Biofuels: Producing Ethanol from Cellulosic Material
Overview
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
In this experiment, cellulosic material (such as corn stalks, leaves, grasses, etc.) will be used as a feedstock for the production of ethanol. The cellulosic material is first pretreated (ground and heated), digested with enzymes, and then fermented with yeast. Ethanol production is monitored using an ethanol probe. The experiment can be extended to optimize ethanol production by varying the feedstock used, pretreatment conditions, enzyme variation, yeast variation, etc. An alternative method of monitoring the reaction is to measure the carbon dioxide produced (using a gas sensor) instead of the ethanol. As a low-tech alternative, glucose meters (found in any drug store) can be used to monitor the glucose during the process, if an ethanol probe or carbon dioxide gas sensor is not available.
With an increased emphasis on ‘inquiry-based learning”, scientific probes are becoming more popular. Handheld devices like the Vernier Lab Quest used in conjunction with a variety of probes (such as those for conductivity, dissolved oxygen, voltage, and more) allow for less focus on collecting data and/or making graphs and more on analyzing the data and making predictions. Another advantage is that these are small and lightweight and can be taken into the field for measurements.
Principles
The United States is looking to wean itself off of fossil fuels, especially petroleum used in gasoline. Global climate change, dependence on foreign countries, and increased political instability around the world are only a few reasons why. One possible way to decrease dependence on petroleum as a transportation fuel is by using more ethanol. Currently, regular gasoline contains approximately 10% ethanol as an additive. Special flex-fuel vehicles can use E85 gasoline, which is 85% ethanol.1
In Brazil, ethanol is made by using sugarcane as the feedstock. The main product of sugarcane is sucrose, which is a disaccharide of glucose and fructose. Most species of yeast have the enzyme sucrase and are able to cleave the glucose – fructose bond. The sucrose (C12H22O11) is fermented by yeast to produce ethanol and carbon dioxide. The overall chemical reaction is shown in Equation 1.2,3
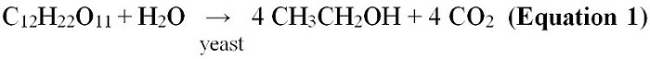
However, in the US, ethanol is made from corn. Corn stores carbohydrates as a polysaccharide called starch. This requires more effort than making ethanol from sugarcane because yeast cannot cleave the bonds in starch. To make ethanol from corn, first corn kernels need to be ground up, then the ground corn treated with enzymes to convert the starch in the corn to glucose. After this step, the process continues as in the sugarcane method above by using yeast to ferment the glucose into ethanol and carbon dioxide. The chemical reaction is shown in Equation 2.2

The production of ethanol from corn is problematic, however. For one thing, it takes some corn out of the food supply, especially feed for livestock, thus driving up prices. It is also energy and fertilizer intensive to produce corn, decreasing its desirability as a transportation fuel alternative to petroleum. Therefore, scientists are increasingly turning to cellulosic material to make ethanol. These materials include wood, grasses, and non-edible parts of plants. These are more desirable as they do not impact food supply. However, in order to release the glucose from the cellulosic material, much more effort is needed, as the glucose from cellulosic material is bound up in cellulose, which is then wrapped with hemicelluloses and lignin. First the cellulose needs to be extracted from the hemicelluloses and lignin bindings. This is done through a pretreatment of grinding and acid hydrolysis. Then, the cellulose is treated with enzymes to break it up into its component glucose. Finally, the glucose can be fermented with yeast to produce ethanol and water. The process can be summarized as shown in Equation 3.

This is currently too energy intensive to make it a practical method for large-scale ethanol production. However, research is underway to make the process better.2,3
Procedure
1. Sample Preparation
- Select cellulosic material to be used as feedstock. This can be corn stalks, grasses, leaves, pet bedding, or paper.
- Using a ball mill grinder (or coffee grinder if ball mill grinder is not available), grind feedstock into a fine powder with no large pieces remaining.
- Measure 1.0 g of feedstock and place in a 50-mL centrifuge tube. Label the tube with the feedstock chosen.
- Label a second 50 mL centrifuge tube as “Control”. Do not put any feedstock in this tube.
2. Pretreatment
- Set up a 500-mL beaker with approximately 400 mL of water on a hotplate and heat to a gentle boil.
- Add 25 mL of distilled water to the 2 centrifuge tubes. Swirl to mix. Put the cap on the centrifuge tubes loosely.
- Put the centrifuge tubes in the beaker full of gently boiling water. Be sure that water from the water bath does not leak into the tubes. Let boil for 30 min.
- Let tubes cool to room temperature.
3. Enzymatic Digestion
- Add 1 mL of cellulase enzyme to both tubes.
- Put tubes in an incubator at 50 °C for 24 h.
- Allow the tubes to cool to room temperature.
4. Fermentation
- Add 1.0 g of active yeast (regular grocery store yeast is ok) to each of the centrifuge tubes. Swirl to mix.
- Put an airlock on top of the centrifuge tube. The airlock allows CO2 to escape, keeping the pressure low in the centrifuge tube.
- Place the centrifuge tubes in a rack and put in an incubator at 37 °C for 24 h.
- Using an ethanol sensor, measure the ethanol concentration in the control tube (Figure 1). Ethanol sensors can be purchased through Vernier or PASCO for approximately $100 each.

Figure 1. Ethanol probe measuring the ethanol concentration in the control tube. - Using an ethanol sensor, measure the ethanol concentration in the sample tube (Figure 2).

Figure 2. Handheld tablet displaying % of ethanol.
Results
The % ethanol in the solution will be displayed on the handheld tablet screen using the software related to the brand of the ethanol sensor used (Figure 2).
Representative results of the percent ethanol produced by various feedstocks can be seen in Table 1.
| Feedstock | Ethanol produced |
| Sawdust | 0.70% |
| Corn Stalks | 0.60% |
| Cardboard | 1.67% |
| Switchgrass | 0.37% |
| Control | 0.11% |
Table 1. Representative results of the percent ethanol produced by various feedstocks.
Application and Summary
The Energy Independence and Security Act of 2007 set into law a renewable fuel standard. It created a phase-in for renewable fuel volumes starting at 9 billion gallons in 2008 and ending at 36 billion gallons in 2022. Of that 36 billion, it was expected that 16 billion of that would come from cellulosic materials. For 2014, the original proposal was for 18.15 billion gallons of renewable fuel, 1.75 billion of that coming from cellulosic material. Unfortunately, based on the volume of cellulosic ethanol that is feasible to be produced currently, this number has had to be reduced to 17 million gallons according to a recent EPA proposal.1 Improving the process of creating ethanol from cellulosic material is currently a very hot area of research. In this experiment, students will be emulating the scientific practices that scientists in the top research labs are following.
A variety of biomass feedstock materials can be used to produce cellulosic ethanol for transportation. The U.S. Department of Energy’s Bioenergy Technologies Office is focused on developing cellulosic feedstock from non-food based plant material and the related technologies that will allow an economically feasible conversion of this biomass to transportation fuel. They are investigating biomass sources ranging from agricultural residue, herbaceous energy crops, and forest materials to waste materials and algae. In this laboratory activity, students can vary the feedstock they use and compare the amount of ethanol that results. Possibilities include corn stover, grasses, leaves, cardboard, newspaper, paper, flowers, etc.
One roadblock to large scale production of cellulosic ethanol is the costly nature (both in terms of money and energy) of the pretreatment process. Much research is being done on reducing these costs and making the breakdown of the cellulosic material easier. Enzyme companies are spending a lot of time and money developing new enzymes to increase the yield of ethanol. In this laboratory activity, students can vary the enzymes they use and compare the amount of ethanol that results. A variety of Cellulase enzymes can be purchased from chemical companies that sell to schools, such as cellulase from Aspergillus niger or cellulase from Trichoderma virde. Or proprietary enzymes can be purchased from specialty enzyme companies, however these are costly. Other enzymes can be used, like amylase, to compare their ability of producing ethanol from cellulosic material to that of the cellulases.
Another emerging area of research is improving the ability of yeast to convert cellulosic biomass into ethanol. Lignocellulose has evolved in plants to provide stability. This is due to the crosslinking between cellulose and hemicellulose and the ester linkages in lignin. It is necessary to separate the cellulose from the lignin and then treat the cellulose to break it down into a monosaccharide. In addition, the hemicellulose has a high percentage of pentoses like xylose, which is more difficult to ferment than a hexose like glucose.4
References
- The Energy Independence and Security Act of 2007. United States Congress, Washington DC. January 4, (2007).
- Balat, M., Balat, H., Oz, C. Progress in bioethanol processing. Progress in Energy and Combustion Science. 34 (2008).
- Ragauskas, A.J., Williams, C.K., Davison, B.H., Britovsek, G., Cairney, J., Eckert, C.A., Frederick Jr, W.J., Hallett, J.P., Leak, D.J., Liotta, C.L., Mielenz, J.R., Murphy, R., Templer, R., Tschaplinski, T. The Path Forward for Biofuels and Biomaterials. Science. 311 484 (2006).
- Demirbas, A. Bioethanol from Cellulosic Materials: A Renewable Motor Fuel from Biomass. Energy Source. 27 327-337 (2005).
Skip to...
Videos from this collection:

Now Playing
Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.1K Views

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.1K Views

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.4K Views

Using GIS to Investigate Urban Forestry
Environmental Science
12.6K Views

Proton Exchange Membrane Fuel Cells
Environmental Science
22.0K Views

Testing For Genetically Modified Foods
Environmental Science
89.7K Views

Turbidity and Total Solids in Surface Water
Environmental Science
35.8K Views

Dissolved Oxygen in Surface Water
Environmental Science
55.7K Views

Nutrients in Aquatic Ecosystems
Environmental Science
38.8K Views

Measuring Tropospheric Ozone
Environmental Science
26.4K Views

Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.0K Views

Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
125.4K Views

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.4K Views

Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
215.7K Views

Analysis of Earthworm Populations in Soil
Environmental Science
16.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved