Method Article
从尸检人脑软脑膜捐助外植体培养的推导
摘要
The leptomeninges explant culture protocol from human postmortem brain is a technically robust and simple way to derive fibronectin-positive meningeal fibroblasts within 6-8 weeks and cryopreserve approximately 20-30 million cells.
摘要
即使很大的进展已在帕金森氏病的临床表征而作出的,一些研究报告,帕金森氏病的诊断没有病理证实在高达临床诊断帕金森氏病的25%。因此,从组织临床确诊患者原发性帕金森病可有误诊率高收取;因此在从这些组织体外研究来研究帕金森氏病作为临床前模型中可以成为徒劳。
通过收集死后人软脑膜与帕金森氏病的一个证实神经病理学诊断和特征在于黑质纹状体细胞损失和细胞内蛋白质的夹杂物称为路易体,人们可以肯定,临床观察帕金森未被另一个潜在的疾病进程( 例如肿瘤,动脉硬化)引起的。
该协议presenTS解剖,并准备为脑膜成纤维细胞培养的推导死后人类软脑膜的。此过程是健壮和具有高的成功率。培养物的挑战是无菌的脑采购一般不无菌条件下进行。因此,要在培养介质用青霉素,链霉素和两性霉素B的鸡尾酒补充是很重要
脑膜成纤维细胞的尸检确诊病例与帕金森氏症的推导为帕金森氏病的体外模型的基础。脑膜成纤维细胞出现制备样品后3-9天,约20-30亿个细胞可在6-8周冷冻保存。脑膜成纤维细胞培养是同质和细胞表达纤维连接蛋白,一种常用的标记来识别脑膜。
引言
脑膜由保护大脑三种细胞膜的:硬脑膜,蛛网膜和软脑膜。最近,已经认识到,脑膜也起到大脑发育和脑稳态1中起重要作用。脑膜从间充质和神经嵴源性细胞衍生和有趣的是,它已被证明存在于脑膜该祖细胞可以产生的神经元在体外和体内 2,3,4移植后。脑膜培养已还成功地用作饲养层,因为它们具有基质细胞衍生诱导活性为胚胎干细胞分化为多巴胺能神经元5。此外,软脑膜必须直接分化成神经元,星形胶质细胞和少突胶质缺血条件6下的可能性。
此协议,人死后的脑膜样本从蛛网膜和软脑膜收集,统称为软脑膜,并且采购作为人脑捐赠用于研究目的的一部分。大脑的解剖是死亡的24小时之内进行,并且如所示在这里这个协议软脑膜样品置于冷生长培养基用于进一步的处理的下一个6-8小时内。
这个协议描述了用于患者主软脑膜细胞培养的发展解剖和制备的人类脑膜样品。将组织切成25-30片大约3mm×3mm的正方形。三片置于每个6孔明胶包好,并与圆形盖玻片按住。脑膜解剖大约需要25-35分钟。这种文化的主要挑战是,一般不会无菌条件下进行无菌为大脑采购,运输和清扫。 ŧherefore,到培养基用青霉素,链霉素和两性霉素B的鸡尾酒补充并使用多孔培养皿分别培养组织片是很重要的。
脑膜成纤维细胞的生长通常在第一个星期内发生。介质每两至三天更换直到细胞汇合,细胞酶促传代。脑膜成纤维细胞是在每毫升/瓶1百万个细胞在冷冻保存介质冻存。有了这个协议,20-30万元脑膜成纤维细胞可在6-8周衍生为冷冻保存。这些脑膜成纤维细胞的下游应用是用于疾病研究,直接的神经元分化,或从软脑膜为疾病机制的理解以及对药物开发诱导多能干细胞的衍生初级培养物。
研究方案
The brain donation registration includes documentation by the registrant of their intent to donate. The autopsy permission for tissue retrieval is provided by the next of kin as permitted by law. Research studies using collected autopsy specimens are reviewed by the institutional review board (IRB) to ensure compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
NOTE: Leptomeninges samples are collected by the brain dissector or neuropathologist during a brain dissection and are stored in 50 mL conical tubes containing 25-30 mL meninges growth media. The sample is stored at 4 °C until sample preparation. The processing should be performed as soon as possible as the viability of cells decreases with time post mortem.
1. Set-up before Starting the Leptomeninges Dissection
NOTE: Steps 1.1 - 1.3 are to be performed inside a biosafety cabinet.
- Prepare meninges growth media by combining 375 mL of Dulbecco's Modified Eagle Media, 100 mL of fetal bovine serum, 5 mL 10 mM non-essential amino acids, 5 mL 200 mM L-alanyl-L-glutamine, 5 mL 100 mM sodium pyruvate, 5 mL 10,000 U/mL penicillin/streptomycin and 5 mL 250 µg/mL Amphotericin B in 500 mL filter unit. Filter and mix. Use media for up to four weeks.
- Place all materials in the biosafety cabinet before starting the dissection: 4x Petri dishes, 2x 6-well plates, 2x scalpels, 15-20 sterilized 15-mm cover slips, phosphate buffer saline (PBS), serological pipettes, aspirating pipettes.
- Add 1 mL of sterile-autoclaved 0.1% gelatin solution to each well of a 6-well plate. Set the plate aside for 30-60 min. Prepare 2x 6-well plates.
2. Preparation of Leptomeninges Sample
NOTE: Steps 2.1 - 2.3 are to be performed inside a biosafety cabinet.
- Add approximately 10 mL of PBS to a sterile 10-cm tissue culture dish and place the meninges sample in the dish. Using forceps, gently wash leptomeninges with PBS to remove blood.
- Transfer the meninges into a new 10-cm tissue culture dish containing 10 mL of fresh PBS and continue washing. Repeat as necessary to remove as much blood as possible.
- Using two scalpels, cut leptomeninges tissue into an approximately 6 cm x 6 cm large piece.
- Place lid on a culture dish and transfer to a dissecting microscope in a horizontal laminar flow hood.
3. Dissection of Leptomeninges Tissue
NOTE: Steps 3.1 - 3.3 are to be performed inside a horizontal laminar flow hood using a dissecting microscope.
- Remove blood vessels from a region on the leptomeninges piece (step 2.3) large enough to create at least 20-25 3 mm x 3 mm pieces. Hold the leptomeninges in place with one scalpel and with the other, use a gentle and shallow scraping motion to separate the vessels.
- Cut small squares of tissue from the prepared region. Cut in a stepwise process by first making larger pieces and then slicing those into smaller sections.
- Use one scalpel to hold the tissue in place and another scalpel to cut the tissue with a rocking motion. Due to the consistency of the tissue, it is difficult to slide the scalpels against each other. This will only tear the tissue apart and results in ragged edges.
- Continue cutting the pieces in half until there are about 20-25 3 mm x 3 mm pieces.
4. Transfer of Dissected Meninges Pieces onto Tissue Culture Plates
NOTE: Step 4 is to be performed inside a biosafety cabinet.
- Aspirate gelatin from 6-well plate (from step 1.3). Add 1 mL of meninges growth media to each well. Using sterile forceps, place 3-4 biopsy pieces into each well.
5. Placement of Cover Slips over Meninges Pieces
NOTE: Steps 5.1 - 5.2 are to be performed inside a horizontal laminar flow hood using a dissecting microscope.
- Cover the pieces with 1-2 sterile 15-mm circular coverslips per well.
- Press down firmly with forceps to ensure the pieces are touching the bottom of the plate. This allows attachment of tissue pieces to the bottom of the well.
- Place the 6-well plate into a 37 °C, 5% CO2 incubator.
6. Cell Culture Maintenance
NOTE: Steps 6.1 - 6.3 are to be performed inside a biosafety cabinet. Handle culture dishes with care as coverslips should not move and dislodge the meninges pieces.
- After 2 days in culture, add an additional 1 mL of fresh meninges growth media to each well.
- At Day 7, carefully aspirate the media and add 2 mL of fresh media to each well.
- After Day 9, continue to change growth media every other day until culture becomes confluent.
7. Passaging
NOTE: All steps are to be performed inside a biosafety cabinet. Once meningeal fibroblasts have migrated out of the meningeal tissue pieces and start to grow towards the edges of the culture vessel, expand meningeal fibroblasts into larger culture dish.
- Before starting the passaging, coat the culture dish with 1% gelatin for 15-30 min.
- Aspirate media from 6-well dish and wash cells with 2 mL PBS.
- Add 1 mL of trypsin/EDTA to each well and incubate at 37 °C for 5-10 min. It is not necessary to remove the cover slips or the meningeal tissue for this step.
- When cells are rounded and start to detach, add 2 mL of culture media.
- Pipette cells up and down to completely disrupt all clumps and transfer the cell solution to larger culture dish.
- Use a ratio of 1:3 to 1:4 to combine cells from three 6-well dishes into one T75 culture dish.
- Once meningeal fibroblasts cells are confluent in T75 culture flasks, trypsinize the cells, and cryopreserve in cryopreservation media at 1x106 cells/mL per vial. One T75 culture flask with confluent meningeal fibroblasts contains about 5-7 million cells.
8. Characterization of the Meningeal Fibroblasts by Immunostaining
NOTE: Before starting the procedure, prepare 4% paraformaldehyde solution, 5% normal goat serum in PBS (blocking buffer), 0.3% Triton X-100 in PBS (permeabilization buffer), primary and secondary antibody solutions, and 1 µg/mL of Hoechst 33342 diluted in PBS from 10 mg/mL stock.
- Seed 15,000 to 30,000 meningeal fibroblasts in 8-well chamber slide coated with 0.1% gelatin.
- After 24 h when the cells are attached, fix cells in 100 µL 4% paraformaldehyde per well for 10 min at room temperature. Wash the wells 3 times with 1x PBS.
- Permeabilize the cells with 150 µL of 0.3% Triton X-100 for 5 min at room temperature. Wash the wells 3 times with 1x PBS.
- Add 200 µL blocking solution (PBS + 5% normal goat serum) for 1 h at room temperature.
- Aspirate the blocking solution, and add 150 µL of desired primary antibodies diluted in blocking solution. For double-staining, prepare working dilutions of primary antibodies in separate centrifuge tubes on ice as follows:
- Prepare 1:300 dilution of anti-Fibronectin + 1:200 dilution of anti-nestin in blocking solution. Prepare 1:250 dilution of anti-SERPINH1 + 1:1,000 dilution of anti-TUJ1 in blocking solution. Prepare 1:250 dilution of anti-SERPINH1 + 1:100 dilution of anti-SOX2 in blocking solution
- Incubate at 4 °C overnight.
- Wash the wells 3 times with 1x PBS.
- Prepare a working 1:400 dilution of fluorescently labeled secondary antibodies goat anti-rabbit (green; Ex/Em2 495/519 nm) and goat anti-mouse (red; Ex/Em2 590/617 nm) in blocking solution and add 100 µL to each well. Incubate for 1 h, in the dark, at room temperature. Then wash wells once with 1x PBS.
- Add 100 µL of 1 µg/mL Hoechst 33342 (blue; Ex/Em2 358/461 nm) solution to each well and incubate in the dark for 3 min. Wash wells 3 times with 1x PBS, leaving in the final 1x PBS wash in the well, and cover the slide with aluminum foil.
- Analyze cells under fluorescent microscope.
结果
When the leptomeninges processing protocol has been successful, outgrowth of meningeal fibroblasts is first observed three to nine days after dissection, although this can depend on the length of post-mortem interval for the brain. Figure 1 demonstrates meningeal fibroblast cultures of four different donors. Figure 1A shows a leptomeninges piece held down by a glass cover slip (dark diagonal line) and fibroblast outgrowth around the tissue four days after processing from an 88-year-old donor with 10 h 20 min post-mortem interval. Figure 1B shows sparse fibroblast outgrowth seven days post processing from a 70-year-old donor and 11 h 45 min post-mortem interval. Figure 1C shows fibroblast outgrowth 13 days after dissection from an 88-year-old donor and 24 h post-mortem interval. Figure 1D shows a confluent culture that was passaged into a T75 vessel and cells can be cryopreserved at this stage.
Meningeal fibroblasts are bipolar or multipolar and have elongated or irregular shapes (Figure 2). They are characterized by the expression of glycoprotein fibronectin and fibroblast marker SERPINH which is localized to the endoplasmic reticulum (Figures 2A-2C). Meningeal fibroblasts are not immuno-reactive to transcription factor SRY (Sex Determining Region Y)-Box 2 (SOX2) which is a marker for undifferentiated stem cells (Figure 2A). However, a subset of leptomeningeal fibroblasts is positive for type VI intermediate filament nestin, a neural stem cell marker (Figure 2B), and neuron-specific class III beta-tubulin (TUJ1) (Figure 2C).

Figure 1. Examples of outgrowth of meningeal fibroblasts from four different donors. A) Leptomeninges tissue piece is held down by a glass cover slip (dark diagonal line) and fibroblast outgrowth around the tissue was observed four days after processing from an 88-year-old donor with 10 h 20 min post-mortem interval. B) Sparse fibroblast outgrowth seven days post processing from a 70-year-old donor and 11 h 45 min post-mortem interval. C) Meningeal fibroblast outgrowth 13 days after dissection from an 88-year-old donor and 24 h post-mortem interval. D) Confluent meningeal fibroblast culture passaged into a T75 vessel. Please click here to view a larger version of this figure.
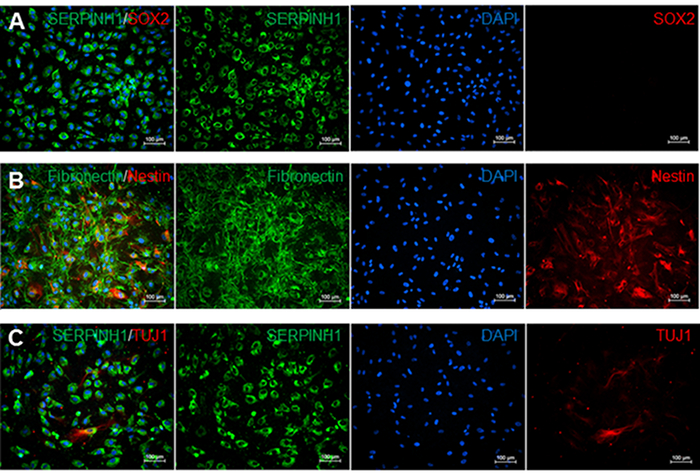
Figure 2. Immunostaining for meninges, stem cell, neural stem cell, and neuron markers. A) Immunofluorescence staining for fibroblast markers SERPINH1 (green) and stem cell marker SOX2 (red). SERPINH1 was expressed in all counted cell nuclei. Stem cell marker SOX2 was not detected in these leptomeninges culture. B) Immunofluorescence staining for meninges-specific marker fibronectin (green) and neural stem cell marker NESTIN (red). 52.1% of counted cell nuclei were positive for both NESTIN and Fibronectin. C) Immunofluorescence staining for SERPINH1 (green) and neuronal marker TUJ1 (red). 6.9% of counted nuclei were positive for SERPINH1 and TUJ1. Cell count data were determined by using the manual Cell Counter Multi-Select tool on ImageJ. Single stained images were assessed by counting the number of DAPI-positive cells compared to the number of cells positive for each marker in percentages. Please click here to view a larger version of this figure.
| Day | Expected results | |||||
| 3-9 | Outgrowth of first meningeal fibroblasts. | |||||
| 7-18 | Meningeal fibroblasts expand, and culture becomes denser. Media change of 2 mL every other day. | |||||
| 18-25 | 6-well plate becomes confluent, once meningeal fibroblasts are confluent combine three wells into a T75 culture flask (passage at 1:3 to 1:4). | |||||
| 26-45 | One confluent T75 culture flask results in 5-7 million cells. Cryopreserve meningeal fibroblasts at 1 million cells/vial. | |||||
Table 1: Timeline of cellular outgrowth.
讨论
This protocol describes a simple and robust protocol to derive a meningeal fibroblast culture from human postmortem leptomeninges collected in conjunction with a brain donation. There are very few descriptions of protocols to derive cell cultures from postmortem human material. Two studies describe skin-derived fibroblast cultures7,8,9, one study describes dura samples10, and another describes non-cryopreserved frozen dura samples11.
We prepare two 6-well plates with 2-3 tissue pieces (about 3 mm x 3 mm) in each well to ensure sufficient outgrowth and expansion before cryopreservation. It is critical that the meninges pieces are attached to the culture dish by gentle pressing a sterile glass cover slip onto the tissue pieces to hold them in place. Without a coverslip, the meninges pieces will not attach to the bottom of the culture dish. Successful outgrowth is only observed when the tissue pieces are in direct contact with the plastic bottom. If the tissue pieces float in the culture media, no cell outgrowth from the tissue is observed. This should be performed with about 500 µL of growth media to not dry out the samples and then add up to 1 mL of growth media to the vessel. It is not necessary to further use silicone to hold the cover slips in place.
If the pieces are smaller than 3 mm x 3 mm, the outgrowth is slower and in some cases no confluent cultures can be established. It is critical to not grow the cells too sparse and we recommend a split ratio of 1:3 to 1:4. Individual small wells allow for better managing potential contamination. Wells can be fed individually and pipettes should be changed between the wells.
We have successfully derived meningeal fibroblasts from 9 postmortem samples with a postmortem interval between 10-24 h. The donors were between 70 and 88 years of age and have disease durations of Parkinson's disease between 8-31 years. We notice that tissues with a longer post-mortem interval take longer before the first cells grow out (up to 9 days). In samples with shorter post-mortem interval, we observe outgrowth as soon as 3 days after preparation. We have not noticed a difference in growth based on age of donor, but we only had donors >70 years.
The significance of the method is the derivation of a primary cell culture for a sporadic neurodegenerative disease like Parkinson's disease, which can only currently be confirmed at autopsy12,13,14 since there are no fully defining clinical characteristics, imaging techniques or biomarkers that can provide a definite diagnosis during life15. We have previously developed a protocol for the derivation of fibroblasts from skin biopsies primarily for genetically confirmed cases of Parkinson's disease16 which have allowed us to generate induced pluripotent stem cells and study disease mechanisms17,18, but this new protocol will be crucial to directly compare neuropathological changes with the findings in vitro for sporadic Parkinson's disease. To our knowledge, this is the first protocol deriving meningeal fibroblasts from post-mortem human leptomeninges.
Meningeal fibroblast cultures can directly be used for studying molecular mechanisms relating to disease or injury or as a source for nuclear reprogramming into induced pluripotent stem cells or direct conversion into neuronal cultures as advanced preclinical models for sporadic Parkinson's disease or other neurodegenerative disorders19. These human patient-derived models provide the foundation and accelerate advancements in personalized and regenerative medicine.
披露声明
The authors have nothing to disclose.
致谢
Development of this protocol was funded by private donations directed to the Parkinson's Institute Brain Donation Program.
材料
| Name | Company | Catalog Number | Comments |
| Corning Petri dishes | Fisher Scientific | 351029 | |
| Nunc 6-well plate | Fisher Scientific | 14-832-11 | |
| 15-mm cover slips | Fisher Scientific | 12-545-83 15CIR-1D | |
| Scalpels, sterile blade, No. 15 | Miltex | 4-415 | |
| Curved precision tip forceps | Fisher Scientific | 16-100-122 | |
| Serological pipettes | Fisher Scientific | 13-678-11E | |
| Pasteur pipettes | Fisher Scientific | 22-230490 | |
| Gelatin | Sigma | G1890-100G | |
| Phosphate Buffer Saline | Fisher Scientific | SH30264.02 | |
| Corning 500 mL filter unit | Fisher Scientific | 430770 | Combine media components and filter. |
| Nunc Cell Culture Treated Flasks with Filter Caps, T175 cm2 | Thermo Scientific | 178883 | |
| Name | Company | Catalog Number | Comments |
| Growth Media | |||
| Hyclone DMEM | Fisher Scientific | SH30081.02 | |
| Hyclone FBS | Fisher Scientific | SH30910.03 | |
| MEM Non-Essential Amino Acids Solution (100x) | Thermo Fisher | 11140-050 | |
| GlutaMAX Supplement (100x) | Thermo Fisher | 35050-061 | |
| Sodium Pyruvate (100 mM) | Thermo Fisher | 11360-070 | |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher | 15140-122 | |
| Amphotericin B (Yellow Solution/250 µg/mL) | Fisher Scientific | BP264520 | |
| Bambanker Freeze 120 mL | Fisher Scientific | NC9582225 | |
| Name | Company | Catalog Number | Comments |
| Fibronectin Staining | |||
| 8 well chamber slides | Fisher Scientific | 1256518 | |
| 20% paraformaldehyde | Electron Microscopy Sciences | 15713 | |
| Triton X-100 | Sigma | T8787 | |
| 100% Glycerol | BioRad | 9455 | |
| 100% normal goat serum | Fisher Scientific | 101098-382 | |
| Anti-Fibronectin antibody [F1] | Abcam | ab32419 | 1:300 dilution in blocking solution |
| Anti-SERPINH1 | Sigma | S5950-200ul | 1:250 dilution in blocking solution |
| Anti-SOX2 | Millipore | MAB4343 | 1:100 dilution in blocking solution |
| Anti-Nestin | Millipore | MAB5326 | 1:200 dilution in blocking solution |
| Anti-TUJ1 | Covance | MMS-435P | 1:1,000 dilution in blocking solution |
| Alexa Fluor 488 anti-rabbit | Thermo Fisher | A11029 | 1:400 dilution in blocking solution; (green channel; Ex/Em2 495/519 nm) |
| Alexa Fluor 555 anti-mouse | Thermo Fisher | A21424 | 1:400 dilution in blocking solution; (red channel; Ex/Em2 590/617 nm) |
| Hoechst 33342 stain | Thermo Fisher | H3570 | dilute to a final concentration of 1.0 μg/mL; (blue channel; Ex/Em2 358/461 nm) |
| Suppliers are suggestions, similar products from alternative vendors can be used as well. | |||
参考文献
- Decimo, I., Fumagalli, G., Berton, V., Krampera, M., Bifari, F. Meninges: from protective membrane to stem cell niche. Am J Stem Cells. 1 (2), 92-105 (2012).
- Bifari, F., et al. Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J Cell Mol Med. 13 (9B), 3195-3208 (2009).
- Bifari, F., et al. Meninges harbor cells expressing neural precursor markers during development and adulthood. Front Cell Neurosci. 9, 383(2015).
- Decimo, I., Bifari, F., Krampera, M., Fumagalli, G. Neural stem cell niches in health and diseases. Curr Pharm Des. 18 (13), 1755-1783 (2012).
- Hayashi, H., et al. Meningeal cells induce dopaminergic neurons from embryonic stem cells. Eur J Neurosci. 27 (2), 261-268 (2008).
- Nakagomi, T., et al. Leptomeningeal-derived doublecortin-expressing cells in poststroke brain. Stem Cells Dev. 21 (13), 2350-2354 (2012).
- Hjelm, B. E., et al. Induction of pluripotent stem cells from autopsy donor-derived somatic cells. Neurosci Lett. 502 (3), 219-224 (2011).
- Hjelm, B. E., et al. In vitro-differentiated neural cell cultures progress towards donor-identical brain tissue. Hum Mol Genet. 22 (17), 3534-3546 (2013).
- Meske, V., Albert, F., Wehser, R., Ohm, T. G. Culture of autopsy-derived fibroblasts as a tool to study systemic alterations in human neurodegenerative disorders such as Alzheimer's disease--methodological investigations. J Neural Transm (Vienna). 106 (5-6), 537-548 (1999).
- Bliss, L. A., et al. Use of postmortem human dura mater and scalp for deriving human fibroblast cultures. PLoS One. 7 (9), e45282(2012).
- Sproul, A. A., et al. Generation of iPSC lines from archived non-cryoprotected biobanked dura mater. Acta Neuropathol Commun. 2, 4(2014).
- Adler, C. H., et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 83 (5), 406-412 (2014).
- Hughes, A. J., Daniel, S. E., Kilford, L., Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 55 (3), 181-184 (1992).
- Hughes, A. J., Daniel, S. E., Lees, A. J. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 57 (8), 1497-1499 (2001).
- Langston, J. W., Schüle, B., Rees, L., Nichols, R. J., Barlow, C. Multisystem Lewy body disease and the other parkinsonian disorders. Nature Genetics. 47 (12), 1378-1384 (2015).
- Vangipuram, M., Ting, D., Kim, S., Diaz, R., Schüle, B. Skin punch biopsy explant culture for derivation of primary human fibroblasts. J Vis Exp. (77), e3779(2013).
- Byers, B., et al. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidative stress. PLoS One. 6 (11), e26159(2011).
- Sanders, L. H., et al. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol Dis. 62, 381-386 (2014).
- Beevers, J. E., Caffrey, T. M., Wade-Martins, R. Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson's disease. Biochem Soc Trans. 41 (6), 1503-1508 (2013).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。