Method Article
Evaluation and Quantification of Micro Epithelial Gaps in the Colonic Mucosa using Immunofluorescence Staining
* These authors contributed equally
In This Article
Summary
Here, we describe a new method to visualize the specific location of where transcellular and paracellular permeability is enhanced in the inflamed colonic mucosa. In this assay, we apply a 10 kDa fluorescent dye conjugated to a lysine fixable dextran to visualize high permeability regions (HPR) in the colonic mucosa.
Abstract
Epithelial cells lining the intestinal mucosa create a physical barrier that separates the luminal content from the interstitium. Epithelial barrier impairment has been associated with the development of various pathologies such as inflammatory bowel diseases (IBD). In the inflamed mucosa, superficial erosions or micro-erosions that corrupt epithelial monolayers correspond to sites of high permeability. Several mechanisms have been implicated in the formation of micro-erosions including cell shedding and apoptosis. These micro-erosions often represent microscopic epithelial gaps randomly distributed in the colon. Visualization and quantification of those epithelial gaps has emerged as an important tool to investigate intestinal epithelial barrier function. Here, we describe a new method to visualize the specific location of where transcellular and paracellular permeability is enhanced in the inflamed colonic mucosa. In this assay, we apply a 10 kDa fluorescent dye conjugated to a lysine fixable dextran to visualize high permeability regions (HPR) in the colonic mucosa. Additional use of cell death markers revealed that HPR encompass apoptotic foci where epithelial extrusion/shedding occurs. The protocol described here provides a simple but effective approach to visualize and quantify micro-erosions in the intestine, which is a very useful tool in disease models, in which the intestinal epithelial barrier is compromised.
Introduction
The gastrointestinal (GI) mucosa creates a physical barrier that separates the extracellular environment and the internal host milieu, and is involved in the absorption of nutrients, water and electrolytes. The intestinal barrier encompasses a mucus layer constituted of glycoproteins, a monolayer of epithelial cells, and the underlying lamina propria are immune and stromal cells reside. Intestinal epithelial cells forming the physical barrier are linked together by different protein complexes, which includes the adherens junction (AJ), the tight junction (TJ) and the desmosomes (DMs). Impairment in the epithelial barrier function augments intestinal permeability and allows the translocation of harmful substances and pathogens from the lumen to the interstitium1. There is an increasing number of illnesses where the epithelial barrier is compromised, such as the inflammatory bowel diseases (IBD) like Crohn's disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC). The incidence of IBD is increasing worldwide, with a prevalence approaching 0.5% in the West. Although the causes of IBD are unclear, the excessive immune/inflammatory response triggered in the gut wall directly contributes to the epithelial barrier disruption by limiting the reestablishment of intestinal epithelial homeostasis2,3,4. In addition, patients with long-standing colonic inflammation are at high risk of developing colorectal cancer (CRC)5. Other pathologies associated with intestinal epithelial barrier disruption are irritable bowel syndrome, obesity, celiac disease, non-celiac gluten sensitivity, and food allergies6. For these reasons, there is an urgent need for the development of experimental approaches that allow analysis of the integrity of the intestinal epithelial barrier in animal models mimicking the pathogenesis occurring in humans.
Here, we evaluated the gastrointestinal passive paracellular and the transcellular permeability associated to an inflammatory process in the colonic epithelium using a simple technique. To investigate the transmural flow of macromolecules, we measured the passive diffusion of FITC-dextran (4 kDa) and RITC-dextran (10 kDa) in colonic sacs ex vivo. Furthermore, by injecting a fluorescent 10 kDa lysine-fixable dextran into the lumen of the intestine sacs, we specifically identified the areas with high permeability in the inflamed mucosa. The use of apoptosis markers and antibodies against AJ proteins allowed us to demonstrate that high permeability areas in the inflamed mucosa correspond to specific regions where epithelial cells undergo apoptosis and cell-cell junctions are disrupted. This new technique can be used to evaluate the integrity of the epithelium in any model where the intestinal epithelial barrier is compromised.
Protocol
All procedures were reviewed and approved by the CINVESTAV Institutional Committee for Care and Use of Laboratory Animals (CICUAL).
1. Preparation of materials and reagents
- Pre-warm Hartmann's solution (130 mM NaCl, 28 mM lactate, 4 mM KCl, 1.5 mM CaCl2) to 37 °C while bubbling with 95% O2/5% CO2. Maintain physiological pH (7.4) for the solution.
- For analyzing the passive paracellular permeability, prepare a working solution by dissolving 1 mg/mL of FITC-Dextran (4 kDa) and 1 mg/mL of RITC-Dextran (10 kDa) in pre-warmed Hartmann´s solution.
- Prepare a 4 µg/mL solution of Alexa Fluor647 Fixable-Dextran (10 kDa) in Hartmann's solution. Store working solutions in a 15 mL conical tube and protect from the light until use.
NOTE: 300 µL of working solution per colon will be necessary. - Prepare a surgical suture by cutting two 5 cm sections for each large intestine. Loop the sutures into an unclosed knot.
2. Dissection and preparation of the gastrointestinal trac
- Withhold solid food for 6 hours before euthanizing the mice. Provide ad libitum drinking water.
NOTE: If possible, place the mice on nutrient gel supplements (purified Water, Molasses, Pumpkin, Corn Syrup, Sunflower Seeds, Wheat Protein, Vegetable Oil, Food Acid, Hydrocolloids, Electrolytes, Corn Fiber, NIH-31M Mineral Mix, NIH-31M Vitamin Mix). - Euthanize mice in a CO2 chamber followed by cervical dislocation in accordance with institutional ethics protocols.
- Sterilize the abdomen and thorax with 70% ethanol.
- Using a pair of scissors, make an incision in the middle of the abdomen and expose the peritoneal cavity.
- For orientation purposes, separate and dissect the large intestine by cutting at the end of the small intestine (end portion of the ileum) right before the cecum and then at the anal verge. Use surgical forceps to gently remove the mesentery and place the colon in Hartmann's solution.
- Importantly, in order to maintain consistency between animals, identify similar sections and use to evaluate permeability. Using regions close to the cecum is highly recommendable.
- Use an insulin syringe equipped with a blunted plastic cannula to gently flush the luminal content present in the colon. If the stool is firm, carefully push with the help of blunt forceps. After the feces have been removed, wash 3 times with 400 µL of Hartmann's solution.
- Tie the proximal region (closest one to the cecum) and place a pre-tied suture-loop in the distal region of the colon. With the help of a syringe equipped with a blunted plastic cannula fill the intestinal sac with the solution containing the desire probe. Carefully remove the plastic cannula and tie the loop in the distal region.
- Place the intestinal sac in a 15 mL conical tube with 6 mL of Hartmann's solution and incubate for 1 h to evaluate the passive paracellular flow of FITC/RITC-Dextran or 30 min to analyze the flow of the Alexa Fluor Fixable-Dextran.
- Maintain the conical tubes containing the intestinal sacs at 37 °C with 5% CO2 and protect from light.
- To measure the passive permeability using FITC/RITC-Dextran. At 0 and 60 min, take a 100 µL sample from the conical tube and transfer to a 96 well plate. Add back 100 µL of fresh media to replace the volume lost.
- Measure samples and standards for FITC/RITC on a fluorescent plate reader (FITC excitation/emission: 495 nm/519 nm; RITC excitation/emission: 570/595 nm).
- To measure the passive permeability using Alexa Fluor 647 Fixable-Dextran, remove the intestines, cut close to the surgical tie knot, and cut the intestine to expose the lumen to remove the solution with the probe. Wash the lumen of the intestine 2 times with cold Hartmann's solution.
- Place the tissue in a tissue-mold previously filled with Optimal Cutting Temperature compound (O.C.T.). Orient the tissue vertically or horizontally according to the side to be sectioned. Store the samples at -80 °C.
3. Immunofluorescent staining
- Fix frozen sections of 20 micrometers with 3.7% paraformaldehyde (PFA) for 20 min at room temperature and then wash 3 times with cold phosphate buffered saline (PBS; 37 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2 PO4).
NOTE: Vertical sections from intestines tend to come off if the washes are very strong. - Permeabilize with 0.2% TX-100/PBS for 12 min at room temperature and then wash 3 times with cold PBS.
- Block with 0.2% BSA/PBS for 1 hour at room temperature.
- Dilute the primary antibody in blocking solution and incubate for 1 h at room temperature. Wash 3 times with cold PBS.
- Incubate for 1 h with secondary antibodies in blocking solution. Wash 3 times with cold PBS.
- Apply mounting medium to the cuts and seal with a coverslip. The slides can be stored for up to 3 months at -20 °C.
Results
In the inflamed mucosa, superficial erosions or microerosions compromise the integrity of the epithelial cell monolayer and represent sites of high permeability7,8. To assess such possibilities, we analyzed the passive permeability in the inflamed colonic mucosa in a dextran sodium sulfate colitis murine model. In brief, for 5 days, C57BL/6J mice received 2.5% DSS (w/v, 40-50 kDa) dissolved in drinking water. This model is characterized by inducing epithelial cell damage and epithelial barrier dysfunction in the large intestine, which in turns allows the entrance of luminal antigens into the lamina propria. Such a process results in stimulation of the immune system, leading to the secretion of proinflammatory cytokines and chemokines that further enhance the epithelial damage9. As shown in Figure 1A, the mice exposed to DSS exhibited a ~12% body weight loss after 5 days of treatment. Fecal blood and diarrhea were also detected in all DSS-treated mice. Additionally, colitic mice displayed a reduction in colon length that was statistically significant (Figure 1B). Thus, the disease activity index (DAI) encompassing animal weight loss, stool consistency, diarrhea, occult blood, and gross rectal bleeding increased after 5 days of DSS treatment (Figure 1C). Histopathological analysis revealed the presence of epithelial erosions, ulcerations, immune infiltrate and edema in the colonic mucosa of colitic mice (Figure 2).
Epithelial barrier disruption, including intestinal epithelial cell apoptosis and dysfunctional intercellular junctions, have been accounted for the increase in passive permeability in the inflamed mucosa10,11. Therefore, we indirectly measured the permeability in the colonic mucosa by monitoring the passive flow of fluorescently labelled probes (FITC and RITC-dextran). As shown in Figure 3A, the inflammatory process induced after DSS administration augmented transmural permeability, as shown by the increase in the passive flow of FITC-dextran (4 kDa) and RITC-dextran (10 kDa). In addition, as shown in Figure 3B, Western blot analysis confirmed the increase of apoptosis in the colonic mucosa of DSS-treated mice as shown by the presence of active caspase 3 (Acasp-3). Thus, the data strongly suggest that during inflammation the apoptotic process triggered in the inflamed mucosa contribute to the disruption of the epithelial barrier function.
Next, we assessed the localization of the areas with high permeability in the inflamed mucosa by analyzing the flow of a fluorescent fixable lysine dextran of 10 kDa. We used a 10 kDa molecule to restrict the flow only to highly permeable regions with high pore size. In control mice, the fixable dextran was retained at the apical surface of the colonic crypts (white arrows) and was delimited by the tight bundle of actin filaments at the brush border microvillus (Figure 4A). However, in colitic mice, the fixable dextran permeated into the cytosol of colonic epithelial cells demonstrating that inflammation induces active transcellular flow (asterisks). Upon further analysis we observed the presence of fixable dextran in the interstitium in colitic mice (arrows) but not in control animals, indicating a net flow from the luminal to the interstitial side (Figure 4B). In the leaky area, the cytosolic fixable dextran was clearly incorporated in epithelial cells grouped in microregions at the luminal side and those cells were positive for the apoptotic marker Acasp-3 (Figure 4C). Importantly, also at the surface in the areas of high permeability where the epithelial cells undergo apoptosis (apoptotic foci) we noticed an aberrant distribution for E-cadherin (Figure 4C, Arrows) and effect not observed in control tissue. Additionally, the results showed that the fixable dextran was internalized in anoikis cells present at the lumen, and some of those cells displayed a faint cytosolic staining for E-cadherin (arrowhead) (Figure 4D). Contrary to E-cadherin the intestinal epithelial cells enriched with cytosolic fixable dextran shown a clear staining for ZO-1 at the TJ (Figure 4E), no staining for ZO-1 was detected in detached cell (Data not shown). Thus, in conclusion we demonstrated that fixable dextran is a good tool to identify sites of high permeability in the gut and is internalized in apoptotic and anoikis cells.

Figure 1. Induction of acute colitis using DSS. (A) Daily changes in body weight of C57BL/6J mice treated with 2.5% DSS for 5 days. Body weight is expressed as a percentage of the body weight at day 0. (B) Macroscopic changes observed in the colons of mice treated with DSS for 5 days. Upper panel. Representative image of colons harvested from control and colitic mice. Lower panel. Shortening of the colon length was observed in DSS-treated mice. (C) Clinical score (Disease Activity Index; DAI) over 5 days following water (controls; n 5) or 2.5% dextran sodium sulphate (DSS) oral treatment. The DAI encompassing body weight loss, consistency stool and blood in feces was increased in colitic mice. Data are presented as the means ± SEM (n=5). P values were calculated using a two-way ANOVA with Sidak´s post hoc test (A) and Student's t test (B, C). **p < 0.01; ***p < 0.001. Please click here to view a larger version of this figure.
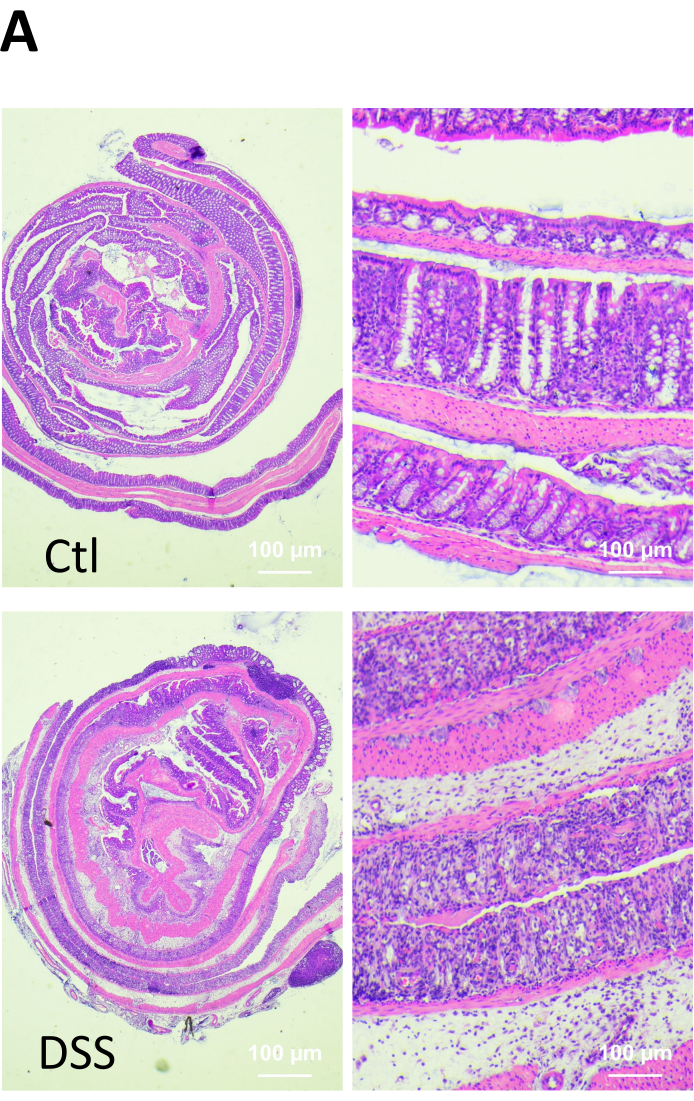
Figure 2. Microscopic changes in the colonic mucosa of healthy and DSS-treated mice. At the end of the experiment, the animals were euthanized, and the tissue specimens were fixed, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Infiltration of inflammatory cells (arrow), ulcerations (arrowhead) and loss of cryptal architecture (erosion, e) were observed in the colon of DSS-treated mice. Please click here to view a larger version of this figure.

Figure 3. Passive paracellular transport of Fluorescent labeled Dextran is enhanced during colitis. (A) Analysis of passive paracellular transport of fluorescent labeled dextran in intestinal sacs of healthy and colitic mice. Intestinal sacs were prepared from colons harvested from age matched C57BL/6J mice treated with water or 2.5% DSS for 5 days. Sacs were loaded with a solution containing 1 mg/ml of FITC-dextran-4 kDa (MW 4,400 Da) and 1 mg/ml of RITC-dextran-10 kDa (MW 10,000 Da). Passive paracellular flux of the fluorescent probes was measured after 60 min. Paracellular permeability is augmented after colitis induction. (B) Evaluation of Active Caspase-3 (Acasp-3) in the colonic mucosa of DSS-treated mice. Whole cell lysates were prepared from colonic samples harvested from age matched C57BL/6J mice treated with water or 2.5% DSS for 5 days. 30 µg of protein were loaded and separated in 12% SDS-polyacrylamide gels, transferred to a nitrocellulose membrane and western blotted for Acasp-3. GAPDH was used as loading control. The presence of active Caspase-3 is augmented after colitis induction. n = 5. Data are presented as the means ± SEM (n=5). P values were calculated using a two-way ANOVA with Sidak´s post hoc test (A) and Mann- Whitney test (B). *p < 0.05 ***p < 0.001. Please click here to view a larger version of this figure.
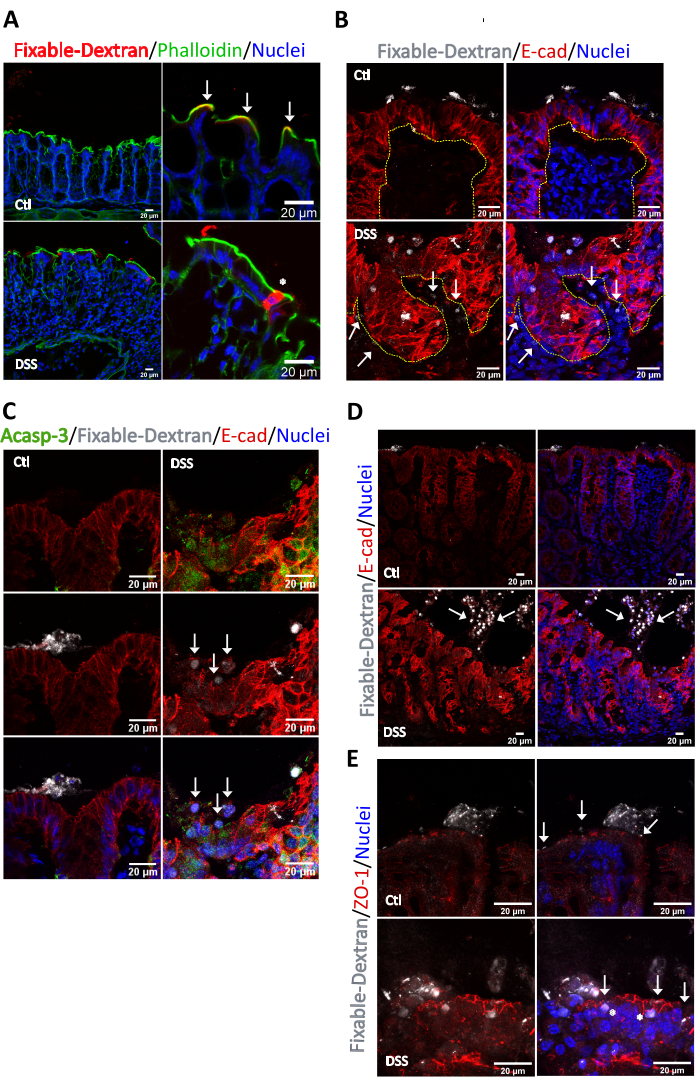
Figure 4. Identification of permeability sites in the colonic mucosa of healthy and DSS-treated mice. Intestinal sacs were prepared from colons harvested from age matched C57BL/6J mice treated with water or 2.5% DSS for 6 days. Sacs were loaded with a solution containing 4 µg/mL of fixable Alexa Fluor 647 dextran, 10 kDa. Passive paracellular flux of the fluorescent probe was stopped after 30 min. The colonic sacs were fixed, embedded in OCT, sectioned and stained for immunofluorescence. (A) In control conditions, the fixable dextran (red) was detected in close apposition with (arrows) the apical actin ring (green) limiting the apical surface of the colonocytes facing the luminal side. After DSS treatment, the continuity of the apical actin ring was disrupted (asterisk). The fixable dextran permeated into the cytosol of surface epithelial cells. (B) Analysis of fixable dextran and E-cadherin at sites of high permeability. In control conditions the fixable dextran was retained at the luminal side. In the colitic mucosa, fixable dextran was detected at the cytosol of epithelial cells lining the crypt surface and in several regions of the interstitium (Arrows). Epithelial cells that incorporated fixable dextran were located at the crypt surface and showed an aberrant distribution of E-cadherin. Dash line delimits the crypt. n = 5. (C) Analysis of Active caspase-3 and E-cadherin at the sites of high permeability identified with the fixable dextran in the colonic mucosa of colitic mice. Active caspase-3 (green) was enriched at the sites of high permeability. Arrows mark epithelial cells with aberrant staining for E-cadherin that were present at the crypt surface. n = 5. (D) Analysis of fixable dextran and E-cadherin. In control conditions the fixable dextran was retained at the luminal side. In the colitic mucosa, fixable dextran was detected at the cytosol of detached epithelial cells located at the intestinal lumen (Arrows). Epithelial cells marked with fixable dextran showed a clear presence of cytosolic E-cadherin. n = 5. (E) Analysis of fixable dextran and ZO-1. In control conditions the fixable dextran was retained at the luminal side above the TJ (marked by ZO-1). In the colitic mucosa, fixable dextran was detected at the cytosol of detached epithelial cells and in epithelial cells at the crypt surface (asterisks). ZO-1 localized at the TJ in control and colitic conditions (Arrows). n = 5. Please click here to view a larger version of this figure.
Discussion
Epithelial homeostasis resulting from balancing cell proliferation and epithelial apoptosis maintains a proper and functional intestinal barrier. Many clinical disorders, such as IBD, are accompanied or characterized by alterations in intestinal permeability, inflammation of the mucosa and disruption of the epithelial homeostasis1. The interplay between those processes is still highly controversial. Therefore, the development of new research approaches to properly investigate those processes is an important subject in the field. There are several published protocols available for indirectly studying the intestinal permeability during inflammation, such as the measurement of transepithelial electrical resistance in epithelial monolayers and quantification of hydrophobic fluorophore-labeled probes in intestinal organoids, mouse intestines and intestinal sacs10,11,12,13. Despite demonstrating that the uptake of a substance increases or decreases in the intestinal mucosa, those methods fall short in analyzing other important events that directly contribute to the changes in the permeability. For example, by failing to identify the specific areas where the integrity of the epithelium is compromised, those studies limit the understanding of the transport mechanisms or the biological functions responsible for affecting the intestinal permeability. Furthermore, given that the intestinal permeability may vary greatly by region, those studies could generate errors such as false positive results. Therefore, complementing those studies with non-quantitative site-specific assessments such as the one described here will allow to determine the exact region where the flow of substances occurs in the damaged epithelium. This new permeability assay not only allows a better understanding of the regional mucosal integrity in digestive diseases but also permits the study of the disrupted mechanisms contributing to such process.
The new technique described is a combination of two previously described techniques, the preparation of intestinal sacs to assess mucosal barrier function ex vivo12 and the use of fixable fluorescent tracers to analyze brain vascular permeability13. By using this technique, we observed that epithelial barrier impairment after IEC damage enhances the general flow of luminal antigens to the interstitial compartment. The use of a 10 kDa fixable dextran was chosen to specifically analyze the localization of highly permeable areas with a big pore size in the colitic mucosa. Therefore, the high permeability sites in the inflamed mucosa that were detected here encompass apoptotic foci where surface colonocytes undergo apoptosis or extrusion, a process known as anoikis. However, the use of probes of different sizes could be a useful tool to discriminate where the paracellular and transcellular flow is affected in the gut. Of note, cells undergoing apoptosis/anoikis always incorporated 10 kDa fixable dextran, making the technique useful to identify the areas where the plasma cell membranes of the epithelial cells are compromised. During anoikis, several cell-cell junction molecules are lost or redistributed, and the technique could also be helpful to identify those proteins. In fact, here we observed that E-cadherin is lost from the lateral plasma membrane or internalized in IEC labeled with fixable dextran. Thus, the results presented here demonstrate that the augment in the intestinal permeability during inflammation is affected by the disruption in the epithelial barrier continuity caused by IEC death (apoptosis/anoikis) and not only by disturbance in the function of the tight junctions14.
To our knowledge, a similar protocol employs confocal laser endomicroscopy in vivo to evaluate the presence of micro-erosions and epithelial damage in the intestine in real time. Such a technique identifies extruding cells in the inflamed and non-inflamed mucosa of the intestine. However, in the absence of specific markers to evaluate the mechanisms, the cell type and even the machinery involved in the disruption of the epithelial integrity this protocol is very limited8. In addition, using this technique we cannot measure the degree of epithelial perturbation, the integrity of the mucous gel layer, or the presence of subtle changes that may not be apparent. Also, the presence of regional changes in barrier function could be lost. Therefore, our protocol appears as a stronger option to investigate the epithelial barrier permeability in the intestinal mucosa. However, it must be taken in account that our method uses fixed tissue and therefore the observations correspond to postmortem or biopsied tissue.
In conclusion, the protocol described here provides a simple but effective approach to visualize and quantify microerosions and epithelial cell death (apoptosis/anoikis) in the intestine, which is a very useful tool in disease models in which the intestinal epithelial barrier is compromised.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The research was partially supported by the SEP-Conacyt grant (No.179 to NV/PND) and supported by the sectorial funding for research and education via the grant for Basic Science from Conacyt (No. A1-S-20887 to PND). We want to extend our gratitude to Norma Trejo, M.V.Z. Raúl Castro Luna, M.C. Leonel Martínez, Felipe Cruz Martínez, Victor Manuel García Gómez and M.V.Z. Ricardo Gaxiola Centeno for their help and technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Active Caspase-3 antibody (1:1000) | Cell signaling | 9664 | Cleaved caspase-3 (Asp175)(5AE1) Rabbit mAb |
| Alexa Fluor 488 anti rabbit (1:1000) | Invitrogen | A21206 | |
| Alexa Fluor 594 anti rat (1:1000) | Invitrogen | A21209 | |
| Confocal microscope (Leica TCS SP8x) | Leica | HyD detectors and White Light Laser | |
| E-Cadherin antibody (1:750) | Sigma | MABT26 | Rat monoclonal Delma-1 antibody |
| Ethanol 70% | Generic | ||
| Fixable-Dextran | Invitrogen | D22914 | Dextran, Alexa Fluor, 10,000 MW, anionic, fixable |
| FITC Dextran | Sigma | 46944 | Fluorescein isothiocyanate–dextran M. Wt. 4 kDa |
| Hartmann's Solution | PiSA | HT PiSA | |
| Incubator (AutoFlow NU-8500) | Nuaire | ||
| Microplate reader (Tecan Infinite 200 PRO) | Tecan | ||
| Nunc F96 MicroWell Black and White Polystyrene Plate | ThermoFisher Scientific | ||
| Paraformaldehyde | Sigma | P6148 | |
| Phalloidin (1:1000) | Invitrogen | A12380 | Alexa Fluor 568 Phalloidin |
| RITC Dextran | Sigma | R8881-100MG | Rhodamine B Isothiocyanate-Dextran. M. Wt. 10 kDa |
| Secondary antibodies (1:10000) | Jackson ImmunoResearch Laboratories | HRP-conjugated secondary antibodies | |
| Suture threads | Generic | Braided silk and braided polyester surgical sutures are prefered. | |
| ZO-1 (1:1000) | Invitrogen | 40-2200 | Rb anti-ZO-1 |
References
- König, J., et al. Human Intestinal Barrier Function in Health and Disease. Clinical and Translational Gastroenterology. 7 (10), 196 (2016).
- Gassler, N., et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. American Journal of Physiology-Gastrointestinal and Liver Physiology. 281 (1), 216-228 (2001).
- Negroni, A., Cucchiara, S., Stronati, L. Apoptosis, Necrosis, and Necroptosis in the Gut and Intestinal Homeostasis. Mediators of Inflammation. 2015, 250762 (2015).
- Nava, P., et al. Interferon-γ regulates intestinal epithelial homeostasis through converging β-catenin signaling pathways. Immunity. 32 (3), 392-402 (2010).
- Choi, C. -. H. R., Bakir, I. A., Hart, A. L., Graham, T. A. Clonal evolution of colorectal cancer in IBD. Nature Reviews Gastroenterology & Hepatology. 14 (4), 218-229 (2017).
- González-González, M., Díaz-Zepeda, C., Eyzaguirre-Velásquez, J., González-Arancibia, C., Bravo, J. A., Julio-Pieper, M. Investigating Gut Permeability in Animal Models of Disease. Frontiers in Physiology. 9, (2019).
- Poulsen, S. S., Pedersen, N. T., Jarnum, S. "Microerosions" in rectal biopsies in Crohn's disease. Scandinavian Journal of Gastroenterology. 19 (5), 607-612 (1984).
- Neumann, H., et al. Assessment of Crohn's disease activity by confocal laser endomicroscopy. Inflammatory Bowel Diseases. 18 (12), 2261-2269 (2012).
- Laroui, H., et al. Dextran Sodium Sulfate (DSS) Induces Colitis in Mice by Forming Nano-Lipocomplexes with Medium-Chain-Length Fatty Acids in the Colon. PLoS ONE. 7 (3), (2012).
- John, L. J., Fromm, M., Schulzke, J. -. D. Epithelial barriers in intestinal inflammation. Antioxidants & Redox Signaling. 15 (5), 1255-1270 (2011).
- Su, L., et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 145 (2), 407-415 (2013).
- Mateer, S. W., Cardona, J., Marks, E., Goggin, B. J., Hua, S., Keely, S. Ex Vivo Intestinal Sacs to Assess Mucosal Permeability in Models of Gastrointestinal Disease. Journal of Visualized Experiments: JoVE. (108), e53250 (2016).
- Devraj, K., Guérit, S., Macas, J., Reiss, Y. An In Vivo Blood-brain Barrier Permeability Assay in Mice Using Fluorescently Labeled Tracers. Journal of Visualized Experiments: JoVE. (132), e57038 (2018).
- Stamatovic, S. M., Johnson, A. M., Sladojevic, N., Keep, R. F., Andjelkovic, A. V. Endocytosis of tight junction proteins and the regulation of degradation and recycling. Annals of the New York Academy of Sciences. 1397 (1), 54-65 (2017).
- Srinivasan, B., et al. TEER Measurement Techniques for In Vitro Barrier Model Systems. Journal of Laboratory Automation. 20 (2), 107-126 (2015).
- Pearce, S. C., et al. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biology. 16 (1), 19 (2018).
- Laukoetter, M. G., et al. JAM-A regulates permeability and inflammation in the intestine in vivo. The Journal of Experimental Medicine. 204 (13), 3067-3076 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved