Structure Of Ferrocene
Overview
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
In 1951, Kealy and Pauson reported to Nature the synthesis of a new organometallic compound, ferrocene.1 In their original report, Pauson suggested a structure for ferrocene in which the iron is singly bonded (sigma bonds) to one carbon atom of each cyclopentadiene ligand (Figure 1, Structure I).1,2,3 This initial report led to wide-spread interest in the structure of ferrocene, and many leading scientists participated in the structure elucidation of this interesting new molecule. Wilkinson and Woodward were quick to suggest an alternative formulization where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II).4 Here, we will synthesize ferrocene and decide, based on experimental data (IR and 1H NMR), which of these structures is observed. In addition, we will study the electrochemistry of ferrocene by collecting a cyclic voltammogram. In the course of this experiment, we introduce the 18-electron rule and discuss valence electron counting for transition metal complexes.
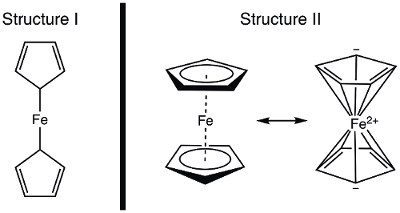
Figure 1. Two proposed structures of ferrocene.
Principles
18-electron Rule and Total Valence Electron Counting:
When drawing Lewis dot structures, it is important to remember the octet rule, which states that atoms of main group elements have 8 electrons in their valence shell. However, the octet rule does not hold for transition metals, which have nine valence orbitals (one s, three p, and five d orbitals) and can thus accommodate as many as 18 electrons. Therefore, an 18-electron rule applies for transition metal complexes. Like the octet rule, there are exceptions to the 18-electron rule, but in general, transition metal complexes with 18 valence electrons are considered highly stable compounds.
There are two methods that can be used to determine the total valence electron count of a transition metal complex: the ionic (charged) model and the covalent (neutral) model.5 Proper application of either method should give rise to the same total electron count. Both models use three ligand classifications called X-, L-, and Z-types. Each ligand type contributes a different number of electrons to the total electron count and is dependent on the method used. X-type ligands include anionic groups such as halides, hydroxide, and alkoxides. L-type ligands include lone-pair donors such as amines and phosphines. Finally, Z-type ligands are electron pair acceptors; therefore, Z-type ligands are neutral Lewis acids, such as BR3. We will consider the molecule Co(NH3)3Cl3 in order to demonstrate the two methods (Figure 2).

Figure 2. Electron counting example, Co(NH3)3Cl3.
Ionic Model:
First, consider the number of electrons contributed by the central atom. Co is in Group 9 of the periodic table and thus has 9 valence electrons. In the ionic model, the oxidation state of the metal needs to be considered. Since the oxidation state of the Co in Co(NH3)3Cl3 is +3, the total number of valence electrons contributed by the metal in the ionic model is 6 e− (Table 1). In the ionic model, both X- and L-type ligands donate 2 e− to the total electron count while Z-type ligands do not contribute any electrons. For the example Co(NH3)3Cl3, there are two ligand types present. Cl is an X-type ligand and NH3 is an L-type ligand. Using the ionic model, the total electron count is 18 e− (Table 1).
Table 1. e− counting of Co(NH3)3Cl3 using the ionic and covalent models
| Ligand/central atom | Ligand type | e− contribution
(ionic model) |
e− contribution
(covalent model) |
| Co | n/a | 6 e− | 9 e− |
| 3 NH3 | L | 3 x (2 e−) | 3 x (2 e−) |
| 3 Cl | X | 3 x (2 e−) | 3 x (1 e−) |
| Charge of ion | n/a* | − (0) | |
| Total e− count | 18 e− | 18 e− | |
*The charge of the metal-containing ion is NOT included in the total electron count using the ionic model. The charge is accounted for in the oxidation state of the metal (electrons contributed by the central atom).
Covalent Model:
For the covalent model, the e− contribution from the central atom is equal to the total number of valence e− for the neutral atom (in this case 9 e−). X-type ligands donate 1 e−, L-type ligands donate 2 e−, and Z-type ligands donate 0 e−. Finally, if the molecule is ionic, the charge of the ion containing the metal center needs to be subtracted from the metal plus the ligand electrons. Using the covalent model, the total electron count for Co(NH3)3Cl3 is also 18 e− (Table 1).
Electron Counting for Ferrocene and Ferrocenium Cation:
Using the covalent model, Fe has 8 e−. If we regard the bonding in ferrocene as that in Structure I, the cyclopentadiene (Cp) ligands are X-type donors and therefore each contribute 1 electron (Table 2). Thus, we would have 10 valence electrons. However, in Structure II, each Cp ring donates 5 electrons (L2X-type ligand), where we have two double bonds (each are 2-electron, L-type donors), and one X-type donor (the radical). This gives a total of 18 electrons (Table 2).
Table 2. Electron counting for ferrocene (Fc) and ferrocenium (Fc+) cation using the covalent model
| Ligand/ central atom | Structure I | Structure II | ||||
| Ligand type | Fc | Fc+ | Ligand Type | Fc | Fc+ | |
| Fe | -- | 8 e− | 8 e− | -- | 8 e− | 8 e− |
| Cp | X | 2 x (1 e−) | 2 x (1 e−) | L2X | 2 x (5 e−) | 2 x (5 e−) |
| Charge of ion | − (0) | − (+1) | Charge of ion | − (0) | − (+1) | |
| Total electron count | 10 e− | 9 e− | Total electron count | 18 e− | 17 e− | |
Ferrocene readily undergoes a 1 e− oxidation to yield ferrocenium cation (Equation 1).
 (1)
(1)
Let's consider the resulting electron count for the ferrocenium cation of Structures I and II. Upon a 1 e− oxidation, ferrocene becomes ionic. Therefore, the charge (+1) of the ferrocenium cation needs to be subtracted from the valence electrons (Table 2). Subtracting the charge results in total electron counts of 9 e− and 17 e− for Structure I and Structure II, respectively.
Which Structure of Ferrocene is Correct?:
If Structure I is correct, with only 10 valence electrons, ferrocene would be highly reactive at the Fe. Oxidation to yield the ferrocenium cation would be quite challenging in this case, since Structure I of ferrocene is electron deficient. On the other hand, Structure II of ferrocene obeys the 18-electron rule and is therefore consistent with ferrocene being a stable compound. In this case, oxidation of ferrocene to ferrocenium cation would yield a 17 e− species. Based on electron counting, one may predict that ferrocene exhibits Structure II; let's think about how to demonstrate the correct structure spectroscopically.
Before structural data was published, the structure of ferrocene was deduced from its magnetic and spectroscopic properties, as well as its reactivity. Let's consider the IR spectra that we would predict for the two structures. Structure I displays two chemically inequivalent protons and should thus give rise to more than one C-H stretching mode in the IR spectrum. In contrast, Structure II displays a single type of C-H bond and should thus display a single C-H stretch. Had NMR spectroscopy been readily available in the 1950's, this too would provide a clue: two resonances in the 1H spectrum for Structure I, and one resonance for Structure II would be predicted. In this experiment, we will synthesize ferrocene and use spectroscopic data to provide evidence for its structure.
Procedure
1. Cracking the Cyclopentadiene Dimer (Figure 3)
Cyclopentadiene undergoes a Diels-Alder reaction with itself to give dicyclopentadiene. This reaction is reversible, so cracking is accomplished using La Châtelier's principle to drive the reverse reaction by distilling the cyclopentadiene monomer (b.p. 42 °C) away from the dicyclopentadiene dimer (b.p. 170 °C). The dimerization reaction is slow when the cyclopentadiene is kept cold, but it must be freshly prepared to successfully synthesize ferrocene.
- To a 25-mL round-bottom flask, add a stir bar and 10 mL of dicyclopentadiene dimer.
CAUTION: Be careful to only use dicyclopentadiene and cyclopentadiene in a fume hood because they are both stench chemicals. - Attach the round-bottom flask to a fractional distillation apparatus (see "Fractional Distillation" module in the Essentials of Organic Chemistry series), and place in an oil bath on a stirring hot plate. Be sure to have the collecting flasks in an ice bath. Clamp the apparatus in place.
- Set the hot plate to 160 °C and gently stir the solution.
- Fractional distil ~ 5 mL of the Cp monomer from the dimer (39-42 °C).

Figure 3. Cracking of dicyclopentadiene.
2. Synthesis of Ferrocene (Figure 4)
- To a 100-mL Schlenk flask, add a stir bar and 15 g of finely ground KOH.
CAUTION: KOH is very corrosive and hygroscopic! The KOH should be ground in a well-ventilated hood and used immediately after preparation. - Add 30 mL of 1,2-dimethoxyethane to the flask while stirring.
- Connect the flask to nitrogen and place a rubber septum over the neck of the flask.
- While stirring under nitrogen, add 2.75 mL of cyclopentadiene via syringe. Allow this to stir for at least 10 min prior to addition of the iron.
- While the reaction is stirring, add a stir bar, 3.25 g of ground FeCl2·4H2O, and 12.5 mL DMSO to a separate 50-mL Schlenk flask. Place a septum on the neck and stir under nitrogen until all of the iron has dissolved.
- Cannula transfer the iron solution to the cyclopentadienyl solution under nitrogen dropwise over the course of 30 min. For a more detailed procedure, please review the "Synthesis of a Ti(III) Metallocene Using Schlenk Line Technique" video in this Inorganic Chemistry series.
- Once the addition is complete, stir for an additional 30 min.
- Meanwhile, in a beaker cool 45 mL of 6 M HCl by adding crushed ice (50 g) directly to the solution.
- Once the reaction is complete, pour the mixture onto the slurry, and stir for a few minutes. Orange crystals should form.
- Collect the crystals on a Büchner funnel (fitted with filter paper), and wash the precipitate with water.
- Allow the solids to dry in the air.
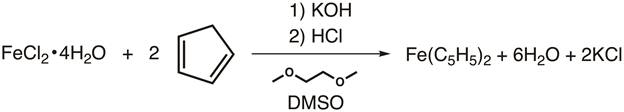
Figure 4. Synthesis of ferrocene.
3. Purification of Ferrocene. Purify the product by sublimation (for a more detailed procedure, please see the "Purification of Ferrocene by Sublimation" video).
4. Characterization of Ferrocene
- Prepare and collect a 1H NMR spectrum of ferrocene.
- Collect an IR spectrum of ferrocene.
- Collect a cyclic voltammogram of ferrocene (see the "Cyclic Voltammetry" video in the Essentials of Analytical Chemistry series).
Results
Ferrocene Characterization:
1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.15 (s).
The 1H NMR spectrum of ferrocene clearly shows a single resonance, consistent with structure II.
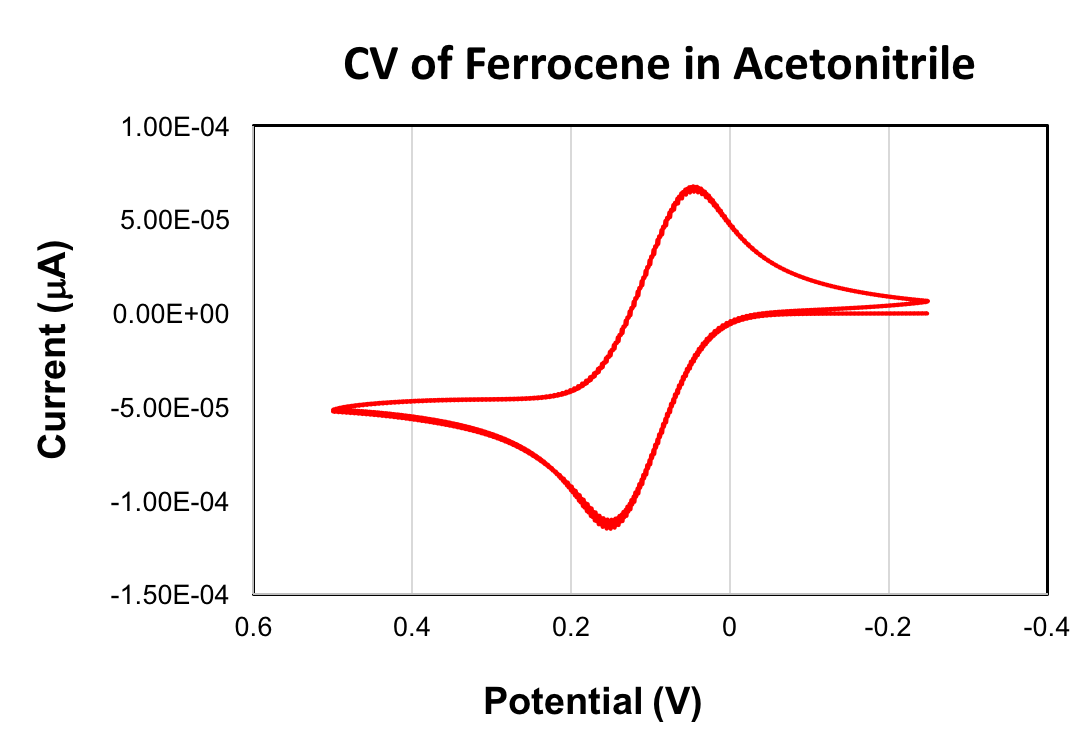
A CV of ferrocene is given below. The E1/2 value obtained for the oxidation of ferrocene was +90 mV (acetonitrile, scan rate 100 mV/s, 0.1 M (Bu4N)PF6, glassy carbon working electrode). The ferrocene/ferrocenium redox couple is commonly used as a reference in cyclic voltammetry. When used as a reference, the E1/2 value of ferrocene is set equal to 0 V.
Application and Summary
In this video, we discussed ferrocene and the role it played in the development of organometallic chemistry. Ferrocene was synthesized and characterized by 1H NMR and IR spectroscopy. Both spectra are consistent with the 18 e− Structure II, where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II). Oxidation of ferrocene to ferrocenium cation was observed electrochemically.
In 1973, following the structural characterization of ferrocene, Wilkinson was one of two chemists awarded the Nobel Prize in Chemistry "for [his] pioneering work … on the chemistry of the organometallic, so called sandwich complexes".6 His work greatly influenced and expanded the emerging field of organometallic chemistry. While the first organometallic compound was prepared in 1849, it was only in the 1950's that significant advancements were made to understanding how metals can bond to carbon atoms. Today, the field of organometallic chemistry, or the chemistry of compounds that form metal-carbon bonds, is central to many applications. This includes: energy, dye-sensitized solar cells, catalysis, polymerization, drug discovery and synthesis, bioinorganic systems, and organic light-emitting diodes (OLEDs).7
Ferrocene itself also plays an active role in the field of organometallic chemistry. Ferrocene readily participates in electrophilic aromatic substitution; in fact, it is 100,000 times more reactive than benzene in these reactions. Ferrocene has found widespread application as a structural component of bidentate ligands in organometallic catalysis. For example, 1,1'-bis(diphenylphosphino)ferrocene (dppf) is a chelating ligand used in homogeneous catalysis. The ligand dppf chelates 1st, 2nd, and 3rd row transition metals including Ni, Pd, and Pt. [1,1'-Bis(diphenylphosphino) ferrocene]palladium(II) dichloride is an example of a palladium cross-coupling catalyst for C-C and C-heteroatom bond formation (Figure 5).8 In the video "MO Theory of Transition Metal Complexes", we will synthesize two metal complexes featuring dppf.
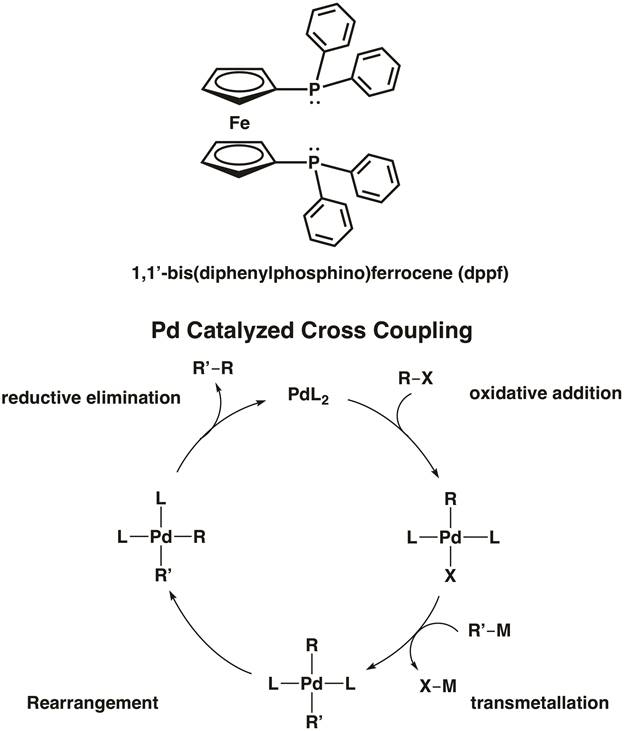
Figure 5. [1,1'-Bis(diphenylphosphino) ferrocene]palladium(II) dichloride is a cross-coupling catalyst for C-C and C-X bond formation.
References
1. Kealy, T. J., Pauson, P. L. A New Type of Organo-Iron Compound. Nature. 168 (4285), 1039-1040 (1951).
2. Pauson, P. L. Ferrocene—how it all began. J Organomet Chem. 637, 3-6 (2001).
3. Seeman, J. I., Cantrill, S. Wrong but seminal. Nat Chem. 8 (3), 193-200 (2016).
4. Wilkinson, G., Rosenblum, M., Whiting, M. C., Woodward, R. B. The Structure of Iron Bis-cyclopentadienyl. 74, 2125-2126 (1952).
5. Green, M. L. H., Parkin, G. Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry. J Chem Educ. 91 (6), 807-816 (2014).
6. Press Release. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1973/press.html.
7. Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 6th ed. John Wiley & Sons. Hoboken. 2014.
8. Gildner, P. G., Colacot, T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics. 34, 5497-5508 (2015).
Skip to...
Videos from this collection:

Now Playing
Structure Of Ferrocene
Inorganic Chemistry
79.1K Views

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.5K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.3K Views

The Evans Method
Inorganic Chemistry
67.9K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
103.9K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.3K Views

Mössbauer Spectroscopy
Inorganic Chemistry
21.9K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.7K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
44.9K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.1K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.7K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.5K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved