Case Report
Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion
In This Article
Summary
The protocol offers a valuable method detailing each step of the FE-TLIF procedure. With proper education, FE-TLIF can be effectively learned, leading to favorable clinical outcomes.
Abstract
Uniportal full endoscopic posterolateral lumbar interbody fusion (FE-TLIF) has recently shown promising results. However, beginners may face challenges in mastering the technical skills required to overcome the learning curve for a more efficient and safer procedure. The goals of this study are to provide a detailed FE-TLIF procedure and provide a step-by-step explanation of all methods, as well as to use written text to describe key techniques and precautions for ensuring a safer and more efficient procedure. We present a case of L4-L5 degenerative spondylolisthesis with spinal stenosis syndrome and right sciatica. The study offers valuable educational video footage detailing each step of the FE-TLIF procedure. The protocol incorporates the use of several instruments common to conventional TLIF procedures, an efficient outside-in technique with a trephine for IAP resection, endoscopic visualization for endplate preparation, and nerve protection. With proper education, FE-TLIF can be effectively learned, leading to favorable clinical outcomes while minimizing complications.
Introduction
Lumbar fusion is considered the standard treatment for various degenerative lumbar conditions1. With the increasing prevalence of minimally invasive spinal surgery, advancements in endoscopic techniques and instruments have expanded the indications for endoscopic spinal surgery2. Endoscopic-assisted fusion has recently demonstrated promising results, including faster recovery, reduced blood loss, and minimized back muscle injury3,4,5. Compared to facet-preserving trans-Kambin endoscopic fusion, facet-sacrificing posterolateral transforaminal lumbar interbody fusion (TLIF) has the benefit of relatively familiar corridor as minimally invasive tubular approach TLIF (MIS-TLIF), direct visualization during spinal decompression and less exiting nerve root injuries6.
Uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion (FE-TLIF) differs significantly in surgical technique and instrument compared to unilateral biportal endoscopy-assisted TLIF (UBE-TLIF)3,6,7. Although both endoscopic fusion techniques have shown similarly favorable early and medium-term postoperative outcomes5,8, the learning curve for FE-TLIF is steeper. Beginners may face challenges in mastering the technical skills required to overcome the learning curve for a more efficient and safer procedure4.
The protocol of FE-TLIF outlined below incorporates techniques described by the Kim and Wu group6,7,9,10,11 with some modifications. In addition to the use of smaller endoscopic equipment with a longer lever arm7, the procedure presents challenges such as equipment limitations, particularly instruments for discectomy and cartilage endplate preparation12, as well as the lack of visualized nerve protection when using specialized cage gliders during adjacent procedures, increasing the risk of nerve root injury13. Wu et al.11 reported a 6% complication rate involving traversing root injuries among 35 patients undergoing FE-TLIF, even in the hands of experienced surgeons. Conversely, Zhao et al.14 observed a 9.6% revision rate in the earliest third of treated patients, along with significantly increased X-ray exposure time during the learning curve.
To overcome these challenges, in the protocol, we incorporate the use of several instruments common to conventional TLIF procedures, endoscopic visualization for nerve protection during endplate preparation and cage insertion. The advantages over applicable references mentioned above7,11,14 were twofold: first, familiarity with instruments such as an endplate shaver, a funnel, and a standard TLIF non-expandable cage enhances procedural safety; and second, visualized nerve protection ensures that neural structures are properly shielded.
The purposes of this study are to video-record the FE-TLIF procedure and provide a step-by-step explanation accompanied by video clips and to use written text to describe key techniques and precautions for ensuring a safer and more efficient procedure.
CASE PRESENTATION:
We present a 68-year-old male with lower back pain, right calf pain, and difficulty walking. The associated symptoms included numbness of the right L5 territory and intermittent claudication. Imaging revealed L4-L5 degenerative spondylolisthesis with spinal stenosis syndrome (Figure 1). After a thorough discussion, the patient was scheduled for right L4-L5 uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion (FE-TLIF).
Protocol
This study (Ref. No. 202500125B0) was approved by the institutional review board of Chang Gung Medical Foundation, Taiwan, and appropriate informed consent was obtained.
1. Positioning, skin marking, and patient preparation
- Patient position: After general anesthesia, put the patient prone on a Wilson frame with slight flexion for better decompression efficiency.
- Perform fluoroscopy-guided skin marking as described below.
- Mark the right L4 transpedicular screw entry point neighboring the right isthmus in the AP view as an endoscopic working portal.
NOTE: The traditional transpedicular screw entry point is usually located near the L4 pedicle eye in the anteroposterior fluoroscopic view. The modified entry, positioned more medially and caudally, allows for better access to contralateral decompression and disc preparation, respectively. - Mark the other three transpedicular screw entry points as usual region, neighboring to bilateral L4 and L5 pedicle eye in the anteroposterior fluoroscopy view.
- Mark the right L4 transpedicular screw entry point neighboring the right isthmus in the AP view as an endoscopic working portal.
- Perform surgery preparation as described below.
- Perform disinfection with Povidone-iodine from the middle back to the buttock.
- Drape the surgical field aseptically. Prepare the surgical field with a dam and water bag, which are needed for endoscopic surgery water outflow. Position the water bag as an irrigation pouch on the side of the square-shaped surgical field closest to the surgeon. Elevate the other three sides using a surgical drape to create a dam, allowing water to flow into the bag. Finally, secure the setup with a waterproof antimicrobial incise drape.
- Set the instruments and normal saline bag hanging about 2 m above to allow gravity flow. The instruments include: An endoscope connected to a camera system, an optic fiber cable, and an irrigation tube, an endoscopic burr, an endoscopic radiofrequency ablator. Securely fix these instruments to the drape using clamps, allowing for a good range of motion.
2. Creating working space and identifying landmarks
- Set up the endoscopic working portal as described below.
- Create an endoscopic portal by making a 1.2 cm long longitudinal incision by scalpel at the first mark in step 1.2 and a wider fascia incision beneath. The fascia is the first firm layer located just beneath the subcutaneous tissue. Make a cranial and caudal incision in the fascia using a scalpel with a total length of 2.5-3.0 cm.
- Dock the obturator on the right L4 isthmus region by checking the fluoroscopy. Use the obturator to contact the bone and confirm its position with fluoroscopic imaging.
- Put serial dilators and, finally, the open bevel working tube (11.2 mm outer diameter/ 10.2mm inner diameter; Figure 2A). Insert the dilators while using the steadily held obturator as a guide. If there is any doubt, reposition the obturator to contact the bone again and verify the position using fluoroscopy.
- To find landmarks, introduce a 15° angled endoscope (10 mm outer diameter). Dissect soft tissue using a radiofrequency ablator to clear the space around the right L4/L5 facet and identify Wu's and Kim's points7 with fluoroscopic images (Figure 2B-C). Use burr to create a bony surface as landmarks after checking position by fluoroscopy to avoid disorientation.
3. Ipsilateral decompression
- Inferior articular process (IAP) removal: Use the outside-in technique10 to remove IAP efficiently from Wu’s point to Kim’s point with a trephine and osteotome. When the IAP bone piece in the trephine or from the osteotome is fractured, save the piece as autograft.
- Change the working tube to a larger one for the reamer (12.5 mm outer diameter/ 11.5mm inner) and advance the sheath of the working tube into the joint space for stabilization when using the trephine to remove the inferior articular process. Rotate the trephine gently while keeping the working tube steady with the other hand. When the IAP bone piece in the trephine is fractured, the bone will rotate during trephine rotation.
- Remove the residual IAP with an endoscopic osteotome. Save the bone piece as an autograft.
- Perform dura decompression and ipsilateral flavectomy as described below.
- Identify the origin and insertion of the ipsilateral ligamentum flavum by removing the caudal lamina of right L4, the cranial lamina of right L5, and the medial base of the superior articular process (SAP).Use either a high-speed 4 mm diamond burr or Kerrison Rongeur.
NOTE: Rather than an outside-in technique in decompression15, we use an inside-out technique and use the neural structure as an anatomical reference for decompression. - Remove ipsilateral ligamentum flavum piece by piece to expose right L5 traversing root and foraminal disc with endoscopic pituitary or Kerrison Rongeur.
- Identify the origin and insertion of the ipsilateral ligamentum flavum by removing the caudal lamina of right L4, the cranial lamina of right L5, and the medial base of the superior articular process (SAP).Use either a high-speed 4 mm diamond burr or Kerrison Rongeur.
4. Contralateral decompression
- Over-the-top technique: Remove the spinous process base until the contralateral cranial lamina, caudal lamina, and contralateral facet are visible.
- Dura decompression and contralateral flavectomy: Remove origin and insertion of contralateral ligamentum flavum. Remove the contralateral medial base of the SAP to release the left L5 traversing root.
- Identifying these structures is the most critical step. Remove the cranial and caudal lamina until the origin and insertion of the contralateral ligamentum flavum are clearly visible. Carefully extract the freed ligamentum flavum piece by piece using either an endoscopic pituitary rongeur or a Kerrison rongeur until the dura is exposed.
- The lateral portion of the ligamentum flavum extends beneath the medial base of the SAP. Remove the medial base of the SAP while preserving the outermost portion of the ligamentum flavum to protect the contralateral traversing root, which lies lateral to the dura. Finally, remove the entire contralateral ligamentum flavum. Please refer to the video for more anatomical details.
5. Disc space clearance and endplate preparation
- Ensure there is enough craniocaudal cage entry space by removing the cranial tip of the superior articular process by osteotome until the cranial edge of the pedicle. Ensure there is enough mediolateral space by clearing the epidural space close to the right L5 traversing root.
- Perform endoscopic discectomy by traversing root protection as described below.
- Withdraw the original working tube and change to a larger working tube with a handle and long lip (16 mm outer diameter/ 15mm inner diameter). Use the dissector to protect the right L5 traversing root and gently rotate the long tip the retract the traversing nerve root.
- Perform annulotomy using hook scissors at the planned cage entry site. Insert endplate shavers in series following the conventional transforaminal lumbar interbody fusion procedure. Change the fluoroscope to lateral projection to monitor position of the endplate shaver (Figure 3A).
- Hold the working tube steadily to ensure the neural structures are safely protected under endoscopic visualization. Withdraw the endoscope to allow the endplate shaver to pass through. When retrieving each endplate shaver, release the nerve root by rotating the long lip. Use the endoscope to inspect the disc material and assess the condition of the nerve.
- Remove disc material and cartilage by pituitary clamp by direct visualization.
- Perform trial cage insertion as described below.
- After preparing the desired endplate with punctate bleeding of the subchondral bone, insert the cage trial serially to determine the cage size while protecting the L5 traversing root.
- Determine the ideal cage height using serial cage trials of 1 mm increment starting from 8 mm (Figure 3B). The ideal cage size can be determined based on the tension required to move the cage trial, bone quality, and the disc height of the adjacent levels. When removing each cage trial, use a slap hammer to apply axial force with one hand while carefully avoiding any impact on the working tube, which should be held steadily with the other hand.
6. Interbody fusion with bone graft and cage
- Bone grafting: Hold the working tube steadily and withdraw the endoscope to allow passage of the bone grafting funnel into the disc space. Using a funnel-shaped bone grafting device, confirm its ideal placement in the disc space fluoroscopically (Figure 3C). Insert autologous bone graft followed by artificial bone substitute in sequence.
- Hold the working tube steadily to ensure the neural structures are safely protected under endoscopic visualization. Withdraw the endoscope to allow the funnel-shaped bone grafting device to pass through and position it within the disc space. The optimal depth is between the anterior third and half of the L5 vertebral body.
NOTE: Bone graft material can be delivered through the funnel and placed into the anterior disc space using a bone graft impactor. In this procedure, 2.5 cc of artificial bone substitute made of demineralized bone matrix putty was used. The volume of autologous lamina chips is not routinely measured. However, several rounds of funnel impaction are typically required to insert the full amount of bone graft.
- Hold the working tube steadily to ensure the neural structures are safely protected under endoscopic visualization. Withdraw the endoscope to allow the funnel-shaped bone grafting device to pass through and position it within the disc space. The optimal depth is between the anterior third and half of the L5 vertebral body.
- Insert the TLIF cage as described below.
- Insert a conventional intervertebral fusion cage (TLIF cage, bullet-shaped, 26 mm in length, PEEK). Hold the working tube steadily to ensure the neural structures are safely protected under endoscopic visualization. Withdraw the endoscope to allow the cage to pass through. When engaging the cage to the posterior disc space, check the position and axis by lateral fluoroscopic image, to avoid any danger to the neural structure and endplate.
- Check the position of the cage by fluoroscopy (Figure 3D). The optimal position is centered within the disc space. On the lateral view, ensure the posterior marker of the cage is positioned anterior to the posterior vertebral body line, while on the anteroposterior view, the anterior marker is aligned with the spinous process.
7. Final check
- Use the endoscopy again after cage insertion for adequate dura and root decompression (Figure 4A-C). Inspect and remove any freed disc tissue or blood clot. Close the water inflow to check dura and root expansion and pulsation.
- Stop the bleeding by cancellous bone and epidural vessels. Inspect any bleeding point and stop it with a radiofrequency ablator. Close the water inflow to check for excessive bleeding. A hemostatic agent can be used if needed.
8. Application of pedicle screws and rods
- Insert percutaneous pedicle screws under fluoroscopy. Reduce flexion of the Wilson frame to restore the lordosis of the patient by circulating nurse. Insert bilateral L4 and L5 percutaneous pedicle screws with a typical technique (Figure 5A-B).
- Use the cannulated needle to insert a K-wire. Leave the K-wire in place once the cannulated needle is removed. Place the cannulated pedicle screw over the k-wire once the soft tissue has dilated. To overcome the skin tension in the relative caudal and medial skin incision or right L4 entry, create a subcutaneous tunnel and another fascia entry from the original skin incision.
- Tighten the rod and reduce the spondylolisthesis. Apply the rod percutaneously and fit the distal screws first. While applying ipsilateral screw compression, tighten the distal screws first and then the proximal screws for rod reduction of the spondylolisthesis (Figure 5C-D). Perform screw compression manually. Specialized instruments pertaining to the percutaneous screw system could also be used.
9. Closing the skin in layers with drain inserted
- Insert one Hemovac drainage tube with the tip in the cage region (Figure 4D). Close the skin in layers. Suture the fascia with no.1 Vicryl, and the subcutaneous layer with 2-0 and 3-0 Vicryl. Fix the drainage tube on the skin with a 2-0 Vicryl suture.
Results
From September 2024 to March 2025, a total of 10 patients at our hospital were diagnosed with L4-L5 degenerative spondylolisthesis with spinal stenosis and underwent surgery. The cohort included five males and five females, with an average age of 67.0 ± 9.27 years (range: 52–82). The average surgical time was 333.2 ± 47.25 min (range: 274–424). Postoperatively, patients reported significant improvement of both back and leg pain score on a visual analog scale (0-10) from 7.2 ± 1.14 to 1.3 ± 1.34. They were able to sit and stand in the ward on postoperative day 1.4 ± 0.52 and began walking independently with a Taylor brace on day 2.3 ± 0.82. The drainage tube was removed on day 2.6 ± 0.52, and patients were discharged on day 4.1 ± 1.60. According to MacNab’s criteria16, six patients (60%) had excellent outcomes, third (30%) had good outcomes, and one (10%) had a fair outcome. There was no complication such as nerve injury, epidural hematoma and screw misplacement (Table 1). Figure 6 shows the presented case’s postoperative radiographs taken 2 days after surgery and MRI images at 6 weeks, demonstrating the effectiveness of the described protocol.

Figure 1: Operative images of the patient. (A) Asymmetric disc space narrowing of L4-L5. (B, C) L4-L5 degenerative spondylolisthesis with dynamic slip. (D, E, F) MRI T2WI showed L4-L5 spondylolisthesis with spinal stenosis, Schizas grade C. Please click here to view a larger version of this figure.
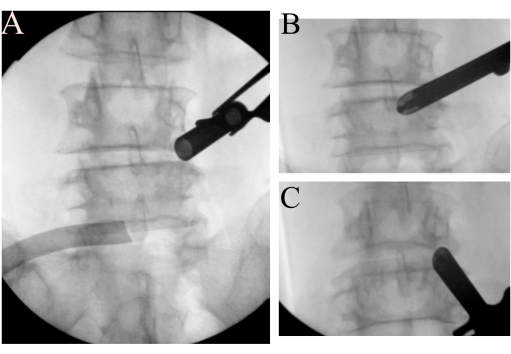
Figure 2: Landmark identification. (A) The open bevel working tube is docking on the right L4 isthmus region. (B) Identification of Wu's point and (C) Kim's point. Please click here to view a larger version of this figure.

Figure 3: Disc and endplate procedures. During the step of disc space clearance, endplate preparation, use of (A) endplate shaver, (B) cage trial, (C) funnel for bone grafting, and (D) TLIF cage. Please click here to view a larger version of this figure.

Figure 4: Final check. (A, B) The L5 ipsilateral traversing root is intact and well decompressed. (C) The dura and contralateral L5 traversing root are also freed. (D) Incision and wound of FE-TLIF procedure. Please click here to view a larger version of this figure.

Figure 5. Percutaneous pedicle screw insertion and rod reduction. (A, B) Apply pedicle screws and rods, and (C, D) use the rod to reduce the spondylolisthesis. Please click here to view a larger version of this figure.

Figure 6: Post-operative images of the patient following FE-TLIF. (A) Anteroposterior and (B) lateral radiograph of the patient showing good implant position and spondylolisthesis reduction at 2 days. (C) Photograph of the lower back wounds of FE-TLIF. (D, E, F) Follow-up MRI T2WI at 6 weeks showed good decompression of the L4-L5 neural structure. Please click here to view a larger version of this figure.
| Parameters | Value |
| Patients number | 10 |
| Age (year) | 67.0 ± 9.27 (52–82) |
| Preoperative pain score on a visualized analog scale | 7.2 ± 1.14 |
| Postoperative pain score on a visualized analog scale | 1.3 ± 1.34 |
| Off bed activity | Day 1.4 ± 0.52 |
| Independent ambulation | Day 2.3 ± 0.82 |
| Drainage tube removal | Day 2.6 ± 0.52 |
| Discharge from hospital | Day 4.1 ± 1.60 |
| Operation duration (minute) | 333.2 ± 47.25 (274–465) |
| Transfusion need (n, %) | 1 (10%) |
| Result of operation based MacNab criteria (n, %) | |
| Excellent | 6 (60%) |
| Good | 3 (30%) |
| Fair | 1 (10%) |
| Poor | 0 |
| Complication | |
| Epidural hematoma | 0 |
| Durotomy | 0 |
| Root injury | 0 |
| Screw misplacement | 0 |
Table 1: Representative result of the good recovery of FE-TLIF.
Discussion
The FE-TLIF procedure has never been straightforward, even as current trends in spine surgery continue to shift toward minimally invasive approaches17. This study is among the first to provide a detailed video demonstration of the FE-TLIF technique. Zhao et al.14 reported that it took 25 cases to reduce operation time and length of hospital stay. Meanwhile, Ali et al.18 found that the learning curve primarily affected certain surgical metrics but did not impact clinical outcomes in endoscopic discectomy. These findings demonstrate that FE-TLIF can be effectively learned and yield favorable clinical outcomes with proper training. The author has trained with several international experts, participated in multiple live and cadaver courses, and performed over 500 endoscopic spine surgeries. In this study, we share the ideal protocol for single-level FE-TLIF to help beginners overcome the learning curve.
Beginners often struggle to become proficient in performing FE-TLIF due to equipment limitations, as standard surgical instruments commonly used in traditional fusion surgery and UBE-TLIF cannot be utilized3,19. Specifically, the design of instruments for discectomy and cartilage endplate preparation varies significantly among brands and depends on surgeon preferences12. To address this challenge, we used several instruments typical of conventional TLIF procedures, including an endplate shaver, a funnel, and a polyetheretherketone (PEEK) non-expandable cage. Du et al.19 also reported that using familiar surgical instruments can reduce costs and improve operational efficiency. Some critics argue that disc shavers and scrapers might be overly aggressive and risk causing endplate injury3. The approach presented emphasizes two key points: first, familiarity with the instruments enhances procedural safety; second, manual force and the condition of the endplate can be carefully monitored through endoscopic visualization after each use.
The outside-in technique with a trephine proved effective for IAP resection while also preserving autologous bones. Kim et al.10 compared the outside-in and inside-out techniques for IAP removal and found the former to be more efficient. Similarly, Du et al.19 reported that the visual trephine enables an efficient and convenient partial facetectomy. While using an endoscopic drill or diamond burr for this step is also effective4,6,9,10,11, these methods may yield less autologous bone for grafting, which is a critical factor in achieving radiographic fusion20,21. For this reason, we advocate for the use of the visual trephine and the outside-in technique to maximize the preservation of autologous bone. Ensure there is no confusion regarding the term outside-in technique in decompression15, which refers to bony decompression performed before the en bloc removal of the ligamentum flavum.
Visualized nerve protection during adjacent procedures represents a critical advancement in the FE-TLIF protocol. In the technique reported by Kim and Wu et al.7, a specialized cage glider was used following endplate preparation, with subsequent bone grafting and cage insertion guided by fluoroscopy. The authors claimed that neural structures were completely safeguarded when appropriately shielded by the specialized instrument. Nevertheless, the same study group reported a 6% complication rate involving traversing root injuries among 35 patients undergoing FE-TLIF. The risk of injury increases in cases of more severe disc space collapse9. Chang et al.13 reported an average distance of 3.3 mm between the cage entry point and the traversing root in FE-TLIF procedures, recommending variations in cage glider strategies to address this challenge. In this protocol, a working tube with a long lip was used to retract the ipsilateral traversing root, held steadily by hand, allowing for safe and visualized nerve protection before cage insertion.
Still, the limitation of surgical equipment existed, as some of the instruments, including the trephine and working tube for reamer in step 3.1, are available only upon request. However, most of the other instruments are familiar to surgeons who have completed spine fellowship training or basic endoscopic spine surgery courses. Additionally, there may be bias in the study, as our case series, with a limited number of cases, has not encountered complications such as hematoma, nerve root injury, screw misplacement, or loosening. Also, in some cases of unilateral laminotomy for bilateral decompression, anatomical reduction of spondylolisthesis is not always necessary. The clinical significance relies on successful nerve decompression but not radiographic reduction. The radiographic lumbar fusion rate for FE-TLIF has been reported to range from 97.5% to 100% when using a combination of autogenous and allogenous bone grafts19,22. Similar to our approach with autogenous bone graft and artificial bone substitute, Tsai and Liu et al.23 reported a 100% fusion rate for FE-LIF. However, in our small case series with a limited number of patients, the fusion rate is not yet available.
In conclusion, this study offers valuable educational video footage detailing each step of the FE-TLIF procedure. The protocol incorporates the use of several instruments common to conventional TLIF procedures, an efficient outside-in technique with a trephine for IAP resection, endoscopic visualization for endplate preparation, and nerve protection. With proper education, FE-TLIF can be effectively learned, leading to favorable clinical outcomes.
Disclosures
All authors disclosed no conflict of interest.
Acknowledgements
Special thanks to Louis Lai for recording the procedure using his smartphone and a tripod. This study received no external funding.
Materials
| Name | Company | Catalog Number | Comments |
| 10mm shaver | REBORN | 420-0710 | |
| 10mm trial | REBORN | 420-0610A | |
| 11mm shaver | REBORN | 420-0711 | |
| 11mm trial | REBORN | 420-0611A | |
| 12mm shaver | REBORN | 420-0712 | |
| 12mm trial | REBORN | 420-0612A | |
| 13mm shaver | REBORN | 420-0713 | |
| 13mm trial | REBORN | 420-0613A | |
| 14mm shaver | REBORN | 420-0714 | |
| 14mm trial | REBORN | 420-0614A | |
| 8mm shaver | REBORN | 420-0708 | |
| 8mm trial | REBORN | 420-0608A | |
| 9mm shaver | REBORN | 420-0709 | |
| 9mm trial | REBORN | 420-0609A | |
| Biopsy Forceps, Blakesley | JOIMAX | BFS323061 | WL 320 mm / OD 3.5 mm / JL 6.0 mm |
| Biopsy Forceps, Spoon | JOIMAX | THF322541 | WL 320 mm / OD 2.5 mm / JL 4.0 mm |
| Biopsy Forceps, Spoon, angled | JOIMAX | THF322041 | WL 320 mm / OD 2.0 mm / JL 4.0 mm / 45° |
| Bone graft impactor | REBORN | 410-1216 | |
| Dissector | JOIMAX | JDA273515 | WL 275 mm / OD 3.5 mm |
| Dissector, angled | JOIMAX | ON REQUEST | WL 280 mm / OD 3.5 mm / 40° |
| Distractor 10mm | REBORN | 420-1610 | |
| Distractor 11mm | REBORN | 420-1611 | |
| Distractor 12mm | REBORN | 420-1612 | |
| Distractor 13mm | REBORN | 420-1613 | |
| Distractor 14mm | REBORN | 420-1614 | |
| Distractor 8mm | REBORN | 420-1608 | |
| Distractor 9mm | REBORN | 420-1609 | |
| Endo-Flexprobe | JOIMAX | TEFP32020 | L 320 mm / OD 2.0 mm |
| Endo-Flexprobe Handle | JOIMAX | TEFH45025 | L 450 mm / OD 2.5 mm |
| Endo-Kerrison-Pistol Handle | JOIMAX | EKH550000 | OD 5.5 mm |
| Endo-Kerrison-Shaft | JOIMAX | EKS24551540 | WL 240 mm / OD 5.5 mm / F 1.5 mm / 40° |
| Endo-Kerrison-Shaft | JOIMAX | EKS24553040 | WL 240 mm / OD 5.5 mm / F 3.0 mm / 40° |
| Funnel for bone graft | REBORN | 410-1215 | |
| Grasper Forceps | JOIMAX | THG323555 | WL 320 mm / OD 3.5 mm / JL 5.5 mm |
| Guiding Rod, conical | JOIMAX | GRD226315 | L 225 mm / OD 6.3 mm |
| Guiding Tube, conical, red | JOIMAX | GTC177010 | L 165 mm / ID 7 mm / OD 10 mm |
| Guiding Tube, conical, violet | JOIMAX | GTC151510 | L 175 mm / ID 10 mm / OD 15 mm |
| Hook Scissor | JOIMAX | JHS243545 | WL 240 mm / OD 3.5 mm / JL 4.5 mm |
| Laminoscope | JOIMAX | LS1006125O | WL 125 mm / OD 10.0 mm / 15° / WChD 6.0 mm / 2x IC 2.0 mm |
| Lumbar implant impactor | REBORN | 420-3303 | |
| Nerve Hook | JOIMAX | TNH322533 | L 320 mm / OD 2.5 mm / JL 3.3 mm |
| Osteotome | JOIMAX | ON REQUEST | WL 260 mm / OD 5.5 mm |
| Peek lumbar 11#-14# implant driver | REBORN | 420-1715 | |
| Peek lumbar 8#-10# implant driver | REBORN | 420-1714 | |
| Reamer Push-Ejector | JOIMAX | RPE280600 | L 280 mm / OD 6.0 mm |
| Semi-Flexible Grasper Forceps, curved, up-biting | JOIMAX | TFG322522U | WL 320 mm / OD 2.5 mm / Helix |
| Slap hammer | REBORN | 420-0401B | |
| T-handle | REBORN | 460-0101A | |
| Working Reamer Tube, put endoscope into for trephining | JOIMAX | ON REQUEST | L 125 mm / ID 10.2 mm / OD 11.2 mm |
| Working Tube with Handle | JOIMAX | ON REQUEST | L 125 mm / ID 10.2 mm / OD 11.2 mm |
| Working Tube with Handle, long Lip | JOIMAX | WTS121602 | L 132 mm / ID 15 mm / OD 16 mm |
| Working Tube, use with reamer | JOIMAX | ON REQUEST | L 120 mm / ID 11.5 mm / OD 12.5 mm |
References
- Ricart, P. H., Gandhi, S. D., Geisinger, J., Baker, K., Park, D. K. Clinical and ct analysis of lumbar spine arthrodesis: Beta-tricalcium phosphate versus demineralized bone matrix. J Am Acad Orthop Surg Glob Res Rev. 2 (9), e024(2018).
- Pholprajug, P., Kotheeranurak, V., Liu, Y., Kim, J. S. The endoscopic lumbar interbody fusion: A narrative review, and future perspective. Neurospine. 20 (4), 1224-1245 (2023).
- Pao, J. L. Biportal endoscopic transforaminal lumbar interbody fusion using double cages: Surgical techniques and treatment outcomes. Neurospine. 20 (1), 80-91 (2023).
- Nakajima, Y., Dezawa, A., Lim, K. T., Wu, P. H. Full-endoscopic posterior lumbar interbody fusion: A review and technical note. World Neurosurg. 189, 418-427.e3 (2024).
- Heo, D. H., Lee, D. C., Kim, H. S., Park, C. K., Chung, H. Clinical results and complications of endoscopic lumbar interbody fusion for lumbar degenerative disease: A meta-analysis. World Neurosurg. 145, 396-404 (2021).
- Kim, H. S., Wu, P. H., Sairyo, K., Jang, I. T. A narrative review of uniportal endoscopic lumbar interbody fusion: Comparison of uniportal facet-preserving trans-kambin endoscopic fusion and uniportal facet-sacrificing posterolateral transforaminal lumbar interbody fusion. Int J Spine Surg. 15 (suppl 3), S72-S83 (2021).
- Kim, H. S., Wu, P. H., Jang, I. T. Technical note on uniportal full endoscopic posterolateral approach transforaminal lumbar interbody fusion with reduction for grade 2 spondylolisthesis. Interdiscipl Neurosurg. 21, 100712(2020).
- Park, M. K., Park, S. A., Son, S. K., Park, W. W., Choi, S. H. Clinical and radiological outcomes of unilateral biportal endoscopic lumbar interbody fusion (ulif) compared with conventional posterior lumbar interbody fusion (plif): 1-year follow-up. Neurosurg Rev. 42 (3), 753-761 (2019).
- Wu, P. H., et al. Uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion with endoscopic disc drilling preparation technique for symptomatic foraminal stenosis secondary to severe collapsed disc space: A clinical and computer tomographic study with technical note. Brain Sci. 10 (6), 373(2020).
- Kim, H. S., et al. Evaluation of two methods (inside-out/outside-in) inferior articular process resection for uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion: Technical note. Brain Sci. 11 (9), 1169(2021).
- Wu, P. H., et al. Prospective cohort study with a 2-year follow-up of clinical results, fusion rate, and muscle bulk for uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion. Asian Spine J. 17 (2), 373-381 (2023).
- Chien, K. T., et al. Optimizing disc and cartilage endplate preparation in full-endoscopic lumbar interbody fusion: An in-depth exploration of surgical instruments with a technique note and narrative review. World Neurosurg. 189, 228-247 (2024).
- Hsu, Y. C., et al. How to prevent nerve root injury in uniportal full endoscopic lumbar fusion surgery? Insights from a cadaveric anatomic study with simulation surgery. Spine. 49 (18), 1301-1310 (2024).
- Zhao, T., Dai, Z., Zhang, J., Huang, Y., Shao, H. Determining the learning curve for percutaneous endoscopic lumbar interbody fusion for lumbar degenerative diseases. J Ortho Surg Res. 18 (1), 193(2023).
- Kim, H. S., Wu, P. H., Jang, I. T. Lumbar endoscopic unilateral laminotomy for bilateral decompression outside-in approach: A proctorship guideline with 12 steps of effectiveness and safety. Neurospine. 17 (Suppl 1), S99-S109 (2020).
- Macnab, I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 53 (5), 891-903 (1971).
- Antonacci, C. L., et al. A narrative review of endoscopic spine surgery: History, indications, uses, and future directions. J Spine Surg. 10 (2), 295-304 (2024).
- Ali, R., et al. Impact of the learning curve of percutaneous endoscopic lumbar discectomy on clinical outcomes: A systematic review. Interdiscip Neurosurg. 32, 101738(2023).
- Du, Y., et al. Full endoscopic posterolateral transarticular lumbar interbody fusion using transparent plastic working tubes: Technical note and preliminary clinical results. Front Surg. 9, 884794(2022).
- Ito, Z., et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (plif): A multicenter study. Eur Spine J. 22 (5), 1158-1163 (2013).
- Yoo, J. S., Min, S. H., Yoon, S. H. Fusion rate according to mixture ratio and volumes of bone graft in minimally invasive transforaminal lumbar interbody fusion: Minimum 2-year follow-up. Eur J Orthop Surg Traumatol. 25 (Suppl 1), S183-S189 (2015).
- Wang, J. C., Cao, Z., Li, Z. Z., Zhao, H. L., Hou, S. X. Full-endoscopic lumbar interbody fusion versus minimally invasive transforaminal lumbar interbody fusion with a tubular retractor system: A retrospective controlled study. World Neurosurg. 165, e457-e468 (2022).
- Tsai, P. C., et al. The novel kambin torpedo full-endoscopic lumbar interbody fusion technique: A case series. Eur Spine J. 33 (2), 417-428 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved