Electron Paramagnetic Resonance (EPR) Spectroscopy
Overview
Source: David C. Powers, Tamara M. Powers, Texas A&M
In this video, we will learn the basic principles behind Electron Paramagnetic Resonance (EPR). We will use EPR spectroscopy to study how dibutylhydroxy toluene (BHT) behaves as an antioxidant in the autoxidation of aliphatic aldehydes.
Principles
Fundamentals of EPR:
EPR is a spectroscopic technique that relies on similar physical phenomena as nuclear magnetic resonance (NMR) spectroscopy. While NMR measures nuclear spin transitions, EPR measures electron spin transitions. EPR is chiefly used to study paramagnetic molecules, which are molecules with unpaired electrons. Recall that an electron has a spin quantum number, s = ½, which has magnetic components ms = ½ and ms = -½. In the absence of a magnetic field, the energy of the two ms states are equivalent. However, in the presence of an applied magnetic field (B0), the magnetic moment of the electron aligns with the applied magnetic field and, as a result, the ms states become non-degenerate (Figure 1). The energy difference between the ms state is dependent on the strength of the magnetic field (Equation 1). This is called the Zeeman effect.
E = m2geμBB0 (Equation 1)
where ge is the g-factor, which is 2.0023 for a free-electron and µB is the Bohr magneton.
At a given magnetic field, B0, the energy difference between the two ms states is given by Equation 2.
ΔE = E½ — E-½ = geμBB0 = hυ (Equation 2)
An electron moves between the two ms states upon emission or absorption of a photon with energy ΔE =hυ. Equation 2 applies to a single, free-electron. However, similar to the way that the chemical shift in 1H NMR depends on the chemical environment of the H atom, electrons within molecules do not behave in the same way as an isolated electron. The electric field gradient of the molecule will influence the effective magnetic field, given by Equation 3.
Beff = B0(1 — σ) (Equation 3)
where σ is the effect of local fields, which can be a positive or negative value.
Plugging Equation 3 into Equation 2, we can define the g-factor for an unpaired electron in a given molecule as g = ge(1 - σ), which simplifies the overall equation to:
hυ = gμBB0 (Equation 4)
During the EPR experiment, the frequency is swept, most commonly in the microwave region ranging from 9,000-10,000 MHz, and the field is held constant at approximately 0.35 T, allowing for the calculation of g. Experimentally determining g using EPR provides information about the electronic structure of a paramagnetic molecule.
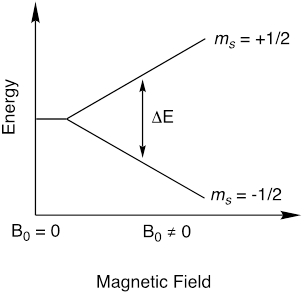
Figure 1. Splitting of magnetic moment states, ms, in the presence of a magnetic field.
Application of EPR:
In this experiment, we will use EPR spectroscopy to investigate the chemistry of antioxidants. O2, which comprises ~ 21% of the Earth's atmosphere, is a strong oxidant. Despite its potential to act as an oxidant, O2 is a ground state triplet and thus only reacts quite slowly with most organic molecules. One important, though often undesired, reaction mediated by O2 is autoxidation. In autoxidation chemistry, O2 initiates radical chain processes, which can quickly consume organic molecules. Figure 2 illustrates a common autoxidation, in which aldehydes are oxidized to carboxylic acids.
Preventing autoxidation chemistry is important to prevent decomposition of many common organic materials, such as plastics, and a large field has developed around identifying effective antioxidants to inhibit autoxidation. One mechanism by which antioxidants can function is by reacting with the radical intermediates to inhibit radical chain processes. Because radical species have unpaired spins, EPR is a valuable tool for understanding the chemistry of antioxidants. In this experiment, we will use EPR spectroscopy to explore the role of BHT as an antioxidant in the autoxidation of aliphatic aldehydes.
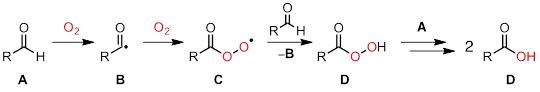
Figure 2. Aldehyde autoxidation proceeds via a radical chain mechanism.
Procedure
1. Autoxidation of Butyraldehyde
- Prepare a solution of butyraldehyde (100 mg) and CoCl2·6H2O (1 mg) in 1,2-dichloroethane (DCE) (4 mL) in a 20 mL scintillation vial. Add a magnetic stir bar and fit the vial with a rubber septum.
- Attach the barrel of a 1 mL plastic syringe to a short piece of rubber tubing. Insert the rubber tubing into a latex balloon and secure the balloon to the tube with a rubber band and electrical tape. Inflate a latex balloon with O2.
- Insert the needle of the O2 balloon into the reaction vial. Insert a second needle into the septum and purge the head-space of the reaction vessel with O2.
- Using a stir plate, stir the reaction at room temperature for 4 h under an O2 atmosphere.
- Concentrate the reaction mixture using a rotary evaporator and take an 1H NMR spectrum of the resulting oily residue in CDCl3.
2. Using BHT as an Antioxidant for the Autoxidation of Butyraldehyde
Set up two vials as described below. One will be used to analyze the product distribution and one will be used in step 3 for EPR spectroscopy.
- Prepare a solution of butyraldehyde (100 mg) and CoCl2·6H2O (1 mg) in DCE (4 mL) in a 20 mL scintillation vial. Add BHT (10 mg) to the solution. Add a magnetic stir bar and fit the vial with a rubber septum.
- Attach the barrel of a 1 mL plastic syringe to a short piece of rubber tubing. Insert the rubber tubing into a latex balloon and secure the balloon to the tube with a rubber band and electrical tape. Inflate a latex balloon with O2.
- Insert the needle of the O2 balloon into the reaction vial. Insert a second needle into the septum and purge the head-space of the reaction vessel with O2.
- Using a stir plate, stir the reaction at room temperature for 4 h under an O2 atmosphere.
- Concentrate the reaction mixture using a rotary evaporator and take an 1H NMR spectrum of the resulting oily residue in CDCl3.
3. Measuring EPR Spectra
- Turn the EPR spectrometer on and let the instrument warm up for 30 min. Set up an EPR acquisition with the following parameters: center field 3,345 G, sweep width 100 G, sweep time 55 s, time constant 10 ms, MW power 5 mW, modulation 100 kHz, and modulation amplitude 1 G.
- Measure an EPR spectrum of an empty EPR tube to ensure that there are no background signals from either the EPR tube or the instrument resonator.
- Prepare a solution of BHT in DCE in an N2-filled glove box. Transfer 0.5 mL of the solution to an EPR tube and measure the EPR spectrum of BHT using the acquisition parameters set up in step 3.1.
- Transfer 0.5 mL of the BHT-added reaction solution from Step 2 to an EPR tube and acquire and EPR spectrum using the acquisition parameters set up in Step 3.1.
Results
The autoxidation of butyraldehyde affords butyric acid. The 1H NMR spectrum obtained from the reaction carried out in Step 1 shows the lack of an aldehydic C-H resonance and the presence of the resonances expected of butyric acid. In contrast, the NMR obtained from the reaction mixture from step 2 (with added BHT) displays signals consistent with butyraldehyde, with no butyric acid present. From these data, we observe the butyraldehyde has served as an antioxidant in aldehyde autoxidation.
The role of BHT in inhibiting aldehyde autoxidation is illuminated by the EPR spectra obtained of BHT and of BHT added to the aldehyde autoxidation reaction. BHT is a diamagnetic organic molecule, meaning that there are no unpaired electrons. Accordingly, the EPR spectrum of BHT displays no signals. In contrast, the EPR spectrum of the autoxidation reaction in which BHT was added displays a strong four-lined pattern, consistent with an organic radical. This spectrum arises because the O-H bond of BHT is weak and in the presence of radicals generated during autoxidation, H-atom transfer from BHT quenches the radical chain mechanism and generates a stable O-centered radical.
Application and Summary
In this experiment, we explored the role of antioxidants in inhibiting autoxidation chemistry. We probed the mechanism of inhibition using EPR spectroscopy, which revealed that BHT serves as an antioxidant by quenching reactive radical intermediates via H-atom transfer.
Molecules with unpaired electrons can be challenging to characterize by NMR and thus EPR spectroscopy frequently provides useful and complementary information regarding these species. EPR spectroscopy is an experimental technique that is frequently used to detect and characterize organic radicals. In addition, paramagnetic inorganic complexes also frequently display EPR spectra that can be instructive for characterization. Experimental EPR spectra delineate the g-factor of the unpaired electron, which provides information about the electronic structure of the paramagnetic center. In addition, the nuclear spins of the nuclei with an unpaired electron as well as neighboring nuclei also influence the magnetic moment of an electron, giving rise to additional splitting of the ms states and multiple lines in the EPR spectrum. The resulting hyperfine and super-hyperfine coupling provides further information about the electronic structure of the molecule.
In addition to characterizing open-shell organic and inorganic species, the exquisite sensitivity of EPR spectroscopy is critical to application to bioinorganic systems, where the concentration of metal cofactors is low. EPR spectra are routinely used in bioinorganic chemistry to provide direct information about the structures and oxidation states of metal ions at the heart of enzymes.
Skip to...
Videos from this collection:

Now Playing
Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.4K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.5K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.3K Views

The Evans Method
Inorganic Chemistry
68.0K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.0K Views

Mössbauer Spectroscopy
Inorganic Chemistry
21.9K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.7K Views

Structure Of Ferrocene
Inorganic Chemistry
79.1K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.0K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.2K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.7K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.5K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved