 mL autoclavable glass bottle, and an L/S 14 tubing with lure fittings using three-way stopcocks as described in Figure 3A. Insert a 20 G needle in a silicon septum of a GL-45 screw cap.
- Fill the media in Tubing A, B, and C with a 10 mL syringe connected to stopcocks i) and iii) (~3-5 mL of media will be needed to fill 1 m of tubing). Repeat the media injection and withdrawal using a 10 mL syringe through a three-way stopcock to ensure that there is no air bubble inside the tubing.
- Attach the PA catheter of the decellularized heart-lung block via a lure fitting connected to Tubing C. Avoid air bubbles in the catheter or the tubing.
- Harvest and resuspend HUVECs at a 0.5-1 × 106 cells/mL density in EGM2. Add the cell suspension from step 2.5 to the cell reservoir.
NOTE: The cell suspension is preferably prepared between steps 3.2.4 and 3.2.5. Put a stirring bar in the cell reservoir.
- Gravity-driven injection of endothelial cells
- Place the cell reservoir containing HUVECs on a magnetic stirrer. Ensure that the bottom of the cell reservoir is 30 cm above the organ chamber (Figure 2D and Figure 3A).
- Turn on the magnetic stirrer at a speed of approximately 120 rpm.
- Open the stopcock i) and ii) so that the cell suspension can be injected into the decellularized scaffold via Tubing A, Tubing C, and the PA catheter.
NOTE: When injecting 3 × 107 cells at a cell density of 1 × 106 cells/mL, the volume of the cell suspension should be 30 mL. A typical injection rate is 1-2 mL/min. - After fully injecting the cell suspension, detach Tubing A from Stopcock ii).
NOTE: Cell count can be performed at this step to measure the cell retention rate in the decellularized scaffold. The typical cell retention rate is 80-90%, regardless of the number of cells injected19.
The quality of the decellularized lung scaffold strictly determines the cell retention rate. Any leakage from the decellularized lung (e.g., from the main PA, lung surface) results in a lower cell retention rate. Make sure that there is no apparent leakage from the decellularized lung by injecting 2-3 mL of culture media with a sterile syringe connected to one of the stopcocks in the perfusion-based bioreactor and confirm that the decellularized lungs slightly expand as the media is injected.
- Perfusion organ culture
- Place the organ chamber in a CO2 incubator.
- Fix Tubing B to a pump head connected to a pulsatile pump.
- Close the CO2 incubator's glass door. Make sure that the tubing is properly placed between the glass door and the rubber seal (Figure 3B).
- Incubate the decellularized scaffold at 37 °C for 3 h to let the injected endothelial cells settle in the scaffold.
NOTE: Make sure that the pulsatile pump is always off between steps 3.4.1 and 3.4.4. - Start the pump at a rate of 6 rpm, which results in 2 mL/min media perfusion using an L/S 14 tubing. Watch the decellularized lung being slightly expanded with the media perfusion.
- Close the incubator door (Figure 3C).
- Perform the media change through Stopcock iii). Change half of the media every 2 or 3 days.
- Stop the pump drive, attach a 50 mL sterile syringe to the stopcock iii), and withdraw 50 mL of media from the chamber.
- Fill another 50 mL sterile syringe with 50 mL of prewarmed media and transfer the media into the chamber via stopcock iii). Switch the stopcock appropriately and then start the pulsatile pump.
- Harvest the recellularized heart-lung block after at least 2 days of perfusion organ culture.
NOTE: In our study, 2 days of perfusion culture was enough to homogeneously revascularize the decellularized lung scaffold using 3 × 107 HUVECs. When using less than 3 × 107 HUVECs, longer incubation could be required to improve the efficiency of recellularization.
4. Orthotopic transplantation of the bioengineered lung
- Drug preparation
- Prepare MMB combination anesthetic agent by mixing midazolam (final concentration, 4 mg/kg), medetomidine (final concentration, 0.75 mg/kg), and butorphanol tartrate (final concentration, 5 mg/kg) with clinical grade normal saline.
- Prepare heparin solution by mixing it with clinical grade normal saline (final concentration, 1000 U/mL).
- Prepare cefazolin sodium by mixing it with clinical grade normal saline (one dose, 30 mg/kg).
- Surgical procedure
- Preparation of cuffs
- Lightly rub the three types of angiocatheter with fine sandpaper to facilitate the vessels to remain cuffed.
- Prepare a bronchial cuff from a 20 G Teflon angiocatheter 1 mm in length using a #11 scalpel. Before cutting the angiocatheter, use the back of the #11 scalpel to make an indentation in the angiocatheter to tie off the 10-0 nylon.
- Prepare the pulmonary vein (PV) cuff from a 22 G Teflon angiocatheter 0.8 mm in length and the PA cuff from a 24 G Teflon angiocatheter 0.6 mm in length.
NOTE: This preparation can be done before the day of the experiment.
- Attachment of cuff to the bioengineered lung
- Place the heart-lung block on a piece of sterile gauze moistened with cold saline, place another piece of dry, clean gauze under it, and put a Petri dish in a Styrofoam box filled with clean ice.
NOTE: Placing the dry gauze prevents the heart-lung block from accidentally freezing. - Place an aneurysm clip to secure the trachea and the gauze (Figure 4A). Place a small piece of gauze on the heart, covering the right lung also. Place another gauze on the left lung. Adjust this retraction with the moistened gauze to expose the left hilum as clearly as possible.
- Carefully dissect the hilar structures from each other using straight or angled micro forceps, depending on preference (Figure 4B). Start dissecting from the pulmonary ligament along the vagus nerve. Incise the visceral pleura under the PV, and move the descending aorta and vagus nerve together through the back of the left pulmonary hilum to the cranial side.
- Dissect the left main PA from the pulmonary trunk to the very edge of the left lung (Figure 4C). Then, divide the PA at the level of the pulmonary trunk to obtain adequate length.
- Dissect the left PV from the left side of the left atrium to the very edge of the left lung (Figure 4D). Divide the left PV at the level of the left atrium to obtain adequate length.
- Suspend the PA cuff just above the PA using the stabilization clamp and insert the PA inside the cuff (Figure 4E). Fold the PA over the cuff), exposing the endothelial surface. Secure around the cuff with a 10-0 nylon tie (Figure 4F). Place the cuff on the PV in an identical fashion (Figure 4G,H).
- Divide the left bronchus at the level of the carina. Place the cuff on the bronchus in an identical fashion (Figure 4I).
- Place the heart-lung block on a piece of sterile gauze moistened with cold saline, place another piece of dry, clean gauze under it, and put a Petri dish in a Styrofoam box filled with clean ice.
- Procedure for the recipient mouse
- Anesthetize the recipient mouse with a mixture of MMB i.p. with a 27 G needle and intubate by inserting a 20 G polyurethane angiocatheter under a microscope. Place the recipient mouse on a temperature-adjustable electric warmer in the right lateral decubitus position and connect the angiochatheter to the respirator. Set the ventilator setting as follows: oxygen 2 L/min, respiratory rate 120 bpm, tidal volume 0.5 mL. Sterilize the chest wall with 70% ethanol and inject the cephazolin mixture s.c.
- Incise the skin with scissors. Cut the subcutaneous tissue and muscles with a cautery. Open the chest through the 3rd intercostal space and place the chest retractors.
NOTE: Although the thoracotomy needs to be large enough for implantation, do not injure the internal mammary artery, which can lead to massive bleeding. - Dissect the pulmonary ligament with a cotton swab and large spring scissors. Place a curved arterial cleanser on the recipient's left lung to retract the lung easily. With an angled micro-forceps, dissect the mediastinal pleura around the left pulmonary hilum.
NOTE: The basis of dissecting the pleura is similar to the anatomical lung resection of a human being. - Dissect the PA from the bronchus using curved micro forceps from the mediastinum to the very edge of the left lung (Figure 5A). Dissect the bronchus from PV in a similar manner.
NOTE: Dissection can be performed easily when the pleura is dissected at the previous maneuver (step 4.2.3.3). - Place a slipknot of 10-0 silk at the base of the PA to occlude (Figure 5B). Place a slim angled aneurysmal clip at the base of the PV and bronchus (Figure 5C).
- Loop 10-0 nylon around the bronchus, PA, and PV, leaving loose to secure cuffs at the following steps.
- Incise the recipient's PA, bronchus, and PV at the edge of the recipient's left lung using microspring scissors (Figure 5D). Gently dilate the PA and PV using straight micro forceps. Remove the blood in the PA and PV with saline using a 1 mL syringe and a 24 G angiocathater.
NOTE: The Incisions of PA, bronchus, and PV are about one-third of the way around. The straight micro forceps are gentle and suitable for dilation. - Place the bioengineered lung, which is covered with moistened gauze, on top of the recipient's left lung (Figure 5E), as close to the recipient's mediastinum as possible.
- Inserting the donor PA cuff into the recipient PA (Figure 5F).
NOTE: There will be some stretch on the donor PA. If the PA incision size is appropriate, the donor's cuff is less likely to be dislodged. Moving the bioengineered lung close to the mediastinum will also prevent the donor's cuff from being dislodged. - Secure around the cuff with a 10-0 nylon tie (Figure 5G). In a similar manner, insert and secure the donor bronchus (Figure 5H) and PV cuffs (Figure 5I,J).
- Remove the slim-angled aneurysmal clip (Figure 5L). Observe that blood is backflowing beyond the PV cuff and remove the silk tie on PA to resume antegrade blood flow to the bioengineered lung.
- Preparation of cuffs
Results
Following the decellularization protocol, mouse lungs are visibly white and translucent (Figure 6A). Cellular components should be entirely removed, but the alveolar structure remains intact in the histological observation (Figure 6B,C). Recellularized mouse lungs using 3 × 107 HUVECs with 2 day perfusion-based bioreactor culture show a homogeneous distribution of HUVECs (Figure 7A). HUVECs migrate into the peripheral alveolar area, forming a capillary network (Figure 7B). After the orthotopic transplantation and reperfusion of bioengineering lungs, blood flow containing red blood cells is homogeneously observed in the bioengineered lungs (Figure 8A,B).

Figure 1: Cannulation of the mouse heart-lung block. (A) Prepared pulmonary artery catheters. (B) Schema of cannulation. (C) Representative image after the completion of cannulation. Scale bars = 1 cm (A,C). This figure was adopted from Tomiyama et al.19. Please click here to view a larger version of this figure.

Figure 2: Preparation of the organ chamber. (A) Holes are cut as described. (B) Tubing is inserted as indicated. (C) Preparation of the cap for an autoclavable glass 250 mL glass bottle for the cell reservoir. (D) The 250 mL glass bottle placed on the magnetic stirrer. This figure was adopted from Tomiyama et al.19. Please click here to view a larger version of this figure.

Figure 3: Perfusion-based bioreactor setup. (A) Parts and assembly. (B) Actual setup. Note that tubing is inserted between a glass door and a rubber seal. (C) A snapshot during the pump-driven perfusion organ culture. This figure was adopted from Tomiyama et al.19. Please click here to view a larger version of this figure.

Figure 4: Preparation of the bioengineered lung for transplantation. (A) Placement of the engineered lung. (B) Dissection of the hilum. (C) Dissection of the main PA. (D) Dissection of the PV. (E) Insertion of the PA into the cuff. (F) Fixation of the PV to the cuff. (G) Insertion of the PV into the cuff. (H) Fixation of the PV to the cuff. (I) Insertion and fixation of the left bronchus to the cuff. Abbreviations: PA = pulmonary artery; PV = pulmonary vein. Please click here to view a larger version of this figure.
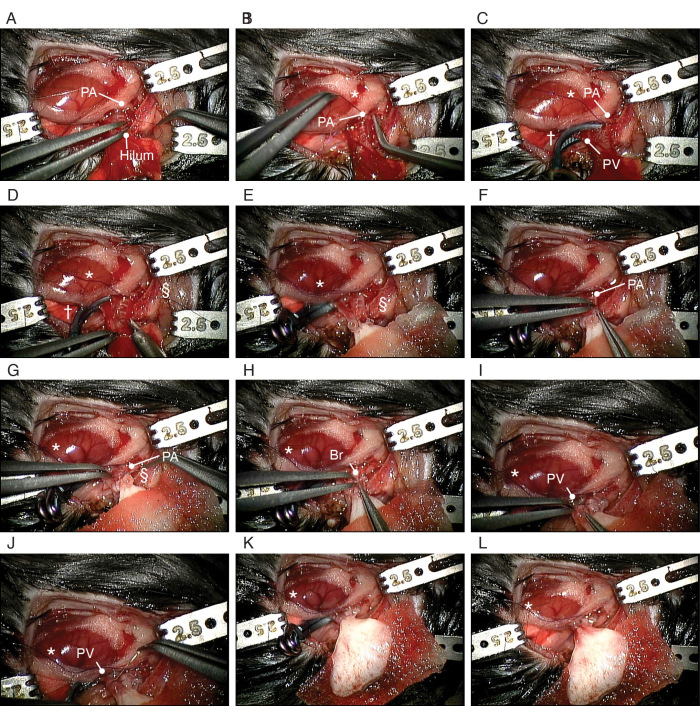
Figure 5: Procedure of orthotopic transplantation of the bioengineered lung. (A) Dissection of the recipient's PA. (B) Making a slipknot around the recipient's PA. (C) Clipping of the PV and bronchus. (D) Incising the PA. (E) Placement of the bioengineered left lung. (F) Insertion of the cuffed PA of the bioengineered lung into the recipient's PA. (G) Securing the PA around the cuff. (H) Insertion of the bronchial cuff of the bioengineered lung into the recipient's bronchus. (I) Insertion of the PV of the bioengineered lung into the recipient's PV. (J) Securing the PA around the cuff. Abbreviations: PA = pulmonary artery; PV = pulmonary vein; Br = bronchus; * = slipknot; † = aneurysmal clip; § = 10-0 nylon tie. Please click here to view a larger version of this figure.

Figure 6: Decellularization of the mouse lung. (A) Macroscopic image of the decellularized lung. (B) Low-power image of the decellularized lung (magnification, 100x). (C) High-power hematoxylin- and eosin-stained image of the decellularized lung. Note that there is no visible cellular component (magnification, 400x). Scale bars = 100 µm (B), 50 µm (C). Please click here to view a larger version of this figure.
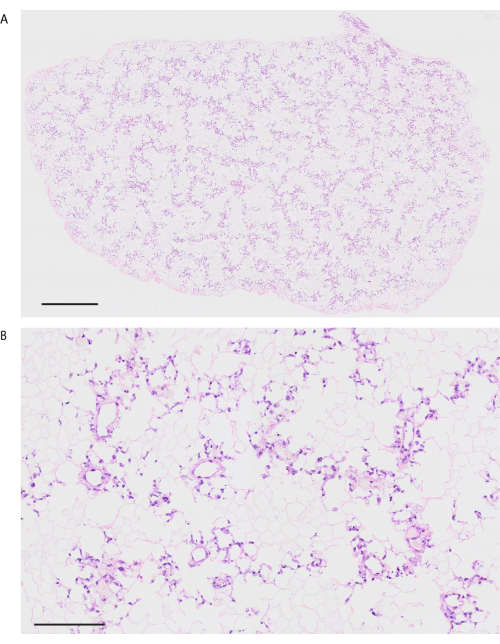
Figure 7: Revascularized mouse lung using HUVECs. (A) Low-power hematoxylin- and eosin-stained image of the revascularized lung (magnification, 200x, tiling). (B) High-power H&E-stained image of the revascularized lung (magnification, 200x). Scale bars = 1000 µm (A), 100 µm (B). Abbreviations: HUVECs = human umbilical cord vein endothelial cells; H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.
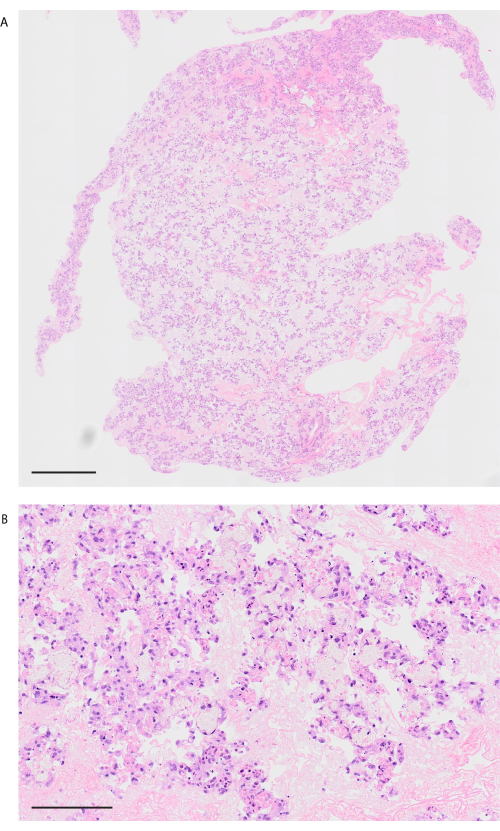
Figure 8: Lung image after transplantation and blood reperfusion. (A) Low-power H&E image of the revascularized lung after 10 min of reperfusion (magnification, 200x, tiling). (B) High-power H&E image of the revascularized lung after 10 min of reperfusion (magnification, 200x). Scale bars = 1000 µm (A), 100 µm (B). Abbreviation: H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.
Discussion
Organ bioengineering is a demanding enterprise. The costly screening process has been hindering this field's research and development cycle. By using mice as an experimental platform, space, cells, and media are significantly reduced compared to the previously used rat platform. Although measuring detailed physical parameters such as gas exchange, vascular resistance, or lung compliance has not been achieved yet, the mouse lung model allows for accelerated research timelines as it enables rapid iteration of experimental protocols and testing of cell viability, integration, and scaffold interaction. Mice reproduce quickly and are available in numerous genetically modified strains, offering flexibility in studying various genetic and cellular modifications in vivo. This ability to rapidly test hypotheses and optimize protocols helps advance our understanding of the optimal cellular environments and culture techniques required for successful lung bioengineering. By refining techniques in mouse models, researchers can establish scalable methods and protocols that can later be translated to larger animal models and, eventually, human applications.
The critical step of the procedure is inserting and fixation a PA catheter. The fixation of the PA catheter is only possible by utilizing a small diameter catheter (<3 Fr) with a collar at the tip. Because of the fragile nature of the lungs, surgery should be performed entirely with caution. No metal instruments should touch the lung surface; otherwise, the lung would suffer significant leakage. Use a cotton swab to maneuver the lungs when necessary. The authors assume that researchers might efficiently perform the canulation procedure after training using 10-15 mice.
The decellularization protocol described here is based on previous reports22,24. Other protocols using different detergent sets can be applicable. The heart-lung block should always be treated with caution. Typical incidents during the decellularization procedure include the penetration of the PA catheter, come-off of the tracheal catheter, and air leakage. The authors have not experimentally confirmed the integrity of the decellularized scaffold after refrigerated storage in PBS. Still, the authors have not experienced problems using decellularized heart-lung blocks stored in PBS for up to 4 weeks.
Avoiding bacterial contamination is crucial. All glass equipment, PVDF, and silicon parts must be autoclaved before the experiment. The other parts should be used only once. To minimize the risk of bacterial contamination, all procedures should be performed in a clean biosafety cabinet. It is desirable to include antimycotics as well as antibiotics in the media. Frequent media changes during the perfusion increase the risk of contamination. In addition, air bubbles must always be avoided in the tubing. Air bubbles in the tubing are subsequently trapped in the decellularized scaffold, which could blockade media perfusion in the peripheral area and result in heterogeneous cell distribution. Moreover, endothelial cells should be thoroughly detached by trypsinization or other appropriate cell dissociation media. Cell pellets should be disrupted well to make a homogeneous single-cell suspension. Too much cell density (e.g., >2 million cells/mL) could promote the formation of cell clumps, which could result in embolism in the proximal vasculature.
We have tested only a short period (2 or 3 days) of perfusion bioreactor culture. In our previous study, we used varying numbers of endothelial cells to revascularize the decellularized mouse lung scaffold and found that there is a threshold where adding more cells does not improve the cell coverage, which was ~3 × 107 endothelial cells per mouse lung-heart block19. We expect that a longer culture duration (e.g., 14 days) will improve the coverage and maturation of recellularized vasculature, as described in the previous lung bioengineering studies9,10,15,25.
The basis of the bioengineered lung graft preparation is similar to that of a regular mouse lung transplantation26,27. The engineered lung tissue is not as fragile as a regular lung graft. The challenge is that the lung tissue, including the hilum structure, is entirely white or almost transparent. A precise understanding of local anatomy is indispensable for successful transplantation. The stable technique should be earned using native lungs. The authors assume that researchers might comfortably perform the transplantation procedure after training using 100 mice.
Transplantation of the bioengineered lung using human-derived cells in the mouse is technically available; however, acute rejection of the graft is inevitable due to the xenotransplantation nature of this model. This model can be used for testing the short-term recellularization efficiency of the bioengineered lungs, and the long-term functionality should be investigated using immunodeficient mice.
Although it was not tested in the current method, whole lung bioengineering using both endothelial and epithelial cells should be technically not that challenging, considering the difficulties in pulmonary vascular engineering described here. Furthermore, this mouse-scale platform can be expanded to other fields of research, such as the investigation of cellular interaction in 3D culture conditions, cell-matrix interaction, ex-vivo cancer modeling, and so on. In summary, this method provides a reasonable and robust lung bioengineering platform.
Disclosures
The authors do not have any conflicts of interest regarding this manuscript.
Acknowledgements
This study was financially supported by the Grant-in-Aid for Scientific Research / KAKENHI (C) #20K09174, #23K08308, the Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)) #22KK0132 for TS, JSPS KAKENHI Grant Number 21K08877 for TW, Leave a Nest Grant Ikeda-Rika award for FT, and the Grant-in-Aid for JSPS Fellows #21J21515 for FT. We greatly appreciate Ms. Maiko Ueda, technical staff in the Biomedical Research Core of Tohoku University Graduate School of Medicine, for her intensive work in histological observation. We also appreciate the technical advice of Ms. Yumi Yoshida and Mr. Koji Kaji in the Center of Research Instruments at IDAC, Tohoku University, for their image processing support.
Materials
| Name | Company | Catalog Number | Comments |
| DECELLULARIZATION | |||
| 27 G x 1/2 in. BD PrecisionGlide Needle | BD | 305109 | Or equivalent 27 G injection needle |
| BD Insyte IV Catheter 20 GA X 1.8 8IN | BD | 381237 | Or equivalent 20 G IV catheter |
| Blade silk suture (4-0) | Nesco | GA04SB | Or equivalent |
| CaCl2 | Sigma-Aldrich | C5670 | |
| Catheter for rat jugular vein, PU 2Fr 10 cm | Instech | C20PU-MJV1301 | Recommended for mice weighs 30 g and under. |
| Catheter for rat jugular vein, PU 3Fr 10 cm | Instech | C30PU-RJV1307 | Recommended for mice weighs over 30 g. |
| DNase I | Sigma-Aldrich | DN25 | |
| MgSO4 | Sigma-Aldrich | M7506 | |
| NaCl | Sigma-Aldrich | S3014 | |
| PinPort injectors | Instech | PNP3M | |
| PinPorts, 22 G | Instech | PNP3F22-50 | Fits C30PU-RJV1307 |
| PinPorts, 25 G | Instech | PNP3F25-50 | Fits C20PU-MJV1301 |
| Sodium deoxycholate | Sigma-Aldrich | D6750 | |
| Sterile syringe, 5 mL | Generic | ||
| Triton X-100 | Sigma-Aldrich | 9036-19-5 | |
| CELL CULTURE | |||
| EGM-2 Endothelial Cell Growth Medium-2 BulletKit | Lonza | CC-3162 | |
| HUVEC – Human Umbilical Vein Endothelial Cells | Lonza | C2519A | |
| PERFUSION-BASED BIOREACTOR | |||
| 20 G needle | Generic | ||
| 3-way stopcock | Generic | ||
| Cork borer | Generic | Boring size, 6-10 mm | |
| EasyLoad III pump head | Cole-Parmer | 243934 | |
| Glass canister | Hario | SCN-200T | Inner diameter: 80 mm |
| Heating magnetic stirrer | Generic | ||
| Lure fitting, PVDF, For Soft Tube | Nordson Medical | 2-9965-01 | Female, fits tubing with I.D. 1.5 mm (L/S 14) |
| Lure fitting, PVDF, For Soft Tube | Nordson Medical | 2-9964-01 | Male, fits tubing with I.D. 1.5 mm (L/S 14) |
| Lure fitting, PVDF, For Soft Tube | Nordson Medical | 2-9965-03 | Female, fits tubing with I.D. 3 mm (L/S 16) |
| Lure fitting, PVDF, For Soft Tube | Nordson Medical | 2-9964-03 | Male, fits tubing with I.D. 3 mm (L/S 16) |
| Magnetic stirring bar | Generic | ||
| Masterflex L/S Digital Precision Modular Drive with Remote I/O and Benchtop Controller | Cole-Parmer | 07557-00 | |
| Masterflex L/S Precision Pump Tubing, PharMed BPT, L/S 16 | Cole-Parmer | 06508-16 | |
| Masterflex L/S Pricision Pump Tubing, Platinum-Cured Silicone, L/S 14 | Cole-Parmer | 96410-14 | |
| Millex-GP Syringe Filter Unit, 0.22 µm, polyethersulfone, 33 mm, gamma sterilized | Millipore | SLGPR33RS | |
| Pyrex 250 mL grass bottle, GL-45 screw cap | Corning | 1395-250 | |
| Silicon Septa for GL45 Open Top PBT Screw Cap | Corning | 1395-455S | |
| Silicone Light Stopper | IMG | 07763-18 | Upper diameter: 87 mm, Lower diameter: 75 mm |
| Sterile syringe, 10 mL, 50 mL | Generic | ||
| MOUSE SURGERY (Isolation of the heart-lung block | Lung transplantation) | |||
| 10-0 Nylon ties | Kono Seisakusho | N/A | |
| 10-0 Silk ties | Kono Seisakusho | N/A | |
| 4-0 Silk ties | Kono Seisakusho | N/A | |
| Arterial clamp, 45 mm curved, grooved | Natsume seisakusyo | C-17-45 | |
| BD Insyte IV Catheter 24GA | BD | 381512 | Or equivalent 24G i.v. catheter |
| Bulldog Vascular Forceps 45mm curved | Natsume seisakusyo | M2 | |
| Butorphanol tartrate | Meiji Seika Pharma | N/A | |
| Cefazolin Sodium | Otsuka Pharmaceutical | N/A | |
| Dumont forceps #5/45 | Fine Science Tools | 1251-35 | |
| Fine vannas style spring scissors | Fine Science Tools | 15403-08 | 45° tip, 0.01 x 0.06 mm |
| Gemini Cautery Kit | Harvard Apparatus | RS-300 | |
| Halsted-Mosquito clamp curved tip, 125 mm | Bioresearch center | 16181670 | |
| Hegar needle holder, 150 mm | B Braun/Aesculap | BM065R | |
| Heparine solution | Mochida Seiyaku | N/A | |
| Medetomidine | Nippon Zenyaku Kogyo | N/A | |
| Micro forceps straight | B Braun/Aesculap | BD33R | |
| Midazolam | Sandoz | N/A | |
| Mouse Ventilator | Harvard Apparatus | Model 687™ | |
| Normal Saline, Clinical grade | Otsuka Pharmaceutical | N/A | |
| Petri dish, 60 x 15 mm | BD | 351007 | |
| Safelet Cath PU 20 gauge polyurethan catheter | Nipro | 09-031 | |
| Sakaki stainless scissors curved 14 cm | Bioresearch center | 64152034 | |
| Scalpel holder | Bioresearch center | 16101040 | |
| Small animal retraction system | Fine Science Tools | 18200-20 | |
| Spare blade scalpel #11 | Muranaka Medical Instruments | 567-001-03 | |
| Spring scissors, 15 cm | Bioresearch center | PRI13-3736 | |
| Stereomicroscope | Leica Microsystems | M525 | Clinical-grade surgical microscope with a flexible arm system is preferable. |
| Sugita titanium aneurysm clip curved slim, No.98 | Mizuho medical | 17-001-98 | |
| Sugita titanium clip applier, 110 mm | Mizuho medical | 17-013-53 | |
| Temperature-adjustable electric warmer | Generic | ||
| Ultrafine cotton swab | Generic | ||
| VASCULAR AND BRONCHIAL CUFF | |||
| Fine sandpaper | Generic | ||
| Venula 20 gauge Teflon angiocatheter | Top | 1160 | |
| Venula 22 gauge Teflon angiocatheter | Top | 1161 | |
| Venula 24 gauge Teflon angiocatheter | Top | 1124 |
References
- van der Mark, S. C., Hoek, R. A. S., Hellemons, M. E. Developments in lung transplantation over the past decade. Eur Respir Rev. 29 (157), 190132 (2020).
- Valapour, M., et al. OPTN/SRTR 2022 Annual Data Report: Lung. Am J Transplant. 24 (2S1), S394-S456 (2024).
- Hoffman, T. W. Waiting list dynamics and lung transplantation outcomes after introduction of the lung allocation score in the Netherlands. Transplant Direct. 7 (10), e760 (2021).
- Wilk, A. R., Edwards, L. B., Edwards, E. B. The effect of augmenting OPTN data with external death data on calculating patient survival rates after organ transplantation. Transplantation. 101 (4), 836-843 (2017).
- Neizer, H., Singh, G. B., Gupta, S., Singh, S. K. Addressing donor-organ shortages using extended criteria in lung transplantation. Ann Cardiothorac Surg. 9 (1), 49-50 (2020).
- Oliveira, P., Yamanashi, K., Wang, A., Cypel, M. Establishment of an ex vivo lung perfusion rat model for translational insights in lung transplantation. J Vis Exp. (199), e65981 (2023).
- Anand, R. P. Design and testing of a humanized porcine donor for xenotransplantation. Nature. 622 (7982), 393-401 (2023).
- Shakir, S., Hackett, T. L., Mostaco-Guidolin, L. B. Bioengineering lungs: An overview of current methods, requirements, and challenges for constructing scaffolds. Front Bioeng Biotechnol. 10, 1011800 (2022).
- Ott, H. C. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 16 (8), 927-933 (2010).
- Petersen, T. H. Tissue-engineered lungs for in vivo implantation. Science. 329 (5991), 538-541 (2010).
- Leiby, K. L. Rational engineering of lung alveolar epithelium. NPJ Regen Med. 8 (1), 22 (2023).
- Kitano, K., et al. Orthotopic transplantation of human bioartificial lung grafts in a porcine model: A feasibility study. Semin Thorac Cardiovasc Surg. 34 (2), 752-759 (2022).
- Ohata, K., Ott, H. C. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg Today. 50 (7), 633-643 (2020).
- Ren, X. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotech. 33 (10), 1097-1102 (2015).
- Doi, R. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep. 7 (1), 8447 (2017).
- Stone, K. C., Mercer, R. R., Gehr, P., Stockstill, B., Crapo, J. D. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 6 (2), 235-243 (1992).
- Basil, M. C., Morrisey, E. E. Lung regeneration: a tale of mice and men. Semin Cell Dev Biol. 100, 88-100 (2020).
- Hsia, C. C., Hyde, D. M., Weibel, E. R. Lung structure and the intrinsic challenges of gas exchange. Compr Physiol. 6 (2), 827-895 (2016).
- Tomiyama, F., et al. Orthotopic transplantation of the bioengineered lung using a mouse-scale perfusion-based bioreactor and human primary endothelial cells. Sci Rep. 14 (1), 7040 (2024).
- Stoian, A., Adil, A., Biniazan, F., Haykal, S. Two decades of advances and limitations in organ recellularization. Curr Issues Mol Biol. 46 (8), 9179-9214 (2024).
- Crabbe, A. Recellularization of decellularized lung scaffolds is enhanced by dynamic suspension culture. PLoS One. 10 (5), e0126846 (2015).
- Daly, A. B. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 18 (1-2), 1-16 (2012).
- . Regulations for Animal Experiments and Related Activities Available from: https://www.clag.med.tohoku.ac.jp/clar/en/ (2024)
- Sokocevic, D., et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 34 (13), 3256-3269 (2013).
- Ren, X., et al. Ex vivo non-invasive assessment of cell viability and proliferation in bio-engineered whole organ constructs. Biomaterials. 52, 103-112 (2015).
- Watanabe, T. Mesenchymal stem cells attenuate ischemia-reperfusion injury after prolonged cold ischemia in a mouse model of lung transplantation: a preliminary study. Surg Today. 47 (4), 425-431 (2017).
- Watanabe, T., et al. Donor IL-17 receptor A regulates LPS-potentiated acute and chronic murine lung allograft rejection. JCI Insight. 8 (21), e158002 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved